INTRODUCTION
The presence or absence of parasites in host populations is the result of a complex of factors, some biotic and others abiotic. Although there is little consensus as to which of these factors are dominant, it is clear that the diversity and abundance of parasites differs from one host population to the next. This variation is to be expected, as habitat characteristics, biotic or abiotic, vary between locations and this can either facilitate or inhibit the establishment and persistence of parasites in host populations. For example, the critical importance of biotic factors on parasite populations has been demonstrated in salmonid fish (Kennedy and Bush, Reference Kennedy and Bush1994), rocky reef fish (Holmes, Reference Holmes, Esch, Bush and Aho1990) and waterfowl (Bush and Holmes, Reference Bush and Holmes1986). On the other hand, it has been argued that the role of biotic factors have been greatly over-emphasized (i.e. Combes, Reference Combes2001) and natural abiotic factors such as ‘harsh’ environmental conditions (Galaktionov, Reference Galaktionov1993; Marcogliese and Cone, Reference Marcogliese and Cone1996; Biserkov and Kostadinova, Reference Biserkov and Kostadinova1998) and anthropogenic perturbations (see MacKenzie et al. Reference MacKenzie, Williams, Williams, McVicar and Sidall1995; Marcogliese and Cone, Reference Marcogliese and Cone1998) may also have large impacts on parasite populations.
In heteroxenous parasite species (those with multiple hosts), it is thought that the dominant drivers of the parasite infracommunities are related to the diversity and abundance of the host community (Huspeni and Lafferty, Reference Huspeni and Lafferty2004; Hechinger and Lafferty, Reference Hechinger and Lafferty2005; Hechinger et al. Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007). The rationale being that as parasite lineages diversify over evolutionary time, they become embedded within the trophic interactions of host food webs, and host-feeding links provide repeatable and dependable interactions for parasites to use as ‘pathways’ for transmission (Marcogliese and Cone, Reference Marcogliese and Cone1997). The number and distribution of pathways within food webs is a direct consequence of the number of species present, as diversity increases so too does the number of trophic connections (MacArthur, Reference MacArthur1955). Thus, diverse communities of invertebrates, fishes and birds have the ability to support multiple parasite species with complex life cycles (Marcogliese, Reference Marcogliese2002, Reference Marcogliese2005; Hudson et al. Reference Hudson, Dobson and Lafferty2006). This is because a typical parasite life cycle may include fish (or bird) definitive hosts and several intermediate invertebrate hosts, and for the parasite to survive, all hosts must co-occur in a stable community structure (Marcogliese and Cone, Reference Marcogliese and Cone1997). Consequently, it is generally accepted that the diversity and stability of the host community, primarily that of the definitive hosts, should impose significant restrictions on species composition of local parasite communities (Rohde, Reference Rohde1993; Combes, Reference Combes2001; Poulin, Reference Poulin2007).
On the other hand, abiotic variables affect the biotic variables, and the interaction between abiotic and biotic variables may be the actual agent of parasite community composition. Indeed, variables such as lake size, water pH and distance between lakes have been associated with the diversity of parasite species in host populations, or with the mean abundance of parasites within given hosts (Kennedy, Reference Kennedy1978; Karvonen et al. Reference Karvonen, Paukku, Valtonen and Hudson2003; Simkova et al. Reference Simkova, Sitko, Okulewicz and Morand2003). Further, there is evidence that seasonal variability in water temperature drives parasite population cycles in temperate and tropical areas (Chubb, Reference Chubb1979; Rohde, Reference Rohde1993). At even smaller spatial scales, there is evidence that water flow rate in streams alters parasite populations in fish (Janovy et al. Reference Janovy, Snyder and Clopton1997; Marcogliese, Reference Marcogliese2001). Additionally, heteroxenous endoparasites are highly susceptible to abiotic conditions through the elimination or reduction of potential intermediate hosts (Poulin, Reference Poulin1992), and direct effects on the population dynamics of definitive hosts (Moller, Reference Moller1987). However, there is no general consensus on the importance of abiotic effects on parasites, and the majority of studies that consider abiotic variables show trends that are positive, negative or neutral depending on the abiotic variable and the parasite taxa (Khan and Thulin, Reference Khan and Thulin1991; Poulin, Reference Poulin1992; MacKenzie et al. Reference MacKenzie, Williams, Williams, McVicar and Sidall1995; Valtonen et al. Reference Valtonen, Holmes and Koskivaara1997; Ondrackova et al. Reference Ondrackova, Simkova, Gelnar and Jurajda2004; Marcogliese, Reference Marcogliese2004, Reference Marcogliese2005).
In this study, we compared the relative importance of abiotic and biotic factors that contribute to the presence of 3 complex life-cycle parasites in a sentinel fish species, Fundulus heteroclitus, using a hierarchical classification tree analysis. We consider F. heteroclitus (common killifish) a sentinel fish because it is a highly abundant resident marsh species (Lotrich, Reference Lotrich1975) along the east coast of North America (Relyea, Reference Relyea1983), and likely plays an important role in marsh food webs. Furthermore, the common killifish was selected for this study because its parasite fauna is well known in New Jersey (Anderson and Sukhdeo, unpublished data) and it is known to harbour multiple heteroxenous parasites (Harris and Vogelbein, Reference Harris and Vogelbein2006). Thus, the presence of parasite species within the common killifish is likely to be sensitive to both biotic and abiotic conditions.
