Introduction
Morphometry aims to quantify, analyse and describe the variations found in biological forms (Roth & Mercer, Reference Roth and Mercer2000). Despite the recent advances in genetics, morphological evaluation has still been an important tool for grouping and classifying species (Doyle et al., Reference Doyle, Gammell and Nash2018). In addition, morphometric data may offer an economical alternative to infer the fundamental biological parameters of populations, because body shape is a product of individual ontogeny (Walker & Grahame, Reference Walker and Grahame2011). Morphometric measurements are used by ecologists as a tool to understand patterns of morphological variation as well as their evolutionary causes, with these being among the main objectives of functional ecology (Pie & Traniello, Reference Pie and Traniello2007).
Morphological variation among individuals of the same species can be used to discriminate ‘phenotypic stocks’, which is defined as groups with similar growth, mortality and reproductive rates (Cadrin, Reference Cadrin2000). The body size of an animal is a trait that influences fitness and determines both its ability to survive and its reproductive abilities (Wang et al., Reference Wang, Johnson, Daane and Yokoyama2009). In molluscs, especially gastropods, sexual dimorphism has been reported in shell size, in the radial features of the shell, and to a lesser extent, in the shape of the shell (Pastorino, Reference Pastorino2007). The description of the population size of gastropods has been based on the length measurements of their shells (Casagranda & Boudoresque, Reference Casagranda and Boudoresque2002).
Shell shapes vary considerably between habitats in response to environmental factors such as temperature or hydrodynamic conditions (Minton & Gochfeld, Reference Minton and Gochfeld2001). Shell shape variation within species can occur either on a microgeographic scale (e.g. between distances of metres or between different coasts within the same site) or on a macrogeographic scale (e.g. between regions) (Avaca et al., Reference Avaca, Narvarte, Martín and van der Molen2013). According to Vermeij (Reference Vermeij1972), the shell sizes of species that inhabit the supralittoral tend to increase along a vertical gradient from lower to higher tide levels, which is an example of microgeographic scale variation. At the macroscale variation, a relationship between latitudinal gradient and body size has been observed for endothermic vertebrates, which increased in body size with increasing latitude or elevation (Mayr, Reference Mayr1956; Olson et al., Reference Olson, Davies, Orme, Thomas, Meiri, Blackburn, Gaston, Owens and Bennett2009). The Brazilian coast has a large latitudinal range and includes three main provinces with five Marine Ecoregions, spread over a wide area with high climatic variability (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). Climatic data show a trend of warming in all five Brazilian Marine Ecoregions in the last four decades (Bernardino et al., Reference Bernardino, Netto, Pagliosa, Barros, Christofoletti, Rosa Filho, Colling and Lana2015). A major influential factor in the different latitudes is temperature e.g. higher temperatures have been shown to increase the metabolism and physiological stress of aquatic organisms (Vaquer-Sunyer & Duarte, Reference Vaquer-Sunyer and Duarte2008).
Littorinidae are widely used as a model in morphometric studies due to their variation in morphological shell plasticity between habitats, and differences in shell size, shape and growth patterns (Chapman, Reference Chapman1995, Reference Chapman1997). The intraspecific shell shape variation among littorinids has been described for different species such as Littorina spp. (Johannesson, Reference Johannesson2003), Littorina saxatilis (Rolán-Alvarez, Reference Rolán-Alvarez2007), Littorina littorea (Doyle et al., Reference Doyle, Gammell and Nash2018), Echinolittorina australis (Johnson & Black, Reference Johnson and Black1999), Littoraria angulifera (Merkt & Ellison, Reference Merkt and Ellison1998; Tanaka & Maia, Reference Tanaka and Maia2006) and Littoraria scabra (Silva et al., Reference Silva, Silva, Madeira, Sallema, Paulo and Paula2013).
