Published online by Cambridge University Press: 03 March 2004
Amylopectin is used for carbohydrate storage in different life-stages of a number of apicomplexan parasites. We have performed an ultrastructural analysis of amylopectin granules from the oocyst residual body and sporozoites of Cryptosporidium parvum. Amylopectin granules were studied in situ and after isolation from ‘French’ press disrupted parasites, by conventional transmission electron microscopy (TEM) of sectioned oocysts and various negative staining and cryoelectron microscopy techniques. Within the membrane-enclosed oocyst residuum large amylopectin granules (0·1–0·3 μm) can be found besides a characteristic large lipid body and a crystalline protein inclusion. Smaller granules were detected in sectioned sporozoites. Negative staining of isolated amylopectin granules revealed some ultrastructural features not readily visible in sectioned material. The large amylopectin granules had a smooth surface with a ‘ball of string’-like inner structure. Granules isolated from sporozoites were more irregularly shaped and showed a rod-like particulate composition. With the exception of α-amylase, which led to some degree of damage of the surface of the particles, treatment of amylopectin granules with other glycohydrolases had little effect on the overall structure. However, granules adhered to one another. Only when the granules were boiled did the ‘ball of string’ structure gradually dissolve.
The oocyst wall of the apicomplexan parasite Cryptosporidium parvum is an extremely robust multi-layer structure that maintains the viability of the 4 internalized sporozoites, even under extreme environmental and experimental conditions. Only when the oocyst is ingested and passes through the mammalian gut does destabilization of the wall occur with the opening of the suture. Excystation of viable sporozoites follows and these invade the enterocytes. In vitro excystation can also be produced experimentally, and has been a useful approach for the study of C. parvum sporozoites and their subcellular components (Reduker, Speer & Blixt, 1985; Tetley et al. 1998; Petry & Harris, 1999; Harris, Adrian & Petry, 2003) as well as the isolated oocyst wall (Harris & Petry, 1999). Also present within the oocyst is a residual body or oocyst residuum, a membrane-bounded structure containing lipid, polysaccharide/amylopectin and possibly protein/amino acids as a storage source of metabolites for the sporozoites (Fayer, Trout & Jenkins, 1998). The formation of the residual body during the late developmental stages of the C. parvum oocyst has been investigated (Bonnin, Dubremetz & Camerlynck, 1991; McDonald, McCrossan & Petry, 1995; Fayer, Speer & Dubey, 1997), and its separation from the sporozoites as a discrete and undoubtedly important metabolic structure is well accepted. The presence of amylopectin granules within C. parvum sporozoites has been suggested (Reduker et al. 1985; McDonald et al. 1995; Fayer et al. 1997), although the detailed ultrastructural analysis of Tetley et al. (1998) did not reveal amylopectin.
Numerous structural studies of other coccidian parasites have been published. Some emphasis has been placed upon the presence of amylopectin granules in the developmental stages of Eimeria sparis (Alvares-Pellitero, Palenzuela & Sitja-Bobadilla, 1997), Neospora caninum and Toxoplasma gondii (Speer, Clark & Dubey, 1998; Speer et al. 1999; Ferguson, Brecht & Sodati, 2000; Medina et al. 2001) and Eimeria bovis (Heise, Peters & Zahner, 1999) and Eimeria sp. (Roberts & Hammond, 1970). A marked difference in the content of amylopectin granules between invasive stages of T. gondii has been reported (Coppin et al. 2003). Whereas the rapidly replicating tachyzoite stage normally lacks polysaccharide granules, the dormant bradyzoite (in tissue cysts) and the sporozoite (in the oocyst) possess numerous amylopectin granules. The latter forms await ingestion by a carnivorous and herbivorous host, respectively, and are dependent on sufficient energy supply to survive the resting periods. Sarcocystis neurona schizonts and merozoites apparently contain rather little amylopectin (Speer & Dubey, 2001). The presence of a residual body in the sporocysts/oocysts of species other than C. parvum has not received as much attention although Speer et al. (1998) commented briefly on a residuum of lipid and amylopectin granules within the T. gondii sporocyst and Zhao & Duszynski (2001) suggested that the oocyst residuum can be used to distinguish two independent lineages of Eimeria spp.
Based on earlier work (Petry & Harris, 1999), the intention of the present paper was to extend our studies on the structure and putative function of the two different types of amylopectin granules in the oocyst stage of the parasite. Following in vitro excystation of C. parvum oocysts we have investigated the presence and biochemical recovery of a mixed population of large and small granules from the residual body and sporozoites. Conventional thin sectioning and the negative staining techniques have been used, bearing in mind the limitation of the latter due to the large size of intact oocysts, sporozoites and residual bodies (Baxby et al. 1984; Harris et al. 2003). From purified sporozoites (i.e. centrifugally separated from the residual bodies), a population of smaller amylopectin granules has been detected. The substructure of the amylopectin granules has been investigated by negative staining and their stability in the presence of glycohydrolases and following brief heat treatment assessed.
