Introduction
In seeds of various species, the embryo is differentiated into organs, but it is small (underdeveloped) and must grow inside the seed prior to germination (Baskin and Baskin, Reference Baskin and Baskin2014). In some species, however, the embryo in freshly matured seeds is not differentiated, but after seed dispersal it differentiates into an underdeveloped embryo (Mondoni et al., Reference Mondoni, Probert and Rossi2012). The requirement for growth of an underdeveloped embryo before the radicle emerges is called morphological dormancy (MD), but these seeds do not require any dormancy-breaking pretreatments, such as warm or cold stratification, to germinate. That is, the dormancy period in seeds with MD is the time required for the embryo to complete growth, which is ≤ 4 weeks (Baskin and Baskin, Reference Baskin and Baskin2014). In addition to MD, the embryo may have a physiological inhibiting mechanism, which includes low growth potential of the embryo as well as restricting effects of the seed coat (Nikolaeva, Reference Nikolaeva and Khan1977), i.e. physiological dormancy (PD). The presence of both MD and PD in the same seed is called morphophysiological dormancy (MPD). Depending on the environmental conditions required to break PD and promote embryo growth and the response of seeds to gibberellins, nine levels of MPD have been identified (Nikolaeva, Reference Nikolaeva1969, Reference Nikolaeva and Khan1977; Baskin and Baskin, Reference Baskin and Baskin2014). If seeds have MPD, treatments such as warm or cold stratification are required to break PD, and the embryo may grow: (1) after all the PD is broken (Baskin and Baskin, Reference Baskin and Baskin1990); (2) after part of the PD is broken (Baskin and Baskin, Reference Baskin and Baskin1989); or (3) during the time PD is being broken (Baskin and Baskin, Reference Baskin and Baskin1994).
MD has been reported in seeds of tropical montane woody plants in various families, including Araliaceae, Campanulaceae, Canellaceae, Garryaceae, Magnoliaceae, Pittosporaceae and Podocarpaceae, but of these families only Araliaceae, Campanulaceae and Pittosporaceae are represented by woody species in the montane zone of Hawaii. MPD occurs in seeds of tropical montane woody plants belonging to various families, including Annonaceae, Aquifoliaceae, Araliaceae, Arecaceae, Campanulaceae, Magnoliaceae, Monimiaceae, Myristicaceae, Papaveraceae, Pittosporaceae, Podocarpaceae, Santalaceae, Schisandraceae, Taxaceae and Winteraceae (Baskin and Baskin, Reference Baskin and Baskin2014), but of these families only Aquifoliaceae, Araliaceae, Arecaceae, Campanulaceae, Papaveraceae, Pittosporaceae and Santalaceae are represented by woody species in the montane of Hawaii (Wagner et al., Reference Wagner, Herbst and Sohmer1999, Reference Wagner, Herbst and Lorence2005). MD has not been studied in detail in woody species in the montane of Hawaii, and MPD has been studied in detail only in six species, all of which belong to the Campanulaceae (Baskin et al., Reference Baskin, Baskin and Yoshinaga2005).
When considering woody plants with MD and MPD in the tropical montane zone of Hawaii, the largest family is the Campanulaceae, with about 140 species, and the second largest is the Araliaceae, with 15 species (Wagner et al., Reference Wagner, Herbst and Sohmer1999, Reference Wagner, Herbst and Lorence2005). Studies on the dormancy-breaking and germination requirements of seeds of six endemic lobelioid shrubs (Campanulaceae) revealed that seeds have the non-deep simple level of MPD (Baskin et al., Reference Baskin, Baskin and Yoshinaga2005). However, no detailed studies have been conducted on Hawaiian montane Araliaceae. Based on occurrence of MD and MPD in the Araliaceae (Baskin and Baskin, Reference Baskin and Baskin2014), we inferred that seeds of Hawaiian Araliaceae have underdeveloped embryos. Thus, the general purpose of our investigation was to determine if seeds of the Hawaiian Araliaceae taxon Cheirodendron trigynum (Gaud.) A. Heller subsp. trigynum have MD or MPD and, if MPD, what level. There are two subspecies of C. trigynum: helleri is restricted to Kauai and trigynum occurs on the other main islands (Wagner et al., Reference Wagner, Herbst and Sohmer1999, Reference Wagner, Herbst and Lorence2005). We studied seeds of C. trigynum subsp. trigynum (hereafter C. trigynum) from two of the islands.