To include a wide range of differences in biotic and abiotic parameters for our analyses, we used a system of estuarine salt marshes located in the Hackensack Meadowlands estuary complex in New Jersey, USA, each with distinct host diversity, abundances and variation in abiotic conditions. Classification tree analysis, a non-parametric technique that hierarchically partitions a data set using dichotomous criteria based upon multiple predictor variables (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984; De'Ath and Fabricius, Reference De'Ath and Fabricius2000) was used to separate the relative effects of biotic and abiotic variables. The output generated is similar to other regression techniques in that it explains the variation of a single response variable through one or more explanatory variables with the added advantage of the predictor variables being assigned a hierarchical order. Trees are constructed by repeatedly splitting the data using a simple rule based on a single explanatory variable. The products of each split are two mutually exclusive groups, each of which being as homogenous as possible. A major advantage of this analysis technique is that it does not rely on the assumptions that are required for the appropriate use of parametric statistics (i.e. Gaussian distribution of predictor variables). Further, regression tree analysis is not restricted by linearity in predictor and response variables or by multicollinearity in predictor variables. In doing this analysis we are able to construct a hierarchical tree that documents the relative importance of abiotic and biotic variables in the presence of parasite populations.
Our a priori hypothesis was that the presence of complex life cycle parasite populations would be dependent on the presence of competent host species. Specifically, we predicted that (1) definitive host presence will be the most important factor (i.e. primary explanatory variable) in determining parasite presence; and (2) intermediate host presence will be assigned a secondary position in the analysis hierarchy.
MATERIALS AND METHODS
Characterization of study sites
Sampling occurred within four salt marshes in the New Jersey Hackensack Meadowlands. These marshes reflect a gradient in time since restoration and a gradient in community diversity: Mill Creek Marsh (20 years since restoration), Harrier Meadow (10 years since restoration), Secaucus High School Marsh (0 years) and Oritani Marsh (unrestored). Mill Creek marsh (20 year) is a 57-hectare tidal marsh bordered by highways and residential land (40°47′45′′ N, 74°02′30′′ W). Harrier Meadow marsh (10 year) is a 32-hectare tidal marsh surrounded by tidal mudflats and urban development (40°47′12′′ N, 74°07′3′′ W). Secaucus High School marsh (0 year) is a 43-hectare tidal marsh bordered by a river and residential development (40°48′17′′ N, 74°02′52′′ W). Oritani marsh (unrestored) is a 224-hectare tidal marsh that has no record of human alteration or use (40°47′57′′ N, 74°05′07′′ W). Though this marsh is much larger than the others, more than 150 hectares are upland area, and a smaller area (~70 hectares) consists of high and low marsh with small tidal channels, making it of similar size to the other marshes.
Free-living community data including species abundances were collected from the literature for birds (Seigel et al. Reference Seigel, Hatfield and Hartman2005; Seigel, Reference Seigel2006; personal communication), fishes (Bragin et al. Reference Bragin, Misuik, Woolcott, Barrett and Jusino-Atresino2005; personal communication) and benthos (Yuhas, Reference Yuhas2001; Yuhas et al. Reference Yuhas, Hartman and Weis2005; personal communication). To control for the influence of abundant species relative to rare species, all species abundances were square root transformed. To determine variation in the compositional structure of the host community between sites, we used non-metric multidimensional scaling (NMDS) with the Bray-Curtis dissimilarity index. Ordination was implemented using the software PC-ORD version 5 (McCune and Grace, Reference McCune and Grace2002) with 500 iterations and 250 runs of both real and randomized data. Abiotic parameters for each marsh were recorded at continuous water monitoring stations located at each of the 4 marshes. Water monitoring stations consisted of Yellow Springs Instruments Model 6600 multiprobes: these probes were configured to measure abiotic water parameters, at approximately 1·0 m depth. The predictor variables used in this analysis are listed in Table 1. Predictor variables included the square root transformed abundance of each potential host species in the marsh (benthic invertebrates, fishes, birds), dissolved oxygen, acidity, specific conductance, temperature, salinity, heavy metals and turbidity.
Table 1. Abiotic and biotic variables sampled within four salt marshes in the New Jersey Hackensack Meadowlands
(These data were used as predictor variables in classification and regression tree analyses.)

Fish sampling and examination for parasites
A sentinel fish, Fundulus heteroclitus (common killifish), and its helminth parasite community were measured every 3 months starting in December 2005 and ending in December 2007 (8 contiguous seasons: 2 fall, 2 spring, 2 winter, 2 summer). Fish were collected using a 4 mm seine and baited minnow traps; all habitats within each marsh were sampled for at least 5 days each season. From each seasonal collection, 15 fish were euthanized and immediately necropsied. Fish necropsy was done using standard parasitological techniques. Helminth parasites collected during necropsy were identified using parasitological keys (Yamaguti, Reference Yamaguti1958; Schell, Reference Schell1970; Schmidt, Reference Schmidt1970; Anderson et al. Reference Anderson, Chabaud and Willmott1974) and primary literature (Harris and Vogelbein, Reference Harris and Vogelbein2006).