Oocysts of C. parvum (Iowa strain) passaged in newborn calves were obtained from Patricia Mason (Pleasant Hill Farm, Troy, Idaho, USA). According to the supplier, calf faeces were passed through a coarse screen to remove solids and extracted twice with ethyl ether to remove lipids. Oocysts were concentrated by sucrose-density centrifugation, washed and resuspended in PBS. The parasite suspension was stored at 4 °C in the presence of 1000 U/ml penicillin and 1000 μg/ml streptomycin. Intact oocysts were further treated with 1[ratio ]20 diluted commercial bleach (conc. 3·8% sodium hypochlorite; 5 min on ice) in order to surface sterilize the parasites and then washed 3 times in water. In vitro excystation was induced in RPMI medium plus 0·75% Na-taurocholate at 37 °C for 2 h.
Sporozoites were separated from intact oocysts and empty oocyst walls by passing the excystation mixture through a membrane filter unit (Schleicher & Schuell, Dassel, Germany, 5 μm pore size). The filtrate contained residual bodies of various degrees of integrity besides intact sporozoites.
Amylopectin granules were prepared as described previously (Petry & Harris, 1999). Briefly, the sporozoites/residual body filtrate was suspended in TEA-buffer (5 mM triethanolamine-HCl, 1 mM EDTA, pH 7·5) containing 0·25 M sucrose and protease inhibitors, and disrupted in a French pressure cell operated at 60 kg/cm2. Unbroken cells were separated from the homogenate by centrifugation (10 min at 600 g). The pre-cleared French press homogenate was fractionated by ultracentrifugation through a sucrose gradient (8 steps from 1·0 to 2·0 M sucrose). Amylopectin granules sedimenting to the bottom of the tube were resuspended in TEA/0·25 M sucrose and re-centrifuged for 1 h at 130000 g. The resulting pellet was resuspended in TEA/0·25 M sucrose and kept at 4 °C. Aliquots were also fixed with 0·1% (v/v) glutaraldehyde (final concentration).
In order to separate intact sporozoites and amylopectin granules derived from the residual body the filtrate of the excystation mixture was concentrated by centrifugation and layered on a step gradient consisting of 0·5 ml of TEA/2·0 M sucrose and 1 ml TEA/1·0 M sucrose. Sporozoites were recovered from the 1·0/2·0 M sucrose interphase. Amylopectin granules sedimented through 2·0 M sucrose to the bottom of the tube. Both fractions were resuspended and centrifuged in TEA/0·25 M sucrose (1 h at 130000 g).
The sporozoites recovered from the 1·0/2·0 M sucrose interphase in the previous step were disrupted and fractionated as described above. Amylopectin granules sedimented to the bottom of the tube.
Aliquots containing an opaque suspension of amylopectin granules were incubated at room temperature and at 40 °C, for periods of 1 h to 24 h, with the following glycohydrolases (0·1 to 0·5 mg/ml), obtained from the Sigma Chemical Company: Amyloglucosidase (EC 3.2.1.3), α-amylase (EC 3.2.1.1), β-amylase (EC 3.2.1.2) and pullulanase (EC 3.2.1.41). Following incubation, samples were taken for the preparation of negatively stained specimens and EM study.
Aliquots of total excystate were centrifuged to pellet the oocyst walls, residual bodies, sporozoites and any remaining intact oocysts, and resuspended in 50 μl of PBS. This suspension was then mixed with 50 μl of 2% (w/v) low-melting-temperature agarose in PBS and centrifuged again while still fluid, to concentrate the parasite material towards the bottom of the tube. After geling, the agarose was removed from the tube, cut into small pieces and the agarose-encapsulated biological material was fixed in 2% (v/v) glutaraldehyde in PBS, overnight at room temperature. The agarose pieces were then washed with distilled water and stained for 2 h in 1% (w/v) aqueous osmic acid, dehydrated and embedded in Araldite resin by conventional procedures. Thin sections were stained with ethanolic uranyl acetate and aqueous lead citrate.
Air-dried negatively stained specimens from the various unfixed and glutaraldehyde-fixed C. parvum sporozoite, residual body and amylopectin granule samples were prepared on continuous carbon films using 1% (w/v) ammonium molybdate (pH 7·0) (Harris, 1997) unless otherwise stated, and on holey carbon support films using 5% ammonium molybdate, 0·1% trehalose (pH 7·0) and 1% (w/v) trehalose alone (Harris & Scheffler, 2002).
Frozen-hydrated unstained specimens were prepared from unfixed and fixed amylopectin granule samples on holey carbon support films by filter paper blotting followed by plunge freezing into liquid ethane (Adrian et al. 1984; Harris & Adrian, 1999). Cryo-negatively stained specimens were prepared by mixing unfixed or fixed amylopectin granule samples with 16% (w/v) ammonium molybdate (pH 7·0) immediately prior to blotting and plunge freezing in liquid ethane (Adrian et al. 1998; Harris & Adrian, 1999).
TEM study of thin sections and air-dried negatively stained specimens was performed using a Zeiss EM900 and a Philips CM12, and electron micrographs were recorded on Kodak type 4489 electron microscope film. Cryoelectron microscopy was performed using a Philips CM12 cryo-electron microscope with a Gatan model 626 cryo-holder. Low-dose electron micrographs were recorded on Kodak SO-163 electron image film.
