Introduction
African yam bean, AYB (Sphenostylis stenocarpa Hochst ex A. Rich. Harms), is a tuberous legume of tropical Africa with considerable potential. The seeds and tubers are of economic importance providing food for humans and livestock. AYB is an affordable source of protein, containing approximately 29 and 19% crude protein in its grain and tuber, respectively (Adewale and Dumet, Reference Adewale and Dumet2011). The amino acid (lysine and methionine) content in AYB grain is higher than that obtainable in pigeon pea, cowpea and Bambara groundnut (Uguru and Madukaife, Reference Uguru and Madukaife2001). These peculiarities encourage a thorough characterization of its germplasm.
Unfortunately, despite the crop's acknowledged potential, it is still classified as neglected and underutilized species (Padulosi et al., Reference Padulosi, Thompson and Rudebjer2013); and little information at the molecular level is available. DNA-based markers previously used in this species include random amplified polymorphic DNA (RAPD) and amplified fragment length polymorphisms (AFLPs) (Moyib et al., Reference Moyib, Gbadegesin, Aina and Odunola2008; Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Nnadi, Kehinde, Ojo, Adegbite and Franco2014; Ojuederie et al., Reference Ojuederie, Morufat, Iyiola, David and Mercy2014); however, there is no report on the use of simple sequence repeat (SSR) primers. SSRs being co-dominant in nature, abundant in genomes and highly polymorphic are markers of choice for crop improvement especially for crops like AYB where single nucleotide polymorphisms are yet to be identified. SSRs can be derived from genomic DNA (genomic SSRs) or from transcribed regions of the DNA, i.e. expressed sequence tags (ESTs).
To our knowledge, no SSR marker has been reported in AYB. Development of SSRs requires prior sequence information, and this is not available for AYB. However, the procedures of identifying species-specific SSRs can be avoided if markers from related species are transferable. Successful transferability of SSRs within the Fabaceae has been reported, from chickpea (Cicer arietinum) to pigeon pea (Cajanus cajan), from cowpea (Vigna unguiculata) to Bambara groundnut (Voandzeia subterranea) and from adzuki bean (Vigna angularis) to black gram (Vigna mungo) (Datta et al., Reference Datta, Kaashyap and Kumar2009; Somta et al., Reference Somta, Chankaew, Rungnoi and Srinives2011; Sanjeev et al., Reference Sanjeev, Debjyoti, Anjum, Aditya and Jitendra2013).
AYB is identified with some limitations, such as hardness of the seed-coat and hence longer time to cook, presence of secondary metabolites, lengthy life cycle, photoperiodic sensitivity, etc. (Adewale and Dumet, Reference Adewale and Dumet2009; Adewale, Reference Adewale2010). To improve any crop species, adequate knowledge of its germplasm is required. In AYB, different approaches have been applied to evaluate genetic diversity within accessions. Adesoye and Nnadi (Reference Adesoye and Nnadi2011) and Adewale et al. (Reference Adewale, Vroh-Bi, Dumet, Kehinde, Ojo, Adegbite and Franco2012) studied AYB intra-specific variation based on chromosome counts and seed characteristics, respectively. Researchers have described AYB diversity based on morphological characters (Akande, Reference Akande2009; Popoola et al., Reference Popoola, Adegbite, Adewale and Odu2011; Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Kehinde, Ojo, Adegbite and Franco2012). Although morphological methods are useful, they have drawbacks, which include the influence of environment (Gepts, Reference Gepts1993). In contrast, molecular markers are uninfluenced by the environment and reveal differences at the DNA level (Vicente et al., Reference De Vicente, Guzman, Engels and Ramanatha2005). Therefore, the objectives of the present research were: to test the amplification ability of 36 cowpea-derived SSRs across AYB genome and to study the genetic diversity within 67 AYB accessions.