C. trigynum is a tree occurring in mesic to wet forests (Wagner et al., Reference Wagner, Herbst and Sohmer1999), and it is one of five species of Cheirodendron in Hawaii. Fosberg (Reference Fosberg1948) concluded that the five Cheirodendron species in Hawaii are derived from a single founding species with southern Pacific affinity. The genus consists of the five Hawaiian species, and another species that is endemic to the Marquesas Islands (Mabberley, Reference Mabberley2008).
Materials and methods
Seeds
Ripe fruits (drupes) were collected on 2 January 2002 from trees growing along the Hawaii Loa Trail on the island of Oahu [315 m above sea level (a.s.l.)], and experiments were started on 24 January 2002. Drupes also were collected from trees growing near Glenwood on the island of Hawaii (915 m a.s.l.) on 22 March 2003 and experiments started on 3 April 2003. A second collection was made at Glenwood on Hawaii (the Big Island) on 15 January 2005 and experiments started on 7 February 2005. After each fruit collection was made, the exocarp and mesocarp were removed from the drupes, leaving the true seed enclosed by the brittle endocarp (hereafter, the seed). Seeds were washed, air-dried at room temperatures for several days and then air-mailed to the University of Kentucky, where experiments were conducted.
Germination conditions
Data from US weather stations at various elevations in the Hawaiian montane zone revealed that the difference between mean daily maximum and minimum monthly temperatures is about 10°C, regardless of month and elevation (see table 2 in Baskin et al., Reference Baskin, Baskin and Yoshinaga2005). Throughout the year, variation in mean daily maximum monthly temperatures and in mean daily minimum monthly temperatures at a given elevation varies from 3 to 5°C. At high and low elevations, mean daily maximum and minimum monthly temperatures are 15/5 and 24/14°C, respectively. For our germination studies, we used daily (12/12 h) temperature regimes of 15/6, 20/10 and 25/15°C to simulate temperatures at high, mid and low elevations in the montane, respectively.
Seeds were incubated on quartz sand moistened with distilled water in 9-cm-diameter plastic Petri dishes at 15/6, 20/10 and 25/15°C at a daily light (c. 40 μmol m− 2s− 1, 400–700 nm of cool white fluorescent light, hereafter light) period of 14 h. The light came on in each incubator 1 h before the daily 12-h high-temperature period began, and it remained on for 1 h after the 12-h low-temperature period began.
A move-along experiment (Baskin and Baskin, Reference Baskin and Baskin2003) was used to determine the dormancy-breaking and germination requirements for seeds collected in 2002, 2003 and 2005. This experiment had three parts. In part one, seeds were incubated on moist sand in light at 15/6, 20/10 and 25/15°C for the duration of the experiment (i.e. the controls). In part two, seeds on moist sand were incubated at a sequence of temperatures, starting with 25/15 (12 weeks) → 20/10 (8 weeks) → 15/6 (12 weeks) → 20/10 (8 weeks) → 25/15°C (12 weeks) → and cycle was repeated (high to low). In part three, seeds on moist sand were incubated at a sequence of 15/6 (12 weeks) → 20/10 (8 weeks) → 25/15 (12 weeks) → 20/10 (8 weeks) → 15/6°C (12 weeks) → and cycle was repeated (low to high). Three replicates of 50 seeds were used for each treatment and control. All seeds were checked at 2-week intervals, at which time seedlings (if present) were counted and removed from the dishes, and water added to the sand if needed. The criterion for germination was emergence of the radicle; after radicle emergence the cotyledons emerged within a few days. In this experiment, the controls provide information on dormancy-break and germination in response to continuous incubation at the three simulated montane temperature regimes. Data from seeds moved from high → low and from low → high tell us if warm (25/15°C) or cool (15/6°C) conditions have an effect on dormancy break and germination. The move-along experiment ran for 60, 80 and 80 weeks for the 2002, 2003 and 2005 seeds, respectively.