The total in vitro excystate from C. parvum oocysts contains a mixture of intact oocysts, partially excysted oocysts, free sporozoites, free residual bodies (some damaged) and empty oocyst walls. Figure 1 shows a thin section of a partly excysted oocyst, with one banana-shaped sporozoite and the residual body within the oocyst wall. The membrane-enclosed residual body contains a characteristic large lipid body, numerous amylopectin granules and a crystalline protein inclusion, along with more finely dispersed material (ribosomes) and cytomembranes.
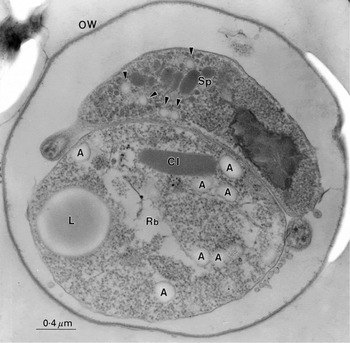
Fig. 1. A thin-sectioned Cryptosporidium parvum oocyst showing the presence of the residual body (Rb) and one of the remaining ‘banana-shaped’ sporozoites (Sp) within the oocyst wall (OW). Within the membrane-bounded residual body, a lipid body (L), protein crystal inclusion (CI) and lightly stained amylopecin granules (A) can be defined, along with internal cytomembranes, vacuoles and particulate material. The sporozoite contains a number of different organelles, particles and granules (e.g. nucleus, micronemes, rhoptry, dense granules, ribosomes, crystalloid body particles (see Petry & Harris, 1999), and possibly also some amylopectin granules (arrowheads) which are significantly smaller than those in the residual body.
Some small particles within the sporozoite are also stained with a similar density to the amylopectin granules in the residual body (indicated by arrowheads in Fig. 1). Whilst amylopectin is often present within the mature and immature apicomplexan parasites, it has not always been convincingly demonstrated within C. parvum sporozoites. As the sporozoite shown in Fig. 1 possesses clearly defined micronemes and dense granules, it is likely that the more lightly stained granules are indeed amylopectin. Figure 2 shows a montage with emphasis upon the residual body remaining within the wall of the excysted oocyst. Variable density of stained amylopectin granules has often been observed; indeed they often tend to appear as electron-lucent particles (Fig. 2A), or as more commonly found in our data, lightly stained but with a more densely stained surface (see below). The crystalline inclusion does not appear in all sections through the residual body, but when it is present it has features characteristic of a protein crystal (Fig. 2B). When the residual body escapes from the oocyst wall it has a tendency to undergo disruption with release of its contents, but many remain intact. Figure 3 shows examples of thin-sectioned externalized residual bodies that are relatively intact. At low magnification (Fig. 3A) the distribution of amylopectin granules, stained relatively intensely in this instance, can be seen. The amylopectin granules have irregular oval shape, but they all exhibit a smooth surface and there is a narrow size range (approx. 0·1–0·3 μm). At higher magnification (Fig. 3B) the amylopectin granules can be seen to possess a fine granular interior which shows some indication of linearity, suggestive of ordered substructure (Fig. 3C).

Fig. 2. Thin sections showing residual bodies still within the Cryptosporidium parvum oocyst wall after excystation. (A) Residual body in the process of exiting through the suture of the oocyst wall (OW). In this instance the surrounding membrane of the residual body is still intact; the amylopectin granules and lipid body (L) are relatively lightly stained. (B) Fragmented residual body exiting the oocyst wall suture, with more densely stained amylopectin granules than in (A) and a crystal inclusion showing parallel dense lines characteristic of a stained protein crystal (arrowheads).

Fig. 3. Thin sections showing Cryptosporidium parvum residual bodies outside the oocyst wall, following excystation. (A) Two residual bodies, each with a relatively intact membrane, well-defined amylopectin granules and lipid body (L). (B) At slightly higher magnification the peripheral densely stained rim of the amylopectin granules is emphasized together with the finely stained nature of the granule content. (C) At high magnification, free amylopectin granules and those within a relatively intact residual body show an internal particulate structure, which occasionally possesses an indication of linearity (view between arrowhead pairs).
The inherent thickness and density of intact C. parvum residual bodies and sporozoites usually prevents the possibility of obtaining information about their contents from the negative staining technique. If, however, the residual body has undergone partial disruption in suspension and/or at the time of producing the negatively stained specimen, then it is possible to define some of the internal components, although excessive stain density can still be a problem (Fig. 4A). When 1% or 0·5% ammonium molybdate is used for negative staining instead of the more usual 2%, then there is a greater chance that detail will be revealed (Fig. 4B). In the total excystate, the protein crystalline inclusions that have been released from disrupted residual bodies are readily revealed by negative staining (Fig. 4C), thereby providing confirmation of the structural interpretation placed on the thin section data (Figs 1 and 2).