Materials and methods
Plant materials and DNA extraction
A total of 67 accessions of AYB previously characterized based on morphological traits (Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Kehinde, Ojo, Adegbite and Franco2012) were collected from the Genetic Resources Centre (GRC) of the International Institute of Tropical Agriculture (IITA), Ibadan. Fresh leaves from 2-week-old seedlings were collected in sample bags properly labelled for each accession. DNA was extracted from the leaves following a modified Dellaporta et al. (Reference Dellaporta, Wood and Hicks1983) protocol. The DNA quality and quantity were determined using the NanoDrop spectrophotometer (ND-1000) (Thermo Fisher Scientific, USA) and visualized by agarose gel electrophoresis (Sunrise 96, Biometra, Germany).
Polymerase chain reaction optimization
A total of 36 cowpea-derived SSR (16 genomic, ten unigene and ten EST–SSR) primers were screened using various reagent concentrations of the polymerase chain reaction (PCR) master mix and sets of cycling parameters, using DNA obtained from two accessions of cowpea (ITK93K-452 and ITK93-452b) and six accessions of AYB (TSs4, TSs57, TSs41, TSs49, TSs64 and TSs7). Amplification was achieved using a touchdown profile between the ranges of 65°C to 55°C and 13 primers with consistent amplification were chosen for the study. The primer sequences used in the study are presented in online supplementary Table S1. The EST–SSR and unigene sequences were based on the primer sequences reported by Pei et al. (Reference Pei, Xiaohua, Baogen, Younghua, Dehui, Jeffery, Timothy, Tingting, Zhongfu and Guoijing2009) and Gupta and Gopalakrishna (Reference Gupta and Gopalakrishna2010).
SSR primer amplification
SSR amplification was carried out with 50 ng/μl of DNA template in two separate 10 μl volume reactions. Both reactions contained 4 μl of DNA template, 0.8 μl of dNTPs (deoxyribonucleotide triphosphate) (2.5 mM), 1.0 μl of 10 × buffer and 0.06 μl of Taq polymerase (Inqaba Biotec, SouthAfrica). Under reaction condition I, 1.0 μl of MgCl2 (50 mM), 1.0 μl of forward and reverse primers (5 μM) and 1.14 μl of ultra-pure water were included. In reaction II, 1.2 μl of MgCl2 (50 mM) and 1.5 μl of both forward and reverse primers were added to make up the master mix. All forward SSR primers were synthesized and fluorescently labelled by Inqaba Biotec. The reaction mixture was loaded in a Veriti 96 Well (Applied Biosystems, USA) thermal cycler according to the following thermal profile: denaturing at 94°C for 2 min; nine cycles at 93°C for 15 s; annealing at 65°C for 20 s and extension at 72°C for 30 s. The annealing temperature of each cycle decreases by 1°C with the final 30 cycles at 55°C and a final elongation step at 72°C for 5 min. PCR products were checked on 2% Agarose gel stained with ethidium bromide in 1 × TBE (Tris Borate Ethylenediaminetetraacetic acid) buffer. Standard size ladder (50 base pair) was loaded alongside. Gel was viewed in a Bio-Rad UV minidark room. The PCR products from specific SSRs were co-loaded for fragment analysis, the fragment analysis was performed using a 36 cm, 16 capillary array and Pop 7 Polymer on an ABI 3130XL Genetic analyzer (Applied Biosystems, USA), and peak and alleles sizes were scored using the GeneMapper V 4.0 software (Applied Biosystems, USA).
The polymorphic information content (PIC) of each SSR marker was calculated as described by Bostein et al. (Reference Botstein, White, Skolnick and Davis1980) using the PowerMarker V3.25 software (Liu and Muse, Reference Liu and Muse2005). Micro-Checker 2.2.1 (Van et al., Reference Van, Hutchison, Will and Shipley2004) was used to check for potential genotyping errors such as allelic dropouts, and stuttering or null alleles using Brookfield's estimate (Brookfield, Reference Brookfield1996). Pair-wise genetic dissimilarity among 67 AYB accessions was obtained using simple matching (SM) coefficient and the SIMQUAL options in NTSYSpc V 2.02j (Rohlf, Reference Rohlf1998). A dendrogram was generated using the unweighted pair-group method with arithmetic average (UPGMA).