The small intertidal gastropod Echinolittorina lineolata (d'Orbigny, 1840) is found on the bare rocks of the supralittoral area, and between barnacles and mytilid bivalves in the upper and intermediate parts of the mesolittoral area (Reid, Reference Reid2009). They can also be found in artificial environments such as piers and bridges. This species is distributed from the Brazilian coast to Uruguay (Reid, Reference Reid2009). These gastropods are dioecious, without the occurrence of sexual dimorphism in their shells (Reid et al., Reference Reid, Dyal and Williams2012). Males actively follow the mucus tracks of females before initiating copulation (Ng et al., Reference Ng, Saltin, Davies, Johannesson, Stafford and Williams2013; Saltin et al., Reference Saltin, Schade and Johannesson2013). Sex ratio is one of the most fundamental reproductive traits in sexual organisms (West, Reference West2009), and the proportion is determined by comparing the number of males to the number of females (Fisher, Reference Fisher1930). This proportion is studied to understand the ecobiological and genetic balance of species in terrestrial and marine ecosystems (Keshavarz & Jahromi, Reference Keshavarz and Jahromi2017). Variation in sex ratio, as well as male patterns in female molluscs, have been documented for bivalves (Yusa et al., Reference Yusa, Breton and Hoeh2013; Zouros, Reference Zouros2013), and gastropods (Yusa, Reference Yusa2007; Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras, Martín, Qiu, Thiengo, Vega, Wada, Yusa, Burela, Cadierno, Cueto, Dellagnola, Dreon, Frassa, Giraud-Billoud, Godoy, Ituarte, Koch, Matsukura, Pasquevich, Rodriguez, Saveanu, Seuffert, Strong, Sun, Tamburi, Tiecher, Turner, Valentine-Darby and Cowie2015; Yusa et al., Reference Yusa, Kitaura and Cazzaniga2016).
Echinolittorina lineolata is an interesting model species to study phenotypic shell plasticity because of its high abundance, extensive longitudinal distribution, and ability to inhabit different kinds of intertidal substrates, both natural and artificial. The objectives of this study were (1) to record the changes in body size in E. lineolata throughout a large latitudinal gradient, considering the hypothesis that size increases as latitude increases; and (2) to provide a morphometric evaluation of the shell shape, applying variables such as latitude and the sex of individuals, based on the hypothesis that females and males differ in terms of shell morphometric variation.
Materials and methods
Study area and sampling
Samples were collected intertidally in the cities of Paracuru – Ceará (3°20′S 39°0′W), Ilhéus – Bahia (14°48′S 39°1′W), Marataízes – Espírito Santo (21°0′S 40°48′W) and Bertioga – São Paulo (23°48′S 46°0′W). Temperature and precipitation for each place were obtained from the WorldClim database (averaged from 1970–2000, Figure 1). In Ceará (3°S), sampling was performed in an artificial environment, on the columns of a pier, because there is no formation of crystalline rocky shores in this area. This sample area is located in the North-eastern Brazilian Marine Ecoregion (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). Its average annual temperature was 26.3°C, with a maximum of 35°C, and an average annual rainfall of 800 mm. This region is prone to irregular rainfall, typical for this semi-arid region (Anjos et al., Reference Anjos, Hernandez, Bañuelos, Dangi, Tirado-Corbalá, da Silva and Filho2018). In Bahia (14°S), sampling was carried out at a crystalline rocky coast, located in the East Marine Ecoregion of Brazil (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). Here, the climate is tropical, with an average temperature of 24.6°C and an annual precipitation of around 2000 mm (Rocha Filho, Reference Rocha Filho1976; Santana et al., Reference Santana, Ramos, Ruiz, Araújo, Almeida, Filho, Mendonça and Santos2003). In Espírito Santo (21°S), sampling was done along the rocky coast, located in the Eastern Marine Ecoregion of Brazil (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). The climate is warm, but with strong sea breezes. The average annual rainfall is around 900 mm (Proater, 2011). In São Paulo (23°S), sampling occurred in the South-east Brazilian Marine Ecoregion (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007). The rocky intertidal coast is generally steep, with no large platforms, and with rocks that are mostly comprised of gneiss and granite (Almeida & Carneiro, Reference Almeida and Carneiro1998) (Supplementary Fig. S1).

Fig. 1. Location map of the sampling areas (Paracuru/CE – 3°S, Ilhéus/BA – 14°S, Marataízes/ES – 21°S and Bertioga/SP – 23°S) of Echinolittorina lineolata (d'Orbigny, 1840) along the Brazilian coast. (A) Precipitation and (B) temperature data were obtained from the WorldClim database in a historical series between 1970–2000.