Fig. 4. (A) A more thinly spread negatively stained residual body undergoing disruption, lying alongside an oocyst wall (OW). The numerous electron-dense amylopectin granules surround an electron-transparent irregular lipid body (L). This electron micrograph serves to show the inherent technical difficulty when applying the negative stain procedure to relatively large and thick samples of biological material such as the oocyst and residual body. (B) Negatively stained Cryptosporidium parvum residual body in the total excystate, undergoing disruption. Although rather electron dense, in this instance the negative stain does reveal more clearly than in (A) the amylopectin granules (arrowheads) surrounding the lipid body (L). (C) A cluster of negatively stained crystal inclusions, present in the total excystate, showing linear periodicity characteristic of a protein crystal (cf. Fig. 2B).
Amylopectin granules isolated as the sucrose gradient pellet following French press disruption of the sporozoites and residual bodies have been found to show a considerable size range. A survey micrograph of a negatively stained sample (Fig. 5A) shows that the population of larger granules exhibit a smooth surface with a compact tightly rolled internal appearance, resembling a ball of string. The smaller amylopectin granules have a more irregular surface and particulate internal content. These structural features of the two classes of amylopectin granules are emphasized at higher magnification in Fig. 5B–D. Sucrose-gradient pellets obtained directly from the total excystate, which contains many disrupted residual bodies, but intact sporozoites, yielded an amylopectin pellet containing only the larger population of granules (Fig. 5B, C). The neatly coiled, smooth-surfaced ‘ball of string’ structure of the larger amylopectin (Fig. 5B, C) contrasts markedly with the more irregular shape and rod-like particulate composition of the smaller granules (Fig. 5D). The smaller amylopectin granules (Fig. 5D) are thought to be derived from the sporozoites, as sucrose gradient centrifugation performed using a purified sporozoite fraction from the C. parvum excystate produced a pellet containing only the population of smaller irregular granules. They could represent a residual amylopectin core that has been metabolically degraded from initially larger granules within the sporozoites or a unique population of smaller undegraded amylopectin granules within the sporozoite.

Fig. 5. (A) Survey electron micrograph showing the mixed population of large and small negatively stained amylopectin granules on a continuous carbon support film. Samples were obtained by sucrose density-gradient centrifugation, following disruption of Cryptosporidium parvum sporozoites and residual bodies in the total excystate using a French pressure cell (Petry & Harris, 1999). Note the compact, smooth-surfaced appearance of the larger granules. Trapped water within the granules often presents a problem, resulting in visible electron beam damage (arrowheads). At higher magnification the structural details of the 2 populations of granules become more apparent. (B) and (C) Larger granules, purified from residual bodies which had previously been separated from sporozoites, which exhibit a tightly coiled ‘ball of string’ morphology, possibly due to the linear structure of the amylopectin polysaccharide chains as flexible coiled ~5 nm diameter strings. (D) The purified population of smaller amylopectin granules, derived from sporozoites first separated from residual bodies by sucrose-gradient centrifugation. The irregularity of the clusters of rod-like particles composing these small granules contrasts strongly with the purified smooth-surfaced larger amylopectin granules from the residual bodies (B, C).
Apart from conventional negative staining, with the sample adsorbed to a carbon support film, several other negative staining and cryoelectron microscopy approaches have been used to study the structure of the C. parvum amylopectin granules. Figure 6 shows a composite with amylopectin granules negatively stained (Fig. 6A) and unstained (in the presence of trehalose; Fig. 6B) after spreading across the holes of a holey carbon support film and air-drying (Harris & Scheffler, 2002). The corresponding cryoelectron microscopy images are shown of cryo-negatively stained amylopectin (Fig. 6C) and unstained frozen-hydrated amylopectin (Fig. 6D), (Adrian et al. 1984, 1998).