Data analysis
Classification and regression tree analysis was performed using the software platform JMP Version 7.0.1 (2007 SAS Institute). We selected 3 representative parasite species (an acanthocephalan Paratenuisentis ambiguous, a nematode Contracaecum sp. and a trematode Ascocotyle diminuta) for this analysis based upon 3 factors: (1) we wished to address the abiotic and biotic factors important in the presence/absence of complex life cycle parasites; (2) these complex life cycle parasites were prevalent enough to warrant statistical inference; and (3) these 3 species occurred at each of our sites. The criterion used for selecting the splits on the nodes was set to ‘Maximise Split Statistic’. This split selection examines all possible splits for each predictor variable at each node. Missing values in our data were assigned ‘Closest’ and the minimum split size was set to 5. We used a k-fold cross-validation procedure to determine the optimal tree size. This process divides the data into a number of mutually exclusive subsets (k subsets) of approximately the same size and builds a series of trees using 90% of the available subsets and uses them to predict the response for the omitted subset(s). It subsequently calculates the error for each tree as the sum of the squared differences between observed trees and predicted trees; for each tree, we ran a series of 10-fold cross-validations and chose the best tree size using the 1-SE rule (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984; De'Ath and Fabricius, Reference De'Ath and Fabricius2000). Trees are represented graphically, with the root node representing undivided data at the top, and the branches and leaves (each leaf represents a final grouping) beneath.
We further explored relationships within the data using alternative splits and surrogate variables. For each of the splits in the data, we compared the strengths of the split due to the selected variable with the best splits of each of the remaining predictor variables. A strongly competing alternative variable was substituted for the original variable to determine whether a tree could be simplified or the number of predictor variables be reduced. Often surrogate variables will give the best alternative split; consequently we generated trees using surrogate variables. Should surrogate variables generate trees in accordance with the tree generated by the primary splitting variable, it is plausible to state that they are equally important in determining the presence/absence of the response variable.
RESULTS
A total of 480 sentinel fish were necropsied: 15 collected in each of the 8 seasons between 2006 and 2007 in each of the 4 marshes. Host species richness was 71 spp. in Oritani marsh, 87 spp. in Secaucus marsh, 112 spp. in Harrier marsh and 122 spp. in Mill Creek marsh. These marshes had significantly different benthic, fish and bird communities with regards to abundance and similarity as measured by non-metric multidimensional scaling analyses. Non-metric multidimensional scaling analyses of birds (r 2=0·81, stress value=0·08), fishes (r 2=0·82, stress value=0·09) and benthic macroinvertebrates (r 2=0·82, stress value=0·07) showed distinct separation in communities between each of the 4 marshes sampled. Within our sentinel species, 11 taxa of metazoan parasites were identified including the nematodes Dichelyne bullocki and Contracaecum sp.; the digenean Lasiotocus minutus and metacercaria of Ascocotyle diminuta, Mesostephanus appendiculatoides, Posthodiplostomum minimum; monogeneans Fundulotrema prolongis and Swingleus ancistrus; acanthocephalans Paratenuisentis ambiguous and Southwellina hispida (cystacanth); the copepod Ergasilus funduli. These taxa infected more than 70% of the killifish examined and parasite intensity per host ranged from 1 to 127.
Of the aforementioned parasite species, only 3 complex life cycle parasites, a trematode A. diminuta, an acanthocephalan P. ambiguous, and a nematode Contracaecum sp., were present in all 4 sites and of sufficiently high prevalence to warrant statistical inference. Consequently, for these 3 parasite populations, we constructed classification trees that assigned each of the 480 host observations of parasite presence/absence to terminal leaf nodes: these terminal leaf nodes categorize broad-scale controls on the establishment of these parasite populations in our salt marsh system. Using a cross-validation procedure (sensuBreiman et al. Reference Breiman, Friedman, Olshen and Stone1984) we obtained estimates of prediction error for trees of a given size (Figs 1b, 2b and 3b). The best tree size was taken as the smallest tree such that its prediction error rate is within 1 standard error of the minimum (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984). For the trematode A. diminuta, cross-validation using the 1-SE rule determined the optimal tree size as a 5-leaf tree (Fig. 1a), and 9-leaf trees for the acanthocephalan P. ambiguous (Fig. 2a) and the nematode Contracaecum sp. (Fig. 3a). The tree selected for A. diminuta explained 31% of the variance in the data and the trees selected for Contracaecum sp. and P. ambiguous explained 36% and 30% of the variance respectively. These r 2 values are acceptable given that our initial analyses included 184 predictor variables.

Fig. 1. (a) Classification tree and decision criteria for predicting the presence or absence of Ascocotyle diminuta in the killifish, Fundulus heteroclitus. Decision criteria, split size and infection status (percentage infected in black, uninfected in white) are identified. (b) Cross-validation relative error plots for a single 10-fold cross-validation including 1-SE estimates for each tree size. Under unstratified cross-validation, a tree size of 5 is selected using the 1-SE rule: the best tree size was taken as the smallest tree such that its prediction error rate is within 1 standard error of the minimum.