The percentage amplification of each cowpea SSR primers was calculated based on the formula described by Ruchi et al. (Reference Ruchi, Bhat and Lakhanpaul2009).
 $$\begin{eqnarray} Percent\,\,amplification = \frac {number\,\,of\,\,accessions\,\,in\,\,which\,\,amplification\,\,occurred}{total\,\,\,number\,\,of\,\,accessions}\times 100. \end{eqnarray}$$
$$\begin{eqnarray} Percent\,\,amplification = \frac {number\,\,of\,\,accessions\,\,in\,\,which\,\,amplification\,\,occurred}{total\,\,\,number\,\,of\,\,accessions}\times 100. \end{eqnarray}$$
Results
PCR optimization
Cowpea SSR primers – VM31, Bmd17, VM40, VM51, CLM0899, CLM0936, VM30, CLM0938, VM54, VM70 and VM74 – amplified AYB DNA under reaction condition I. Primers VuUGM25 and VM71 showed amplification under reaction II. SSR primer pairs, VM31, VM51, VM74 and CLM0899, amplified AYB under both reactions (I and II). The amplification patterns obtained during optimization by three cowpea SSRs (VM31, VM37 and VM51) are shown in Figs 1 and 2. Fig. 2(a) shows inability of SSR primer VM37 to amplify genomic DNA of six AYB accessions, but it amplified two cowpea DNAs under the same reaction condition.

Fig. 1 Amplified bands of two cowpea (Vigna unguiculata) and six AYB (Sphenostylis stenocarpa) genomic DNA obtained with cowpea-derived SSR primer (VM31). This was observed during optimization of PCR reaction I and II respectively (Wells: - 1, 50bp ladder; 2, ITK93-452; 3, ITK93-4523; 4, TSs4; 5, TSs5; 6, TSs41; 7, TSs49; 8, TSs67; 9, TSs7; 10, ITK93-452; 11, ITK93-452; 12, TSs4; 13, TSs57; 14, TSs41; 15, TSs49; 16, TSs67; 17, TSs7).

Fig. 2 Amplified bands of six AYB (Sphenostylis stenocarpa) genomic DNA and two cowpea (Vigna unguiculata) genomic DNA obtained with cowpea-derived SSR markers (VM37 and VM51). This was observed during optimization of PCR reaction I (Wells: 1, 50bp ladder; 2, TSs4; 3, TSs57; 4, TSs41; 5, TSs49; 6, TSs67; 7, TSs7; 8, ITK93-452; 9, ITK93-452; 10, TSs4; 11, TSs57; 12, TSs41; 13, TSs49; 14, TSs67; 15, TSs7; 16, ITK93-452; 17, ITK93-452).
Transferability of cowpea SSR markers and polymorphism
The transferable cowpea markers exhibited extensive polymorphism across 67 AYB accessions. This confirms their usefulness in detecting genetic diversity. Of the 13 SSR primers that showed characteristics bands in AYB; primers VM13, VM31, VM51, VM74, VM54, VM40, VM71, VM30, Bmd17 and VM70 are genomic, CLM0899, CLM0938 and CLM0936 are ESTs and VuUGM25 is unigene (see online supplementary Table S1). Percentage amplification of primers across accessions varied. Three primers, VM54, VM71 and VM40, showed more than 80% amplification across AYB accessions, while four primers VuUGM25, VM31, CLM0936 and VM74 positively amplified between 72 and 79% (Table 1). Similarly, the percentage amplification of Bmd17 was more than 60%. Moreover, the transferability efficiency of VM70, VM51, VM30, CLM0938 and CLM0899 was less than 20% across accessions (Table 1). All the 36 cowpea-derived SSRs amplified cowpea genomic DNA used as control. Out of the 36 primers, 23 fail to show amplification in AYB and were not used further.
Table 1 Summary of cross-amplification of cowpea SSR primers across 80 AYB accessions

PIC, polymorphic information content.