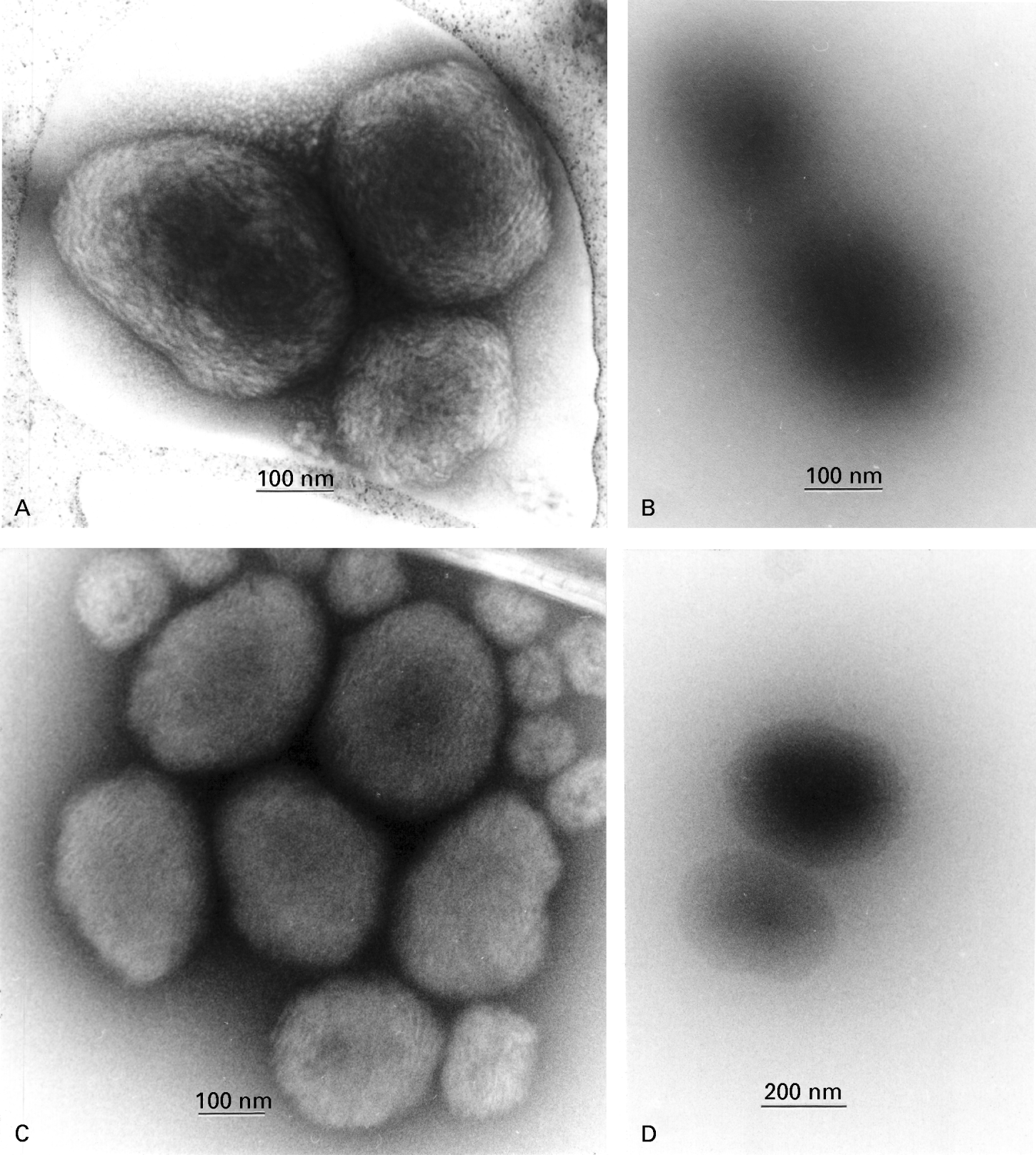
Fig. 6. Amylopectin granules imaged following differing specimen preparation conditions. (A) Negatively stained by spreading in 5% ammonium molybdate, 0·1% trehalose (pH 7·0) across a holey carbon support film and air-dried. (B) Spread across a holey carbon support film in the presence of 1% trehalose and air-dried (i.e. unstained). (C) Cryo-negatively stained with 15% ammonium molybdate (pH 7·0), on a holey carbon support film. (D) Vitrified directly, in the absence of negative stain, on a holey carbon support film (i.e. cryo-electron microscopy). For both the negatively stained examples (A and C) the ‘ball of string’ structure of the oligosaccharide within the amylopectin granules is clearly shown, and with the fully hydrated sample (C) it can be assumed that there is no shrinkage artefact induced by drying. The unstained samples (B and D), show images produced only by the inherent electron density of the amylopectin granules which, in the case of 1% trehalose embedding (B) (perhaps with an element of contrast matching), does not reveal any detail other than the overall size of the particle. However, conventional cryo-electron microscopy (D) does reveal the unstained amylopectin with greater clarity than 1% trehalose and there is a slight indication of the polysaccharide substructure. In general, these alternative technical approaches provide support for the validity of the amylopectin images shown in Fig. 5.
The absence of a carbon support film creates the potential for greater image detail from the negatively stained amylopectin (Fig. 6A). The frozen-hydrated negatively stained images of the amylopectin granules shown in Fig. 6C reveal a more tightly packed internal structure, but in general these images correlate well with those shown above (Figs 5 and 6A). No internal detail within unstained amylopectin is revealed by air drying in a thin film of trehalose (Fig. 6B). Undoubtedly there will be an element of contrast matching between the disaccharide trehalose and amylopectin, which explains the indistinct edge of amylopectin granules. With cryoelectron microscopy, however, unstained vitrified specimens of amylopectin reveal the granule edge more clearly (Fig. 6D), since the density of the surrounding vitreous ice is significantly less than that of the amylopectin. Nevertheless, the internal structure within the amylopectin granules is only just definable under these conditions (Fig. 6D).