Fieldwork was done during summer of January 2017. Individuals of E. lineolata were collected by hand from the supralittoral zone of each consolidated environment in order to standardize sampling in the different areas. Collection during low tide was performed during daytime (Stephenson & Stephenson, Reference Stephenson and Stephenson1949; Magalhães, Reference Magalhães1998; Absalão et al., Reference Absalão, Alves and Roberg1999). The biological material was collected with authorization from IBAMA/SISBIO (No. 53725-1). Individuals with damaged or eroded shells were discarded from the samples.
At the Laboratório de Invertebrados Marinhos from the Federal University of Ceará, the specimens were photographed using a Leica EZ stereoscopic microscope. The shell length (SL), shell width (SW), the length of the shell opening/aperture (AL) and the width of the shell opening/aperture (AW) were measured using the ImageJ program (Figure 2). Each shell was photographed in a ventral view in order to carry out the measurements. In the laboratory, the gastropods were classified based on sex by observing the presence or absence of a penis that can be found behind the right tentacle of the animal's head.

Fig. 2. Schematic representation of the species Echinolittorina lineolata (d'Orbigny, 1840) with the measurements used in the morphometric measurement. SL, shell length; SW, shell width; AW, shell width; AL, aperture length of the shell.
Statistical analysis
For standardization and data organization, the localities were labelled by their latitude. Ceará, Bahia, Espírito Santo and São Paulo were represented by 3°S, 14°S, 21°S and 23°S, respectively.
An exploratory analysis of the morphometric data was performed to verify the existence of outliers, and to determine homogeneity, normality, correlation and collinearity (Zuur et al., Reference Zuur, Ieno and Elphick2010). After checking assumptions, a two-way analysis of variance (ANOVA) was used to evaluate the measurements of the shells between the different latitudes and sexes, and for comparing the proportionalities of the shells. The Tukey HSD test was used to verify differences between the pairs. We analysed shell length (SL) and shell opening length (AL) using ANCOVAs that considered latitude as fixed effect and shell width (SW) and shell opening width (AW) as covariates. Simple plots were done to compare shell length against shell width and aperture height against aperture width for the observed groups of individuals from different latitudes. The same analysis was also carried out to determine the proportionality of the shell, defined as the length-to-width ratio (Merkt & Ellison, Reference Merkt and Ellison1998). Sex ratio analysis was done applying the chi-square test (χ2). All calculations were performed using the R program (R Core Team, 2018), and plots from the ggplot2 package (Wickham, Reference Wickham2016) and the additional hrbrthemes package. In all statistical analyses significance level was considered for P < 0.05.
Results
The individuals presented the following averaged measurements for SL, SW, AL and AW. For latitude 3°S: SL = 3.75 ± 0.07 mm (mean ± SD), SW = 2.70 ± 0.05 mm, AL = 1.85 ± 0.04 mm, AW = 1.58 ± 0.03 mm; latitude 14°S: SL = 5.31 ± 0.07 mm; SW = 3.78 ± 0.04 mm; AL = 2.66 ± 0.04 mm, AW = 2.25 ± 0.03 mm; latitude 21°S: SL = 6.60 ± 0.08 mm; SW = 4.70 ± 0.05 mm; AL = 3.50 ± 0.04 mm and AW = 2.79 ± 0.03 mm; and latitude 23°S: SL = 4.93 ± 0.04 mm; SW = 3.61 ± 0.03 mm; AL = 2.59 ± 0.02 mm, AW = 2.15 ± 0.02 mm.
The SL increases gradually from the lower latitude at 3°S to the higher latitude at 21°S, decreasing again at 23°S (Figure 3A). There was no difference in the mean body length between males and females at different latitudes. Males and females from latitude 21°S had larger SL, SW, AL and AW, than males and females from the other latitudes (Table S1).

Fig. 3. (A) Length of the shell of the males and females of Echinolittorina lineolata (d'Orbigny, 1840) in the different latitudes of the Brazilian coast (3°S, 14°S, 21°S and 23°S). (B) Proportionality of the shell (defined as the ratio between shell length and shell width). Legend: Different letters on the bars indicate significant differences between the averages (P < 0.05) – Tukey HSD test.