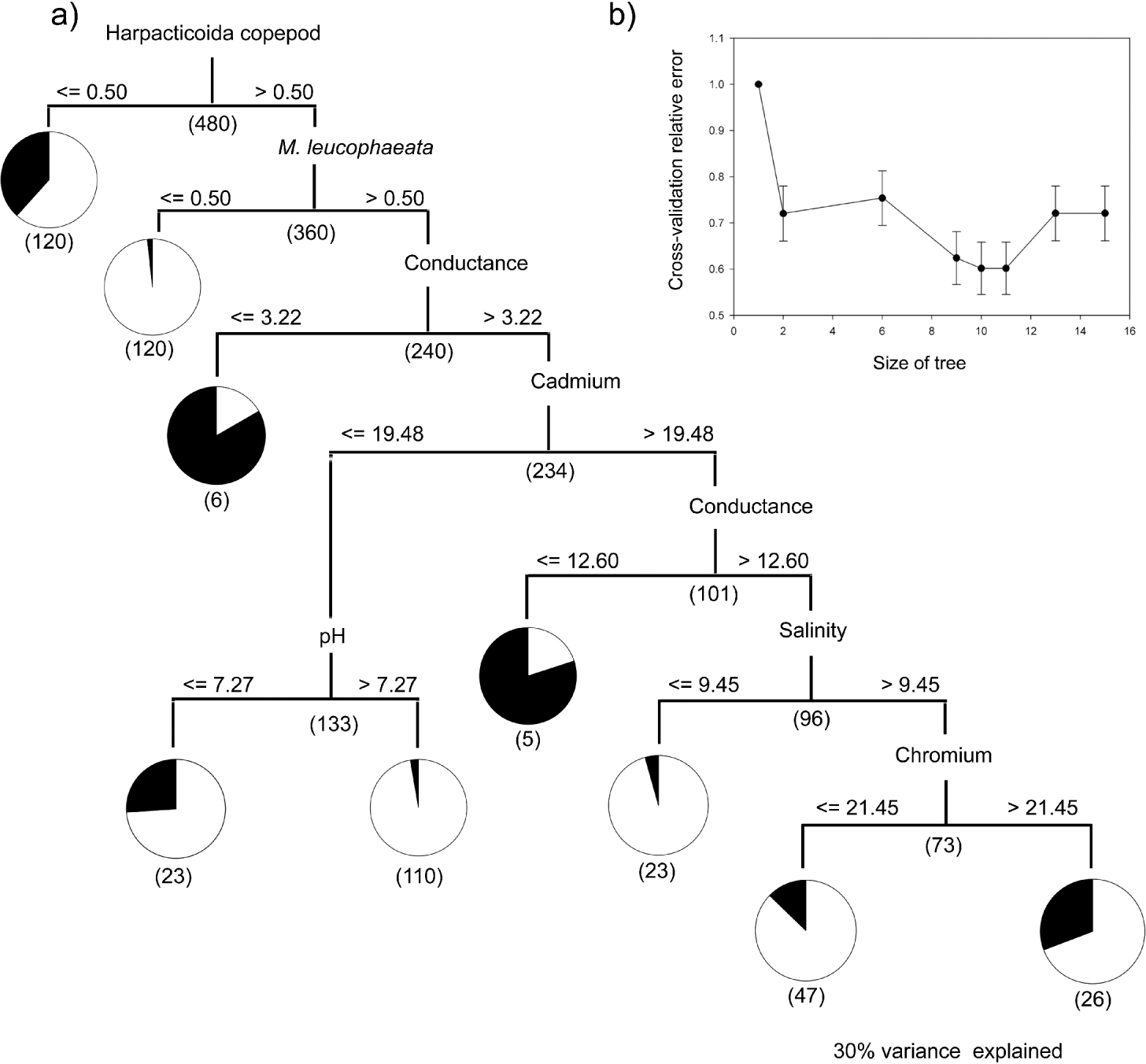
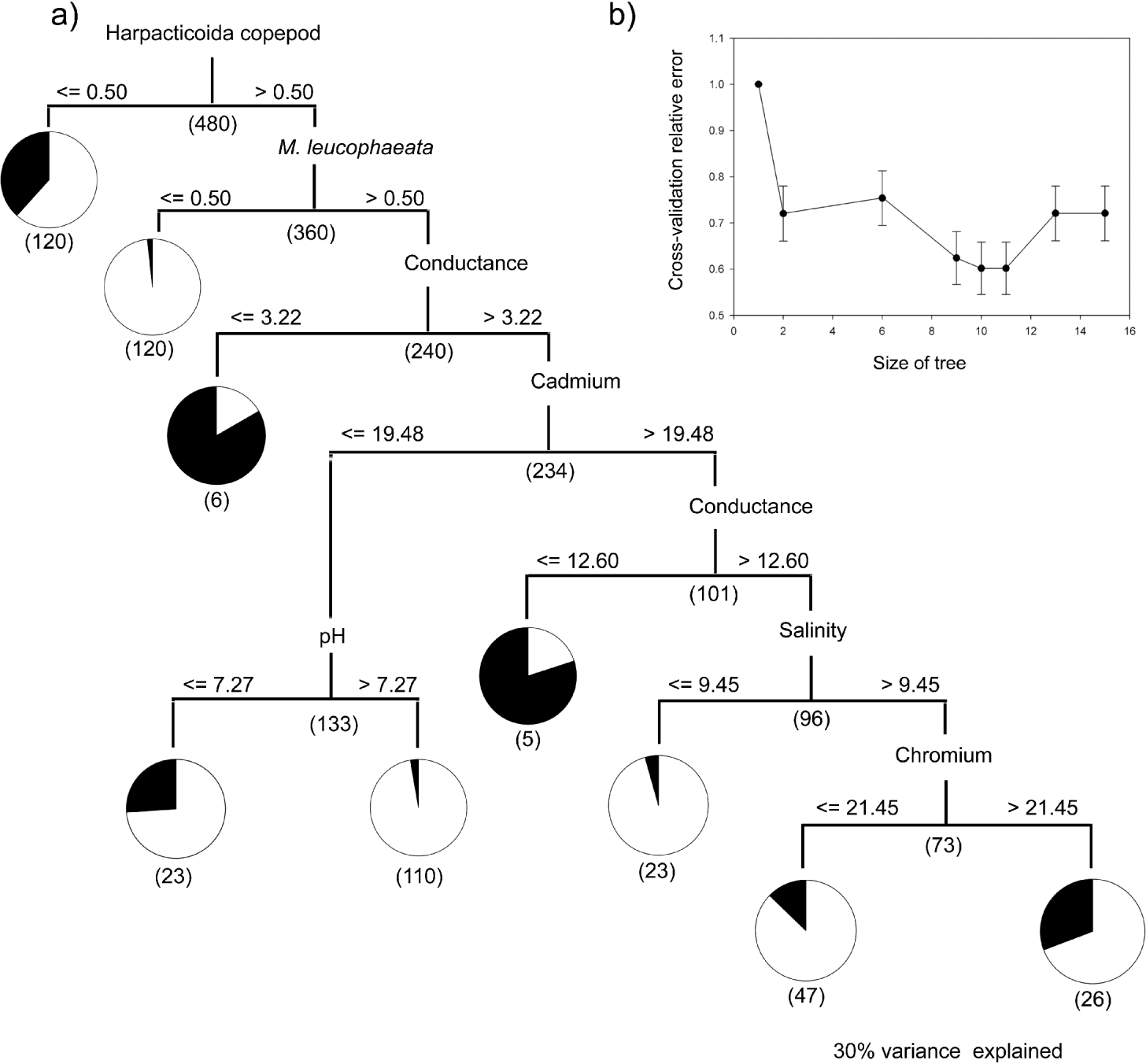
Fig. 2. (a) Classification tree and decision criteria for predicting the presence or absence of Paratenuisentis ambiguous in the killifish, Fundulus heteroclitus. Decision criteria, split size and infection status (percentage infected in black, uninfected in white) are identified. (b) Cross-validation relative error plots for a single 10-fold cross-validation including 1-SE estimates for each tree size. Under unstratified cross-validation, a tree size of 9 is selected using the 1-SE rule: the best tree size was taken as the smallest tree such that its prediction error rate is within 1 standard error of the minimum.

Fig. 3. (a) Classification tree and decision criteria for predicting the presence or absence of Contracaecum sp. in the killifish, Fundulus heteroclitus. Decision criteria, split size and infection status (percentage infected in black, uninfected in white) are identified. (b) Cross-validation relative error plots for a single 10-fold cross-validation including 1-SE estimates for each tree size. Under unstratified cross-validation, a tree size of 9 is selected using the 1-SE rule: the best tree size was taken as the smallest tree such that its prediction error rate is within 1 standard error of the minimum.
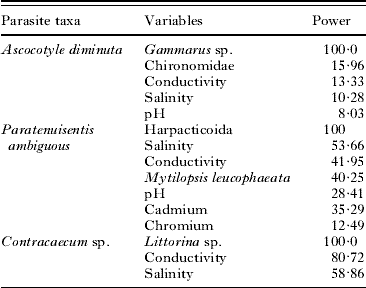
The first split within the A. diminuta tree is the abundance of the amphipod, Gammarus sp. Receiver operating characteristic (ROC) curves, which plot the proportion of correct classifications, demonstrate that a test model including the predominant predictors classified 80·1% of the host observations as infected or uninfected correctly. Though the optimal tree size for A. diminuta (Fig. 1a) included a series of abiotic parameters (pH and conductance) that split the data into progressively more homogenous leaf nodes, the discriminatory power of these secondary predictors was relatively low (less than 15%: Table 2). Surrogate variables were examined to better understand the relative effects of these biotic and abiotic variables. In the classification of A. diminuta, a suite of benthic invertebrates (oligochaetes, Hobsonia florida, Melampus bidentatus, Balanus improvisus) and one fish species (the American shad Alosa sapidissima) were strong surrogates for Gammarus sp. and provided classification trees in accordance with Fig. 1a. For example, dropping the abundance of Gammarus sp. from the A. diminuta model resulted in the same 5-leaf tree with the same splitting criteria and it explained only marginally less of the variance in the data (31% vs 30%).
Table 2. Ranking of factors contributing to infection status of Fundulus heteroclitus for three complex life cycle parasites by overall discriminatory power
(Only variables representing primary splitters are presented.)