The intense blue colour of starch in the presence of iodine is a characteristic colouration reaction for the detection of amylose and amylopectin. C. parvum amylopectin granules do indeed produce this same colouration (data not shown) and from our earlier biochemical analysis (Petry & Harris, 1999) we know that there is no protein in the amylopectin pellet taken from our sucrose gradients. Digestion of the amylopectin granules with enzymes known to cleave complex branched polysaccharide chains is likely to yield useful structural information as to the granule composition. We have investigated the action of amyloglucosidase, α-amylase, β-amylase and pullulanase, with incubation at room temperature and at 40 °C. Amylopectin granules subjected to digestion for several hours, up to 24 h at 40 °C showed a marked tendency to aggregate as small clumps (Fig. 7A, B), with close adherence of one surface to the next, in a viscous manner, but without significant breakdown of the granules. If cleavage of some polysaccharide side-chains occurs, this could create an inherent stickiness causing the granules to adhere to one another, and also account for the intense negative staining (Fig. 7B) and water retention by the particles that can result in electron beam damage (visible in Fig. 7A). Digestion for 24 h at 40 °C with α-amylase produced no aggregation of the amylopectin, instead visible damage to the surface of the particle is clearly apparent (Fig. 7C). The amylopectin granules now have an irregular surface and small rod-like polysaccharide strands are released. There is now an apparent similarity between these large digested amylopectin granules and the population of small possibly sporozoite-derived granules shown in Fig. 5C. β-amylase and pullulanase both produced no significant change to the amylopectin granules, following 24 h digestion (data not shown).

Fig. 7. Amylopectin granules following incubation with glycohydrolases. (A and B) Following incubation at 40 °C for 24 h with amyloglucosidase. The granules in the sample became progressively aggregated, with indication of granule fusion and self-adherence, but without significant breakdown of the granules. The partial digestion of only surface polysaccharide with release of sticky oligosaccharides appears to be responsible for this aggregation, which produces conditions where there is considerable electron beam damage and where there is excessive electron density (B). Following incubation with α-amylase at 40 °C or room temperature for 24 h, some breakdown of the amylopectin granules was detected (C). The granules became irregular in shape, with an uneven surface. Some cleavage of the polysaccharide chains within the granules is apparent, with release of short strands of amylopectin from the granules (arrowheads, C).
Amylopectin (starch) granules are known to become progressively hydrated with increasing temperature and ultimately they form a gel. Treatment of the C. parvum amylopectin granules for short periods of time at 100 °C produced a marked change to their structure (data not shown). After 2 min they lost the compact ‘ball of string’ morphology and had a more globular amorphous structure with fine material radiating out from the centre. After 10 min at 100 °C the particles became swollen, with dispersal of the amylopectin as diffuse globules with an irregular surface. The changes were characteristic of those produced by increasing hydration of amylopectin/starch granules.
Amylopectin contains a highly branched chain of α1, 4- and β1, 6-glucans, arranged in a complex ordered array. Early work has identified amylopectin rather than glycogen as the storage polysaccharide in Eimeria brunetti and E. tenella (Ryley et al. 1969) and a recent detailed biochemical analysis of amylopectin of Toxoplasma gondii has confirmed the branching pattern of the polysaccharide chains to be similar to amylopectin in plant starch (Coppin et al. 2003). The physiological role of amylopectin during the maturation and survival of the C. parvum oocyst, and nutrition of the sporozoites appears to be linked to a population of large amylopectin granules within the residual body and to a population of smaller amylopectin granules within the sporozoites. The former may be looked upon as carbohydrate storage granules, utilized during the transmission period of the oocyst stage in the environment whereas the latter are likely to be closely involved in the immediate metabolism of the sporozoite once liberated from the oocyst in the intestine of a new host. The total amylopectin content of C. parvum oocyst has been measured by Fayer et al. (1998) who showed that oocyst storage at temperatures above 5 °C produced a marked reduction in amylopectin and infectivity over a period of several weeks, whereas at 0 °C and 5 °C the amylopectin depletion was very slow. Amylopectin and the mRNA encoding amyloglucosidase can serve as viability markers of C. parvum oocysts (Jenkins et al. 2000, 2003). Similar studies in which amylopectin was considered as an energy source have been performed with Eimeria sp. (Augustine, 1980; Nakai & Ogimoto, 1989). Such analysis has not been correlated with the ultrastructual detection of amylopectin granules, but it follows that there is likely to be a rapid diminution within both the residual body and sporozoites at the raised temperatures.
Our data has been obtained using oocysts that were stored at 4 °C. Thus, we have found that they possess a high amylopectin content by both biochemical analysis (Petry & Harris, 1999) and in the present paper by ultrastructural analysis. Although no carbohydrate-specific staining has been utilized, amylopectin granules have been found to bind the conventional heavy metal stains used for thin section electron microscopy. The C. parvum residual body contains a uniform population of large smooth-surface amylopectin granules of somewhat variable shape. The presence of smaller amylopectin granules within C. parvum sporozoites is shown by our data; this is supported by that of others (Fayer et al. 1997; McDonald et al. 1995; Reduker et al. 1985), but not by the work of Tetley et al. (1998). Thin sectioning reveals that the larger amylopectin granules within the residual body and those that have escaped from damaged residual bodies during in vitro excystation possess a fine granular structure, with some suggestion of internal organization. This interpretation correlates with the concept that long polysaccharide chains are present within the granules, and is supported by our negative stain data (see below).