In regards to shell proportion, the highest averages (1.4 ± 0.07 mm in both males and females) were also found in individuals from the 14 and 21°S latitudes (Figure 3B). These values were significantly higher for the 23°S latitude individuals (Table S1). On the other hand, shell proportion in males (1.40 ± 0.07 mm) and females (1.39 ± 0.07 mm) presented no significant statistical differences (Table S1).
According to the results of the two-way analysis of variance (ANOVA), the morphometric variables of the shells (SL, SW, AL and AW) were influenced by both latitude and sex of the individuals (Table 1). The animals from 3°S were smaller and those from 21°S were larger considering the morphometric relationships of SL-SW and AL-AW (Figure 4). There was no interaction between the sex and latitude factors in relation to the measurements of the shells. For the covariance analysis (ANCOVA) it was observed that the SL and AL were significantly affected by the covariates of SW and AW under different latitudes (SL-SW: F (1,641) = 6642.38, P < 0.05; AL-AW: F (1,641) = 3266.45, P < 0.05; Tables S3–S4). Regarding proportionality, only latitude showed a significant difference (Table 1).
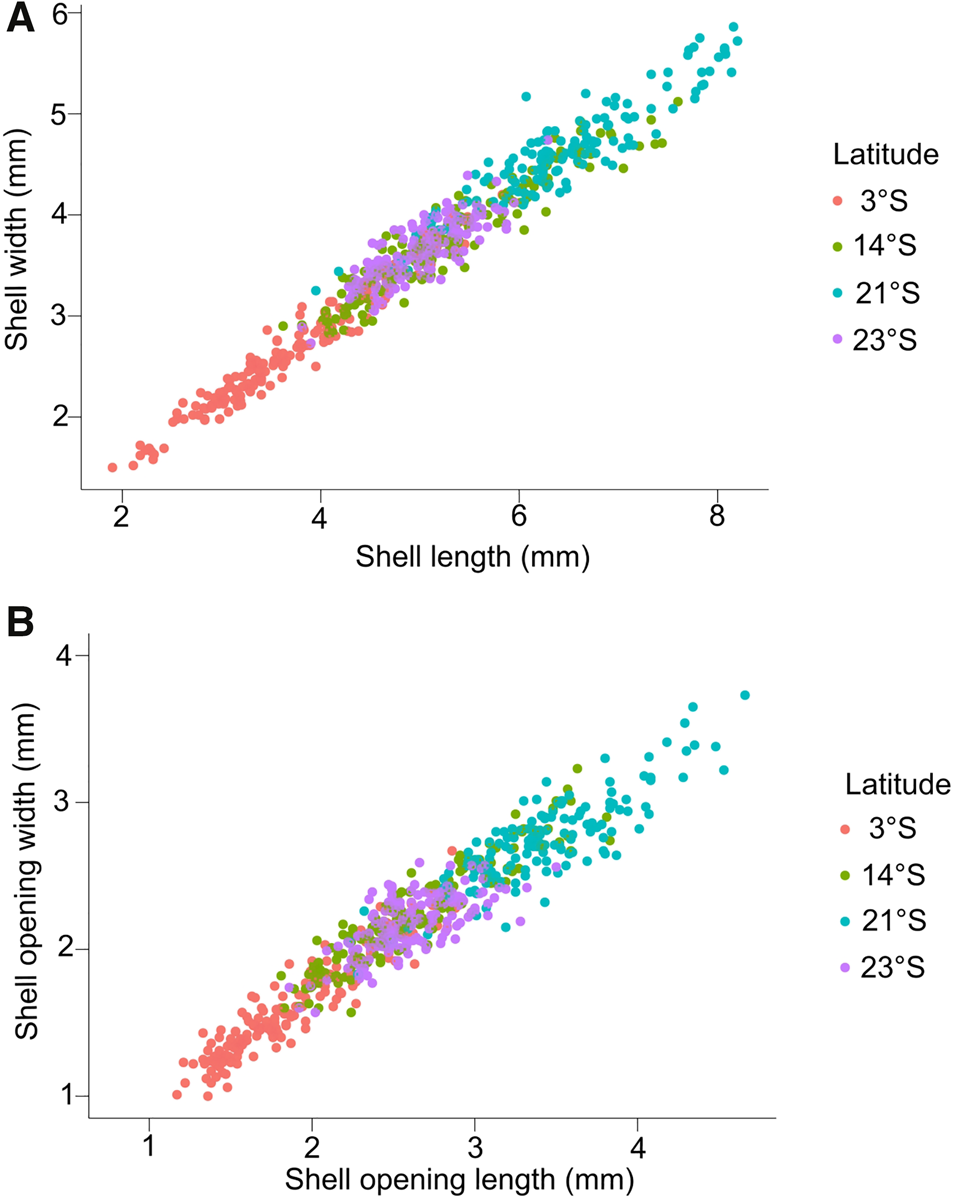
Fig. 4. Relationship between shell measurements: (A) shell length vs shell width (SL-SW) and (B) aperture length of the shell vs shell width (AL-AW) at different latitudes along the Brazilian coast.