To determine if a cold (5°C) or a warm (25/15°C) stratification treatment had an effect on dormancy break and germination, seeds collected in 2003 were incubated in light at 5°C or at 25/15°C for 0, 2, 4, 6 and 8 weeks and then moved to light at 20/10°C, where germination was monitored for 78 weeks.
Embryo:seed ratio
To determine embryo length (E):seed length (S) ratio, imbibed seeds were cut open lengthwise with a razor blade and the embryo excised. Embryo and seed lengths (i.e. thickness of the endocarp not included) were measured using a micrometer in the eyepiece of a dissecting microscope. Extra dishes of seeds were included in the move-along experiments to provide seeds for monitoring changes in the E:S ratio. At time zero, seeds were allowed to imbibe for 24 h in darkness at room temperature, and then the E:S ratio was determined. Embryo growth was studied for seeds collected in 2002, 2003 and 2005. At time zero and each time the E:S ratio was determined, 25 seeds were used, unless stated otherwise. The maximum (critical) E:S ratio to which embryos must grow before the radicle emerges was determined for 15 seeds in which the endocarp had split but no radicle protrusion had occurred.
For seeds collected in 2002, the E:S ratio was determined when seeds were moved from 25/15 to 20/10°C in the high → low move along (see above) and when moved from 15/6 to 20/10°C in the low → high move along (see above); after incubating at 20/10°C for 8 weeks the E:S ratio was determined. For seeds collected in 2003, the E:S ratio was determined after seeds had been incubated at 20/10°C for 3, 6, 10, 12, 15, 18 and 24 weeks. For seeds collected in 2005, the E:S ratio was determined for 15 seeds after 0, 4, 8, 12, 18, 20, 24, 30 and 36 weeks of incubation in the light at 15/6, 20/10 and 25/15°C, and for seeds at the time they were moved from one temperature to the next in the high to low sequence and in the low to high sequence of temperature regimes.
Statistics
Final germination percentages in the three move-along experiments were arcsine transformed before statistical analysis to ensure homogeneity of variance; however, non-transformed data are presented in the figures. A one-way analysis of variance (ANOVA) (P< 0.05) was conducted on the data and, if significant differences were detected, the Duncan New Multiple Range Test was used to determine differences among treatments. Time for 10% and 50% of 2003-collected seeds, given 0–8 weeks of warm or cold stratification, to germinate when incubated at 20/10°C was based on number of seeds sown and was determined from data sheets, i.e. not calculated.
Results
Move-along experiment
For the 2002 seeds, incubation temperature had no effect on final germination percentage, with seeds incubated continuously at 15/6, 20/10 and 25/15°C germinating to 94, 98 and 100%, respectively (Fig. 1a) (F= 3.84, P>0.05). Seeds began germinating first at 25/15°C, but the most rapid germination occurred in those incubated continuously at 20/10°C. Seeds had germinated to ≥ 50% at 20/10, 15/6 and 25/15°C after 14, 16 and 18 weeks, respectively. Moving seeds from high → low or from low → high temperature regimes resulted in 100% germination for both treatments.

Figure 1 Cumulative germination percentages (mean ± SE, if ≥ 5%) of Cheirodendron trigynum seeds collected in (a) 2002 on Oahu, (b) 2003 on the Big Island and (c) 2005 on the Big Island, and incubated continuously at 15/6, 20/10 or 25/15°C, or subjected to a low to high (15/6 → 20/10 → 25/15 → 20/10 → 15/6°C) or to a high to low (25/15 → 20/10 → 15/6 → 20/10 → 25/15°C) sequence of temperature regimes. Means for final germination percentages followed by different letters differ significantly (P< 0.05) according to the Duncan New Multiple Range Test. Arrows along the x-axis indicate the times when seeds were moved.
The 2003 seeds incubated continuously at 15/6 and 20/10°C reached ≥ 50% germination after 30 and 42 weeks, respectively, and after 80 weeks seeds at 15/6, 20/10 and 25/15°C had germinated to 99, 96 and 30%, respectively (Fig. 1b). Moving seeds from high → low or from low → high temperature regimes resulted in final germination of 68% and 99%, respectively. Moving seeds from high → low significantly increased germination percentages compared to that of seeds incubated continuously at 25/15°C (F= 22.26, P< 0.05) with much of the germination occurring in seeds incubated at 25/15°C the second time (40–52 weeks).