An interesting monoclonal antibody that binds species-specifically to amylopectin granules in Eimeria bovis has been utilized by Heise et al. (1999). The granule epitope involved was not destroyed by α-amylase and β-glucosidase digestion, but destruction of O-linked carbohydrate by alkaline treatment did destroy the epitope. Such treatment could, however, also have destroyed a protein epitope. So, because of the species-specificity of the antibody these authors considered that the antibody was likely to be targeting a protein epitope rather than one on the polysaccharide.
Negative staining of intact residual bodies and disrupted residual bodies often presented some difficulty due to excessively dense staining, but has served to provide a bridge between the thin section data and the negative stain data on isolated C. parvum amylopectin granules.
Earlier work on Eimeria tenella (Wang, Weppelmann & Lopez-Ramos, 1975), using surfactant and protease digestion to release amylopectin granules from oocysts, showed that the enzyme amylopectin phosphorylase was present, and likely to be involved in the breakdown of amylopectin. The authors further showed that despite the decrease of the amylopectin source during sporulation, the size and shape of the granules did not change; however, the total number of granules did decline.
Our C. parvum amylopectin granules were obtained by more conventional subcellular fractionation techniques (Petry & Harris, 1999, and this report). The dimensions of our large and small population of amylopectin granules following isolation correlates with the in situ size, thus suggestive of their location in the residual body and sporozoites, respectively. The size and ultrastructure were extremely stable over months stored at 4 °C. Two populations of polysaccharide granules were also described in endogenous forms of Eimeria brunetti (Ferguson et al. 1977). The larger ones (approx. 500 nm by 250 nm) were observed in mature merozoites, macrogamonts, and developing oocysts, a smaller population (15 to 30 nm) was seen at the periphery of the residual cytoplasmic mass of mature microgamonts. Although the morphology of the two populations of amylopectin granules studied in our analysis of C. parvum differs, they are sufficiently similar structurally and both sediment through 2·0 M sucrose to form a pellet. Only the large amylopectin granules exhibit the ‘ball of string’ structure. This is dissimilar to the established concentric lamellar structure of high amylose starch granules, but may be more closely related to the structure of the low amylose waxy maize starch (Atkin et al. 1999; Nordmark & Ziegler, 2002).
Cryoelectron microscopy of fully hydrated unstained and negatively stained amylopectin granules provided supportive data that conforms well with that from air-dry negative staining. Thus, a rigid compact coiled structure for the large isolated amylopectin granules agrees with our interpretation and those of others, derived primarily from thin section EM studies on residual bodies and varying stages of parasite development.
Our preliminary glycohydrolase study provided evidence for partial degradation of the C. parvum amylopectin granules by α-amylase, but the three other enzymes investigated had little effect, in our hands. However, Fayer et al. (1998) used Aspergillus niger amyloglycosidase to determine the total amylopectin content of C. parvum oocysts. Several genes encoding enzymes involved in polysaccharide metabolism have been identified in the genome of C. parvum. Among which are an α-amylase homologue (Accession No. B88566) and an amyloglucosidase homologue (Accession Nos. B88444 and AF268073), (Strong & Nelson, 2000; Jenkins et al. 2000). Expression of functionally active forms of these enzymes will be very useful in further characterization of the polysaccharide metabolism of this parasite.
Our use of heat-treatment was limited to short periods at 100 °C, which rapidly produced a marked swelling of the amylopectin granules, with loss of the ‘ball of string’ structure. It is likely that further investigations using somewhat lower temperatures, e.g. 50–60 °C, might produce an intermediate dispersal of the amylopectin and also be of use for subsequent glycohydrolase studies. Such work is needed in order to correlate the properties of C. parvum amylopectin with the work of Puteaux, Buléon & Chanzy (2000) who solubilized amylopectin from waxy maize at 50 °C, followed by cooling to room temperature, and produced beaded chains of dispersed amylopectin.
Exactly why the apicomplexan parasites utilize amylopectin rather than glycogen as their storage carbohydrate is unclear. In contrast to starch granules of plants which are stored in specialized amyloplasts and also in chloroplasts apicomplexa store amylopectin in the cytoplasm rather than in the apicoplast. That this is a reflection of the presence of a plant-like plastid (Köhler et al. 1997; Roberts et al. 1998) encoding genes for amylopectin production, such as amylopectin synthase rather than glycogen synthase, is a possibility. However, such a plastid has not been detected in all apicomplexa and apparently it is absent in C. parvum.
Part of this work has been supported by DFG grant SFB 490 C5 (F.P.). We acknowledge the skilled technical assistance of Inka Kneib and Elisabeth Sehn. Electron microscope facilities were made available by Professor Albrecht Fischer, Institute of Zoology, University of Mainz; French Press facilities were made available by Professor Gottfried Unden, Institute of Microbiology and Wine Research, University of Mainz.

Fig. 1. A thin-sectioned Cryptosporidium parvum oocyst showing the presence of the residual body (Rb) and one of the remaining ‘banana-shaped’ sporozoites (Sp) within the oocyst wall (OW). Within the membrane-bounded residual body, a lipid body (L), protein crystal inclusion (CI) and lightly stained amylopecin granules (A) can be defined, along with internal cytomembranes, vacuoles and particulate material. The sporozoite contains a number of different organelles, particles and granules (e.g. nucleus, micronemes, rhoptry, dense granules, ribosomes, crystalloid body particles (see Petry & Harris, 1999), and possibly also some amylopectin granules (arrowheads) which are significantly smaller than those in the residual body.