Table 1. Results of two-way analysis of variance (ANOVAs) testing for differences in the shell measurements between sexes and latitude

Significant differences are indicated in bold.
In relation to the observed sexual ratio, there was a statistically significant higher proportion of females than males at all latitudes (Table 2).
Table 2. Sexual proportion of Echinolittorina lineolata in the different latitudes south of the Brazilian coast (3°S – Ceará, 14°S – Bahia, 21°S – Espírito Santo and 23°S – São Paulo)

Significant differences are indicated in bold (P < 0.05).
Discussion
The latitudinal increase in body size of co-specific populations has been tested in freshwater, terrestrial and marine animal groups (Blanckenhorn & Demont, Reference Blanckenhorn and Demont2004; Linse et al., Reference Linse, Barnes and Enderlein2006; Angielczyk et al., Reference Angielczyk, Burroughs and Feldman2015; Malvé et al., Reference Malvé, Gordillo and Rivadeneira2018). According to our hypothesis that size of the gastropods would increase according to the latitudinal gradient in our localities, results showed a moderately positive relationship between the size of the individuals and each latitude. However, when the whole dataset was considered, the largest sized gastropod was not observed at the highest latitude (23°S in this study). The variability of body size has a complex spatial tendency that cannot be easily framed by ecogeographic rules such as Bergmann's rule (Malvé et al., Reference Malvé, Gordillo and Rivadeneira2018).
The latitude, in association with other factors, can directly influence the size of the gastropods (Linse et al., Reference Linse, Barnes and Enderlein2006). These factors may include body temperature (Chapperon et al., Reference Chapperon, Le Bris and Seuront2013; Seuront & Ng, Reference Seuront and Ng2016), food availability (Apolinário et al., Reference Apolinário, Coutinho and Baeta-Neves1999), density-dependent intraspecific competition (Magalhães, Reference Magalhães1998) and phenotypic plasticity (Hollander et al., Reference Hollander, Collyer, Adams and Johannesson2006). According to Bernardino et al. (Reference Bernardino, Netto, Pagliosa, Barros, Christofoletti, Rosa Filho, Colling and Lana2015), the extreme conditions of El Niño, combined with long-term global warming, can lead to thermal stress on benthic assemblages found in the mid-latitudes of the tropics, and short-term osmotic stress of those organisms found in the estuaries of south-eastern Brazil. This was observed in the body sizes of individuals from latitudes of 3 and 23°S (which are part of the Marine Ecoregions of north-east and south-east Brazil), that were observed to have smaller sizes than the animals obtained from the Eastern Marine Ecoregion (14°S and 21°S). Another factor that influences animal size in different latitudes is substrate. In this study, the smaller individuals were observed on artificial substrates at latitude 3°S. Artificial substrates may generate a suboptimal environment that benthic organisms can occupy, with biotic and physical characteristics that are different from natural habitats (Goodsell et al., Reference Goodsell, Chapman and Underwood2007). Moreover, studies have found that when shading is disturbed, primary producer biomass and body size of primary consumers are directly influenced, and these results were consistent for both descriptive and manipulative approaches (Bulleri & Chapman, Reference Bulleri and Chapman2010; Pardal-Souza et al., Reference Pardal-Souza, Dias, Jenkins, Ciotti and Christofoletti2017). Echinolittorina lineolata is a primary consumer that feeds on biofilm in the supralittoral zones. In the case of the population from latitude 23°S, the smaller-sized individuals were associated with the structure and dynamic of local populations. Experiments on the types of substrates observed at latitude 23°S should be carried out, as the rocks are mostly composed of gneiss and granite, and are loose and do not form an intertidal platform (A. Matos, personal communication) (Almeida & Carneiro, Reference Almeida and Carneiro1998).