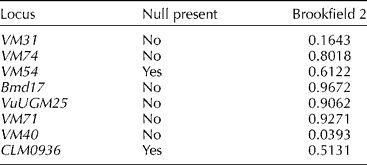
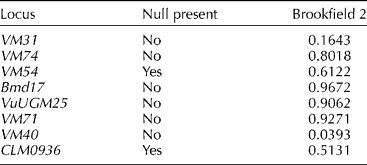
Eight primers that showed above 60% frequency of amplification were used for diversity study. The eight transferable SSRs produced a total of 55 polymorphic fragments in AYB. The number of fragments per SSRs ranged from 4 to 10 with an average of 6.9 per primer (Table 1). The SSR primer VM71 detected the highest number of fragments (ten) and therefore the most informative primer. VuUGM25 gave the least fragments (four). PIC for AYB varied from 0.6691 (VuUGM25) to 0.8857 (VM40) with an average of 0.7791 (Table 1). There was no evidence for large allele drop-out or stuttering for any of the SSR loci. Two loci (VM54 and CLM0936) showed evidence for a null allele (Table 2).
Table 2 Estimated null allele frequency as calculated using Brookfield's method
Diversity among 67 AYB accessions
Based on the 55 polymorphic fragments detected by the eight transferable SSR primers, a dendrogram was generated using the UPGMA method of clustering and three main clusters were obtained (Fig. 3). The mean dissimilarity between the 67 AYB accessions was 0.743 and the least dissimilarity of 0.458 (Table 3) was observed between TSs98 whose origin was not recorded and TSs59 which is of Nigerian origin. The highest dissimilarity of 1.0 (Table 3) was observed between TSs2 and TSs60, TSs47 and TSs67, and TSs61 and TSs76. Cluster 1 had 30 accessions, majority of which are of Nigerian origin; moreover, the only accession (TSs67) from Bangladesh grouped in this cluster. Accessions TSs76 and TSs77 (both from Ghana) had a dissimilarity coefficient of 0.778, and they grouped together with the only accession of DRC (Democratic Republic of the Congo) origin, i.e. TSs65 and other accessions which are mostly of Nigerian origin in cluster 2. Cluster 3 had 2 accessions TSs119 which origin had no passport data and TSs92 a Nigerian accession (Fig. 3). The association of accessions within clusters was not based on their geographical origin.

Fig. 3 Dendrogram showing the clustering pattern among 67 accessions of AYB, Sphenostylis stenocarpa, as dictated by eight cowpea SSR primers. The dendrogram was constructed based on UPGMA, using the SM coefficient option.
Table 3 Genetic information obtained from the association of 67 AYB accessions

DS, dissimilarity.
Discussion
Cross-species molecular marker transferability is important to species with no sequence information. To our knowledge, this study is the first to transfer SSR markers from cowpea to AYB. There have been reports of cross-species transferability of SSRs and their use in understanding diversity within the family Fabaceae. Examples include the following: Glycine max to Arachis hypogaea (He et al., Reference He, Woullard, Marong and Guo2006); V. angularis to V. mungo (Gupta and Gopalakrishna, Reference Gupta and Gopalakrishna2009) and V. unguiculata to Vigna sesquipedialis (Datta et al., Reference Datta, Kaashyap and Kumar2009), etc. The 36% transferability recorded in this study is relatively high. Within pulse crops, SSR marker transferability rates have been shown to be as low as 16% (Choudhary et al., Reference Choudhary, Sethy, Shokeen and Bhatia2008; Reddy et al., Reference Reddy, Rathour, Kumar, Katoch and Sharma2010; Gupta et al., Reference Gupta, Sen, Anjum, Pratrap and Kumar2013). In our study, SSR primers VM40 and VM30 resulted in the highest and lowest amplification of 85 and 6.3%, respectively (Table 1). We obtained genomic SSR and EST–SSR transferability rates of 56 and 35% in this study. However, unigene SSRs exhibited 10% transferability. Datta et al. (Reference Datta, Kaashyap and Kumar2009) recorded a higher transferability rate of 71% with EST–SSRs as against 65% with genomic SSRs. The amplification size produced by transferable SSRs in AYB was different from the size of amplicon produced in cowpea as shown in Fig. 1. This is an indication that some of the cowpea primers resulted in amplifications of different sizes other than the expected amplicon size. Datta et al. (Reference Datta, Kaashyap and Kumar2009) also recorded similar observations. According to Datta et al. (Reference Datta, Kaashyap and Kumar2009), 23 SSR markers failed to amplify any of the AYB DNA; this may be due to the absence of loci or due to mutation in the primer-binding sites. Five SSRs (VM70, VM51, VM30, CLM0938 and CLM0899) with less than 20% amplification frequency were not included for diversity study as they could not generate sufficient information. The amplification of cowpea SSRs in AYB and the average PIC value of 0.7791 (Table 1) clearly suggest the applicability of these SSR markers for germplasm characterization, breeding application, diversity studies and comparative mapping between AYB and cowpea.