Fig. 2. Thin sections showing residual bodies still within the Cryptosporidium parvum oocyst wall after excystation. (A) Residual body in the process of exiting through the suture of the oocyst wall (OW). In this instance the surrounding membrane of the residual body is still intact; the amylopectin granules and lipid body (L) are relatively lightly stained. (B) Fragmented residual body exiting the oocyst wall suture, with more densely stained amylopectin granules than in (A) and a crystal inclusion showing parallel dense lines characteristic of a stained protein crystal (arrowheads).

Fig. 3. Thin sections showing Cryptosporidium parvum residual bodies outside the oocyst wall, following excystation. (A) Two residual bodies, each with a relatively intact membrane, well-defined amylopectin granules and lipid body (L). (B) At slightly higher magnification the peripheral densely stained rim of the amylopectin granules is emphasized together with the finely stained nature of the granule content. (C) At high magnification, free amylopectin granules and those within a relatively intact residual body show an internal particulate structure, which occasionally possesses an indication of linearity (view between arrowhead pairs).

Fig. 4. (A) A more thinly spread negatively stained residual body undergoing disruption, lying alongside an oocyst wall (OW). The numerous electron-dense amylopectin granules surround an electron-transparent irregular lipid body (L). This electron micrograph serves to show the inherent technical difficulty when applying the negative stain procedure to relatively large and thick samples of biological material such as the oocyst and residual body. (B) Negatively stained Cryptosporidium parvum residual body in the total excystate, undergoing disruption. Although rather electron dense, in this instance the negative stain does reveal more clearly than in (A) the amylopectin granules (arrowheads) surrounding the lipid body (L). (C) A cluster of negatively stained crystal inclusions, present in the total excystate, showing linear periodicity characteristic of a protein crystal (cf. Fig. 2B).

Fig. 5. (A) Survey electron micrograph showing the mixed population of large and small negatively stained amylopectin granules on a continuous carbon support film. Samples were obtained by sucrose density-gradient centrifugation, following disruption of Cryptosporidium parvum sporozoites and residual bodies in the total excystate using a French pressure cell (Petry & Harris, 1999). Note the compact, smooth-surfaced appearance of the larger granules. Trapped water within the granules often presents a problem, resulting in visible electron beam damage (arrowheads). At higher magnification the structural details of the 2 populations of granules become more apparent. (B) and (C) Larger granules, purified from residual bodies which had previously been separated from sporozoites, which exhibit a tightly coiled ‘ball of string’ morphology, possibly due to the linear structure of the amylopectin polysaccharide chains as flexible coiled ~5 nm diameter strings. (D) The purified population of smaller amylopectin granules, derived from sporozoites first separated from residual bodies by sucrose-gradient centrifugation. The irregularity of the clusters of rod-like particles composing these small granules contrasts strongly with the purified smooth-surfaced larger amylopectin granules from the residual bodies (B, C).

Fig. 6. Amylopectin granules imaged following differing specimen preparation conditions. (A) Negatively stained by spreading in 5% ammonium molybdate, 0·1% trehalose (pH 7·0) across a holey carbon support film and air-dried. (B) Spread across a holey carbon support film in the presence of 1% trehalose and air-dried (i.e. unstained). (C) Cryo-negatively stained with 15% ammonium molybdate (pH 7·0), on a holey carbon support film. (D) Vitrified directly, in the absence of negative stain, on a holey carbon support film (i.e. cryo-electron microscopy). For both the negatively stained examples (A and C) the ‘ball of string’ structure of the oligosaccharide within the amylopectin granules is clearly shown, and with the fully hydrated sample (C) it can be assumed that there is no shrinkage artefact induced by drying. The unstained samples (B and D), show images produced only by the inherent electron density of the amylopectin granules which, in the case of 1% trehalose embedding (B) (perhaps with an element of contrast matching), does not reveal any detail other than the overall size of the particle. However, conventional cryo-electron microscopy (D) does reveal the unstained amylopectin with greater clarity than 1% trehalose and there is a slight indication of the polysaccharide substructure. In general, these alternative technical approaches provide support for the validity of the amylopectin images shown in Fig. 5.

Fig. 7. Amylopectin granules following incubation with glycohydrolases. (A and B) Following incubation at 40 °C for 24 h with amyloglucosidase. The granules in the sample became progressively aggregated, with indication of granule fusion and self-adherence, but without significant breakdown of the granules. The partial digestion of only surface polysaccharide with release of sticky oligosaccharides appears to be responsible for this aggregation, which produces conditions where there is considerable electron beam damage and where there is excessive electron density (B). Following incubation with α-amylase at 40 °C or room temperature for 24 h, some breakdown of the amylopectin granules was detected (C). The granules became irregular in shape, with an uneven surface. Some cleavage of the polysaccharide chains within the granules is apparent, with release of short strands of amylopectin from the granules (arrowheads, C).