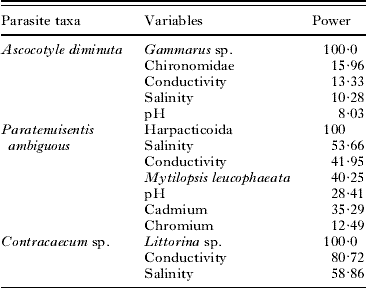
The optimal tree selected for the acanthocephalan P. ambiguous (Fig. 2a) partitioned the data into 2 main branches based on the abundance of harpacticoid copepods, followed by the abundance of an invasive mussel species (Mytilopsis leucophaeta) and then a series of abiotic variables (conductivity, pH, the heavy metals cadmium and chromium, and salinity). ROC curves, demonstrated that a test model including the predominant predictors classified 71% of the host observations as infected or uninfected correctly. The relative discriminatory power for the primary splitter variables (Table 2) used in P. ambiguous revealed an increased importance of abiotic parameters, with salinity (53·66%) and conductance (41·95%) playing a similarly important a role as the secondary biotic variable, M. leucophaeta (40·25%). In the classification of P. ambiguous host infection status, the primary splitter (harpacticoid copepod abundance) had strong surrogates in 3 families of fly larvae (Empididae, Ceratopogonidae and Tipulidae), a polychaete (Glycera sp.), and an unidentified species of amphipod. The surrogates for the secondary splitter, M. leucophaeta, were a diverse group of benthic invertebrates (Littorina sp., Macoma sp., Corophium sp. and Nereis succinea).
The optimal 9-leaf tree that described the presence of Contracaecum sp. (Fig. 3a) partitioned the data on the common periwinkle, Littorina sp., and then a series of abiotic parameters (conductance, turbidity, pH, temperature and salinity). Relative discriminatory power (Table 2) ranked the important variables to Littorina sp. (100%), conductance (81%) and salinity (59%). Using ROC curves, the infection status of the common killifish was correctly determined 67% of the time. The primary predictor splitter in the Contracaecum sp. classification tree, Littorina sp., had surrogates of 2 polychaete species (N. succinea, Streblospio benedecti) and 3 benthic invertebrates (Macoma sp., Nemertea, Palmacorixa sp.): in the case of P. ambiguous and Contracaecum sp. the biotic surrogates provided classification trees in accordance with those presented in Figs 2a and 3a.
Where the surrogates and alternative splits for biotic variables were strong, the surrogates and alternative splits for abiotic parameters included in the models for each of the 3 species were considerably weaker and showed no consistent pattern when removed from analyses. The only consistent trend being that abiotic parameters seemed to be collinear i.e. temperature was collinear with dissolved oxygen and pH, conductance was collinear with salinity and pH.
DISCUSSION
In this study, we constructed hierarchies of biotic and abiotic variables that contribute to the success of 3 representative complex life cycle parasite populations. The key insight provided by our analyses is that a diverse benthic community is more important in determining parasite presence within our sentinel fish than the presence of competent hosts. This is not to say that parasites exist without hosts, but that free-living groupings of organisms are more likely to support competent hosts. Other studies have differed, reporting strong positive relationships between parasite community diversity and the diversity of bird definitive hosts (Smith, Reference Smith2001; Hechinger and Lafferty, Reference Hechinger and Lafferty2005) and large benthic host invertebrates (Hechinger et al. Reference Hechinger, Lafferty, Huspeni, Brooks and Kuris2007). Our data do not support this pattern. Competent hosts were less important in determining parasite presence than non-host benthic invertebrates in our analyses. Thus, our data suggest that it is not simply the presence of competent host species, but the context in which the trophic interaction between fishes and birds, or crustaceans and fishes, occurs that is necessary for the successful completion of each parasite's life cycle.
Earlier studies, largely using digeneans, have elegantly demonstrated the effects of intermediate host density and contact rates on parasite population dynamics (Marcogliese et al. Reference Marcogliese, Dumont, Gendron, Mailhot, Bergeron and McLaughlin2001; Sandland et al. Reference Sandland, Goater and Danylchuk2001). These studies complemented earlier evidence that abiotic factors such as water flow rate and temperature may affect the success of parasite infective stages (Stables and Chappell, Reference Stables and Chappell1986; Janovy et al. Reference Janovy, Snyder and Clopton1997). Further, other studies have correlated the physical structure of the ecosystem (i.e. habitat characteristics such as character of the lake or river bottom, presence or absence of macrophyte vegetation) and the abundance of the digenean Diplostomum spp. in fish hosts (Marcogliese et al. Reference Marcogliese, Dumont, Gendron, Mailhot, Bergeron and McLaughlin2001). However, there is only 1 study (Ondrackova et al. Reference Ondrackova, Simkova, Gelnar and Jurajda2004) that attempted to explain the presence of a parasite species with all potential predictor variables (i.e. intermediate and definitive hosts, community structure of the fish community, and habitat characteristics). Much like the study by Ondrackova et al. (Reference Ondrackova, Simkova, Gelnar and Jurajda2004), the advantage of our study is that it considers a broad suite of abiotic and biotic variables. However, in addition to identifying predictor variables that correlate with the presence of certain parasite species, our method also provides a hierarchical ranking of the relative importance of each variable.