For a germplasm to be effectively conserved and utilized in breeding programmes, it is necessary to assess the genetic diversity (Li et al., Reference Li, Ra, Park, Kwon, Lee, Park and Park2011). This study analyzed the genetic diversity among 67 accessions of AYB using eight cowpea SSR primers. A dendrogram based on the UPGMA method grouped accessions into three major clusters with SM coefficient ranging from 0.458 to 1.000 (Table 3). The clustering of accessions (Fig. 3) clearly showed the extent of genetic diversity among the studied accessions. Similarly, Adewale et al. (Reference Adewale, Vroh-Bi, Dumet, Kehinde, Ojo, Adegbite and Franco2012) recorded a high level of diversity when they morphologically characterized the same accessions. The research conducted by Moyib et al. (Reference Moyib, Gbadegesin, Aina and Odunola2008) using RAPDs revealed considerable genetic diversity among 24 AYB accessions. Furthermore, AFLPs analysis presented a high level of diversity among AYB accessions (Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Nnadi, Kehinde, Ojo, Adegbite and Franco2014; Ojuederie et al., Reference Ojuederie, Morufat, Iyiola, David and Mercy2014).
SSR primer analysis revealed a higher rate of polymorphism (36%) when compared with 26% recorded by Adewale et al. (Reference Adewale, Vroh-Bi, Dumet, Nnadi, Kehinde, Ojo, Adegbite and Franco2014), but the average number of polymorphic fragments per primer was higher with AFLP (11.8) than with SSR primers (6.9). The extent of genetic diversity recorded in the present research also seems to be higher than what has been obtained in previous analysis of morphological and molecular markers (AFLP and RAPD). A dissimilarity which ranged from 0.46 to 1.0 was recorded with SSRs, while, with AFLPs, it ranged from 0.07 to 0.95 (Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Kehinde, Ojo, Adegbite and Franco2012; Ojuederie et al., Reference Ojuederie, Morufat, Iyiola, David and Mercy2014). In RAPD analysis, Moyib et al. (Reference Moyib, Gbadegesin, Aina and Odunola2008) observed a dissimilarity range of 0.42–0.96.
The clustering pattern of accessions as revealed by SSR primers was different from the pattern observed in AFLP analysis of Ojuderie et al. (Reference Ojuederie, Morufat, Iyiola, David and Mercy2014); however, there are differences in the accessions studied. The SSR markers grouped the only Bangladesh accession in the same clusters with those of Nigerian origin. In the work carried out by Ojuderie et al. (Reference Ojuederie, Morufat, Iyiola, David and Mercy2014), the Bangladesh accession grouped together with the two accessions of Ghana origin. Moreover, the clustering of accessions in the present research was not on the basis of geographical origins and this concur with previous reports (Moyib et al., Reference Moyib, Gbadegesin, Aina and Odunola2008; Adewale et al., Reference Adewale, Vroh-Bi, Dumet, Nnadi, Kehinde, Ojo, Adegbite and Franco2014). This result seems to suggest high exchange of AYB germplasm among smallholder farmers across regions.
However, developing an understanding of intra-specific variation of AYB at regional or geographical level is necessary to confirm this possibility. As the accessions considered in this study are mostly of Nigerian origin and therefore probably represent only a proportion of the total diversity within the species, there is a clear need for further germplasm collection and characterization. Hence, increased germplasm size from other regions and more SSR markers would provide clearer understanding of the genetic diversity in this crop.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262115000064
Acknowledgements
The authors thank the seed bank of the GRC, IITA, Ibadan, for providing the accessions used and Pei Xu for providing the sequences of the EST–SSR primers.