The differences observed in the average sizes of E. lineolata along the Brazilian coast can also be explained by physiological responses. For example, species inhabiting the supralittoral regions can suffer from the most extreme thermal stress and are considered more vulnerable to climate change (Somero, Reference Somero2012; Chapperon et al., Reference Chapperon, Le Bris and Seuront2013). The constant regional differences in tidal regimes, climate and other environmental factors act as selective forces that influence the physiology of intertidal species that extend through broad latitudinal ranges (Han et al., Reference Han, Cartwright, Ganmanee, Chan, Adzis, Hutchinson, Wang, Hui, Williams and Dong2019). The simplistic view stating that thermal stress varies as a function of latitude, with populations at higher latitudes experiencing less heat stress than populations at lower latitudes, is therefore challenged (Lathlean et al., Reference Lathlean, Ayre and Minchinton2014). Local-scale effects that influence benthic communities should be considered, as the thermal stress in the intertidal rocky shore is a product of numerous climatic and biological factors that must be accounted for in the estimation of extreme thermal stress (Lathlean et al., Reference Lathlean, Ayre and Minchinton2014; Seuront & Ng, Reference Seuront and Ng2016; Seuront et al., Reference Seuront, Ng and Lathlean2018).
In regards to shell proportionality, differences were observed only between the gastropods at different latitudes, and not between the sex of individuals. In the context of this study, the biological meaning of proportionality can be explained for the larger animals at latitudes 14°S and 21°S, that had greater water storage capacity in their bodies, allowing for more exposure time, reduced water loss from desiccation, and increased ability to defend themselves against predators (Rivest, Reference Rivest1983). The proportionality of the shell (SL/SW) can be related to the water retention capacity of the gastropods (Merkt & Ellison, Reference Merkt and Ellison1998), potentially avoiding mortality by desiccation during extreme tides. Gastropods living in tropical areas may have higher and wider shells, which increases capacity to hold more water reserves, and allow for more efficient cooling and resistance to desiccation (Vermeij, Reference Vermeij1972). While latitudinal gradient, sex and the proportionality of the shell cannot explain the differences in the morphometric variables, these results may indicate an allometry, as observed in the study of the gastropod Buccinanops globulosus (Avaca et al., Reference Avaca, Narvarte, Martín and van der Molen2013).
Different populations of littorinids of the genus Echinolittorina, facing divergent thermal stress along their latitudinal distribution ranges (Han et al., Reference Han, Cartwright, Ganmanee, Chan, Adzis, Hutchinson, Wang, Hui, Williams and Dong2019), show influence in the fitness and trade-off of the species. The shells of individuals at latitudes 21°S are larger and wider (SL-SW), and with greater openings (AL-AW), than snails from 3°S, which were smaller and less wide. Smaller individuals observed at latitudes 3°S and 23°S, may invest less energy in growth, thus leading to smaller shells, when considering the adaptive differentiation of populations to local environmental conditions (e.g. periods of the tide). When resources are limiting, any investment in a specific feature, such as shell growth, must have a cost which could result in compensation of other characteristics such as reproduction (Araújo et al., Reference Araújo, Serrão, Sousa-Pinto, Arenas, Monteiro, Toth, Pavia and Åberg2015).