Classical regression techniques used in abiotic and biotic parasite studies (e.g. Ondrackova et al. Reference Ondrackova, Simkova, Gelnar and Jurajda2004) assume that interactions are linear or additive and that the specified models describing interactions are realistic. In contrast, the classification analysis we use provides a flexible relationship between predictor variables, allows for missing values, is not sensitive to multicollinearity, and controls for outliers in the data (Breiman et al. Reference Breiman, Friedman, Olshen and Stone1984; De'Ath and Fabricius, Reference De'Ath and Fabricius2000). For instance, outliers have little influence on splitting because they are partitioned into unique subsets during analysis. In addition, missing values are estimated using surrogate predictor variables so that missing predictor data do not influence the decision tree output. Furthermore, the relative discriminatory power for each predictor may also be determined while the comparison of relative discriminatory power is not possible in classic multivariate analyses. Classification tree analysis flexibility and power has seen the technique become popular in environmental science studies (e.g. Rothwell et al. Reference Rothwell, Futter and Dise2008), ecology (e.g. De'Ath and Fabricius, Reference De'Ath and Fabricius2000) and has been implemented to describe risk factors in malaria transmission (Thang et al. Reference Thang, Erhart, Speybroeck, Hung, Thuan, Hung, Ky, Coosemans and D'Alessandro2008).
The analyses we present suggest that the presence of 3 complex life cycle parasites in F. heteroclitus is primarily the result of a diverse community of benthic invertebrates. In each of the hierarchical displays of dichotomous decision criteria the abundance of benthic invertebrates is the most important factor. It should be noted that the invertebrates need not be intermediate hosts, and each of the non-host invertebrates had multiple non-host invertebrate surrogates (i.e. a diverse assemblage of benthic invertebrates), and this occurred with both autogenic and allogenic parasite species in our system. Autogenic parasite species complete their entire life cycle within the confines of an aquatic system, whereas allogenic species use hosts that are outside of the aquatic environment e.g. bird or mammal definitive hosts (Esch et al. Reference Esch, Kennedy, Bush and Aho1988). Our results challenge a central tenet in parasite ecology, that the diversity and abundance of definitive and intermediate hosts determines the diversity and abundance of the parasite community (Smith, Reference Smith2001; Hechinger and Lafferty, Reference Hechinger and Lafferty2005), whereas we found these parasite populations were most dependent on the presence of a broad group of non-host benthic macroinvertebrates. From classic studies, evidence suggests that autogenic parasite species are strongly influenced by the trophic ‘condition’ of the ecosystem (Wisniewski, Reference Wisniewski1958; Chubb, Reference Chubb1963; Esch, Reference Esch1971). As an example, Esch (Reference Esch1971) demonstrated that decreased trophic interactions in oligotrophic lakes resulted in an increase in the presence of adult parasites and fewer larval stages than in eutrophic lakes. On the other hand, in allogenic species where trophic limitation is not evident, the density of intermediate hosts, and frequency of contact between definitive and intermediate hosts (a function of population size of susceptible individuals) are thought to be the most important variables (Janovy et al. Reference Janovy, Snyder and Clopton1997; Marcogliese, Reference Marcogliese2001; Bagge et al. Reference Bagge, Poulin and Valtonen2004; Ondrackova et al. Reference Ondrackova, Simkova, Gelnar and Jurajda2004).
We found no association between the abundance of competent hosts and the success of complex life cycle parasites in our sentinel fish. Our data do not include competent intermediate or definitive hosts as primary splitters but it is likely that a high diversity of benthic invertebrates drives the diversity of fishes, which generates a high diversity of birds. Whittaker (Reference Whittaker1972, Reference Whittaker1975) presented 2 different mechanisms by which community diversity may be realized, either diversity begets diversity (e.g. Murdoch et al. Reference Murdoch, Evans and Peterson1972; Rosenzweig, Reference Rosenzweig1995) or that diversity is limited by niche saturation (e.g. MacArthur and Levins, Reference MacArthur and Levins1967; Lawlor, Reference Lawlor1980; Wilson et al. Reference Wilson, Gitay and Agnew1987). Niche saturation is unlikely to be a consideration in our salt marsh system because host community species diversity increases with time post-restoration in our study. Diversity creating more diversity is a more likely mechanism operating within our system. If consumers are to some extent specialized on prey items, whether plant or animal, more consumers will occur in systems with more ‘prey’ species. Diversity at lower trophic levels providing resources and driving diversity at higher trophic levels has been demonstrated (Hutchinson, Reference Hutchinson1959) and positive correlations exist between the species richness of producers and consumers (e.g. Murdoch et al. Reference Murdoch, Evans and Peterson1972; Brown, Reference Brown1995; Rosenzweig, Reference Rosenzweig1995). It is then plausible that the diversity evidenced in our benthic invertebrate community, reflected in its status as a primary splitter and multiple strong invertebrate surrogates, might be a cause of diversity at higher trophic levels.
A cascading effect of free-living diversity at lower trophic levels supporting free-living diversity at higher trophic levels is of particular importance for complex life cycle parasites. This is because high diversity systems have a greater probability of including species necessary for the successful completion of the life cycle (Hudson et al. Reference Hudson, Dobson and Lafferty2006; Lafferty et al. Reference Lafferty, Allesina, Arim, Briggs, DeLeo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008). This situation is analogous to experiments on, and observations of, ecosystem productivity and community dynamics in relation to species diversity. In marine systems, highly diverse communities are more likely to include individual species that have disproportionate effects on community dynamics (Paine, Reference Paine1966; Sala and Knowlton, Reference Sala and Knowlton2006). Further, the likelihood of encountering a competent host is similar to the sampling effect observed in biodiversity-ecosystem functioning studies (Loreau et al. Reference Loreau, Naeem, Inchausti, Bengtsson, Grime, Hector, Hooper, Huston, Raffaelli, Schmid, Tilman and Wardle2001). It is therefore plausible to state that a diverse free-living community not only increases the diversity of parasite community because of simple addition of hosts that act as habitat and dispersal agents, but also because the probability of encountering a suitable host is increased.
It has been argued that complex life cycle parasites rely on stable trophic links (Marcogliese and Cone, Reference Marcogliese and Cone1997), and trophic stability is likely a consequence of a diverse, and functioning ecosystem (Hudson et al. Reference Hudson, Dobson and Lafferty2006; Tilman et al. Reference Tilman, Reich and Knops2006; Allesina and Pascual, Reference Allesina and Pascual2008). Thus, the presence of benthic invertebrates as the most important factor in determining the presence of parasites likely reflects a robust ecosystem that can support multiple parasite life cycles (Huspeni and Lafferty, Reference Huspeni and Lafferty2004; Hudson et al. Reference Hudson, Dobson and Lafferty2006). It is logical to assert that stable communities of free-living organisms provide parasites with predictable host resources to exploit (Rohde, Reference Rohde1993; Combes, Reference Combes2001; Poulin, Reference Poulin2007) because host species will experience fewer fluctuations in abundance, or vary in a predictable manner (i.e. cyclically). For example, stable predator-prey trophic links between host species are a requirement in trophic transmission, and parasites with multiple hosts exploit the stability of ecosystems to ensure successful transmission (Marcogliese and Cone, Reference Marcogliese and Cone1997; Sukhdeo and Hernandez, Reference Sukhdeo, Hernandez, Thomas, Guegan and Renaud2005). There is a growing body of empirical data that suggests that diverse communities provide for more predictable dynamics. Studies of marine sessile invertebrates and seaweed (Stachowicz et al. Reference Stachowicz, Fried, Osman and Whitlack2002; Allison, Reference Allison2004), crustaceans (Duffy et al. Reference Duffy, Richardson and Canuel2003) and predators (Byrnes et al. Reference Byrnes, Stachowicz, Hultgren, Hughes, Olyarnik and Thornber2006) have suggested that a more diverse assemblage of species provides for higher ecosystem productivity and greater resilience to perturbation. Recent evidence also suggests that highly diverse communities exhibit species redundancy (Levin, Reference Levin1999) so that species richness provides a reservoir of biological options that ensure that ecosystems can respond to perturbation without failure; this phenomenon has been empirically validated (Naeem and Li, Reference Naeem and Li1997; Yachi and Loreau, Reference Yachi and Loreau1999; Tilman et al. Reference Tilman, Reich and Knops2006). Although there is still debate on the mechanisms driving these patterns, it appears that a diverse mix of species in ecosystems reduces fluctuations of ecosystem properties (Loreau et al. Reference Loreau, Naeem, Inchausti, Bengtsson, Grime, Hector, Hooper, Huston, Raffaelli, Schmid, Tilman and Wardle2001; Tilman et al. Reference Tilman, Reich and Knops2006).
The classification tree analyses also reveal that abiotic parameters act as a secondary filter, most probably because invertebrate communities are very susceptible to abiotic conditions (Gasith and Resh, Reference Gasith and Resh1999; Hart and Finelli, Reference Hart and Finelli1999; Yuhas et al. Reference Yuhas, Hartman and Weis2005). There is considerable evidence that abiotic variables can drive parasite population dynamics indirectly through their impact on benthic invertebrate communities (Zander and Reimer, Reference Zander and Reimer2002: see review in Marcogliese, Reference Marcogliese2005). An elegant example of this was reported by Esch and colleagues (Esch et al. Reference Esch, Hazen, Marcogliese, Goater and Crews1986; Marcogliese et al. Reference Marcogliese, Goater and Esch1990), whereby the factors driving the abundance and prevalence of a digenean parasite in its mayfly intermediate host were analysed. These data revealed that superficially the spatial coincidence of competent hosts (mayflies and sphaeriid clams) determined the prevalence and intensity of infection. However, it was determined that the patterns of spatial coincidence of hosts were determined by lake eutrophication. Though our data do not show a causal relationship between abiotic parameters and the presence of benthic invertebrates, it does suggest that abiotic conditions act as a secondary filter in the presence of 3 parasite populations.
In our study, we used a non-parametric classification tree technique to hierarchically link biotic and abiotic factors to the presence of 3 complex life cycle parasites. In doing so, we demonstrate that the most important predictor in the presence of a parasite species is the benthic invertebrate community. We suggest that this occurs because a diverse community is more likely to contain competent host species. Further, we suggest that a consequence of a highly diverse community is an increased likelihood for the existence of stable trophic links that parasite species may exploit.
ACKNOWLEDGEMENTS
We acknowledge the advice and support provided by Francisco Artigas and Brett Bragin (Meadowlands Environmental Research Institute). Comments on an earlier draft by Wayne Rossiter, and feedback provided by two anonymous reviewers greatly improved this manuscript. This research was funded in part by a Willis A. Reid Jr. Student research grant (American Society of Parasitology) to T.K.A., and by the Rutgers Foundation Parasite Research Fund to T.K.A. and M.V.K.S.