A variation of the shell morphometric measurements was found for populations of E. lineolata along the Brazilian coast. Our results show that the intraspecific variation of the shape of the shell is affected by shell length and body width. This variation was also observed between the sexes, with females being larger in size and width. There was no interaction between the factors of sex and latitude. Therefore, variations in lengths of male and female shells did not depend on latitude. The shell length of gastropods from populations of different latitudes did not follow a pattern. This result correlates with the study that body size variation in gastropods (and other molluscs) follows a complex pattern, being related with many variables, not only with latitude (e.g. Bergmann's pattern) (Malvé et al., Reference Malvé, Gordillo and Rivadeneira2018). At different spatial scales, factors such as topography, micro-habitat and the slope of the coast can influence the size and shape of the shell as well as animal physiology (Tanaka & Maia, Reference Tanaka and Maia2006; Lima et al., Reference Lima, Gomes, Seabra, Wethey, Seabra, Cruz, Santos and Hilbish2016). Lima et al. (Reference Lima, Gomes, Seabra, Wethey, Seabra, Cruz, Santos and Hilbish2016) observed physiological responses of Patella vulgata, which were strongly associated with local microtopography rather than coastal latitude. Silva et al. (Reference Silva, Silva, Madeira, Sallema, Paulo and Paula2013) observed that shell size and width of Littoraria scabra varied, probably in association with environmental factors along the coast, such as temperature and hydrodynamics. Large-scale variations in the shell morphology of Littoraria angulifera were found by Merkt & Ellison (Reference Merkt and Ellison1998), and these authors suggest that this pattern may be a result of the animals' response to local environmental conditions.
The biometric measurements considered in this study showed a higher correlation between total length and total width (Figure 4). The width seems to be related to the size of the foot: larger individuals have a larger foot, which provides greater adhesion to the substrate (Trussell, Reference Trussell2000; Ito et al., Reference Ito, Ilano, Goshima and Nakao2002). In the intertidal zone, gastropods are hydrodynamically influenced by the waves, with larger shell openings observed as a resultant characteristic. Larger shell openings allow for the maintenance of a larger foot, which, in turn, increases adhesion to the substrate (Ito et al., Reference Ito, Ilano, Goshima and Nakao2002), but also reduces the risk during displacement (Grahame & Mill, Reference Grahame and Mill1989; Trussel, Reference Trussel1997). According to our observations, E. linoleata was actively moving and grazing during high tide when they were submersed, which exposed them to wave action (Matos, personal communication). It was observed that the proportion of females is higher compared with males in all latitudes, which could be influenced by potentially greater adhesion to the substrate, due to their wider shell shape.
Females were different to males in their shell characteristics with larger shell length and shell width. There is no known sexual dimorphism of the shell in this species, but it has been shown that females tend to be larger and wider than males. Future studies on geometric morphometric analysis would complement these results (Doyle et al., Reference Doyle, Gammell and Nash2018). Shell size in females should be important from a reproductive point of view, since males can preferentially mate with larger females (Ng & Williams, Reference Ng and Williams2012). Larger-sized female littorinids are physically able to produce a larger number of eggs, meaning a higher fecundity as shell size increases (Zahradnik et al., Reference Zahradnik, Lemay and Boulding2008; Ng & Williams, Reference Ng and Williams2012). Size in littorinids influences mating, as was observed for Echinolittorina malaccana and Echinolittorina radiata, where males with larger shells were more successful in mating than those with smaller shells (Ng et al., Reference Ng, Davies, Stafford and Williams2016). Echinolittorina malaccana and E. radiata males also preferentially track mucus from females larger than them, leading them to mate with larger females (Ng et al., Reference Ng, Davies, Stafford and Williams2016).
In this study, some of the observed size patterns appear to be influenced by latitude, considering local spatial responses to factors such as the influence of life history changing the morphometric variables of the gastropods. Of these local issues, sex biology should be considered, since males and females have different metabolisms, reproductive strategies, and energy investment in their shell growth. The smaller sized gastropods were found in the Marine Ecoregions of north-east and south-east Brazil, which appeared to be more influenced by air temperature and rainfall. We can consider that total size and width are morphometric variables that can generally vary between males and females, but a complementary analysis with geometric morphometry can increase confidence levels of the variations between the sexes. In addition, analyses of the latitudinal variation of life histories, such as growth rates in the field, supplemented with laboratory experiments, can provide a more robust basis for the latitudinal factors influencing the populations of E. lineolata along the Brazilian coast.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315420000624.
Acknowledgements
We are especially grateful to Poliana Salve Guizardi and Juliana Gaeta for the samples collected from the Marataízes-ES (21°S) and Bertioga-SP (23°S) sites, respectively. We would like to thank Editage (www.editage.com) for English language editing. We extend our sincerest appreciation for the insightful and constructive comments on our manuscript provided by the anonymous reviewers.
Financial support
This work was funded by the ‘Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico’ (FUNCAP – PEP-0094-00001.01.123/15).