The 2005 seeds incubated continuously at 15/6 and 20/10°C reached ≥ 50% germination after 28 and 36 weeks, respectively, and after 80 weeks seeds at 15/6, 20/10 and 25/15°C had germinated to 99, 99 and 45%, respectively (Fig. 1c). Moving seeds from high → low and from low → high temperature regimes resulted in final germination of 96% and 99%, respectively. Moving seeds from high → low significantly increased final germination percentage compared to that of seeds incubated continuously at 25/15°C (F= 20.80, P< 0.05).
Cold and warm stratification
When incubated at 20/10°C for 78 weeks, final germination of seeds warm-stratified at 25/15°C for 0, 2, 4, 6 and 8 weeks was 96, 99, 98, 93 and 97%, respectively, and after 2, 4, 6 and 8 weeks of cold stratification at 5°C it was 98, 98, 100 and 96%, respectively (data not shown). However, the speed at which seeds germinated at 20/10°C varied (Table 1). When starting from the beginning of imbibition, warm stratification for 8 weeks decreased time to 10% and to 50% germination at 20/10°C by 6 and 7 weeks, respectively, and time from start of incubation at 20/10°C was decreased by 6 and 15 weeks, respectively. However, when starting from the beginning of imbibition, cold stratification for 8 weeks had no effect on time to 10% germination (i.e. 29 vs. 29 weeks) but increased time to 50% germination by 4 weeks. When starting from the time of beginning of incubation at 20/10°C, cold stratification for 8 weeks increased time to 10% germination by 6 weeks but decreased time to 50% germination by 4 weeks.
Table 1 Time (weeks) required for 10% (shown in parentheses) and 50% germination of 2003-collected Cheirodendron trigynum seeds given 0–8 weeks of warm (25/15°C) or cold (5°C) stratification and then incubated at 20/10°C

Embryo growth
Mean E:S ratio in fresh 2002 seeds was 0.12 ± 0.01, and critical E:S ratio for germination was 0.76 ± 0.04. After 12 weeks at both 15/6 and 25/15°C, some embryo growth had occurred, and the embryo in 24 and 16% of the seeds, respectively, had reached the critical length for germination (Fig. 2); seeds with the critical embryo length had germinated. Additional embryo growth occurred when seeds were moved from 15/6 and 25/15 to 20/10°C. After 8 weeks at 20/10°C, 84 and 92% of the embryos in seeds previously incubated at 15/6 and 25/15°C, respectively, had reached the critical length for germination; 68 and 48% of the seeds, respectively, had germinated.

Figure 2 Cumulative embryo length:seed length (E:S) ratios (mean ± SE, if ≥ 0.05) of Cheirodendron trigynum seeds collected on Oahu in 2002 and incubated at 15/6 or at 25/15°C for 12 weeks, after which they were moved to 20/10°C for 8 weeks. Numbers in parentheses indicate percentage of 26 seeds in which the embryo had reached the critical E:S ratio (0.76) for germination.
Mean E:S ratio in fresh 2003 seeds was 0.11 ± 0.01, and embryos did not grow. The E:S ratio was 0.12 ± 0.01 after seeds had been incubated at 20/10°C for 24 weeks, and it was 0.12 ± 0.01 and 0.11 ± 0.10 after 12 weeks at 15/6 and 25/15°C, respectively.
Mean E:S ratio in fresh 2005 seeds was 0.11 ± 0.01, and critical E:S ratio for germination was 0.61 ± 0.02. After 12 weeks, little or no embryo growth had occurred at 15/6, 20/10 or 25/15°C, and after 20 weeks the E:S ratio was 0.29 ± 0.04, 0.16 ± 0.01 and 0.13 ± 0.01, respectively (Fig. 3). Embryos in seeds incubated continuously at 25/15°C were alive at 30 weeks, but they were dead at 36 weeks. Embryos grew sooner when incubated continuously at 15/6°C than at any other temperature regime. When seeds were moved from high → low temperature regimes, rapid embryo growth occurred at 15/6 and 20/10°C (second time). In seeds moved from low → high temperature regimes, the most rapid embryo growth was at 25/15°C.
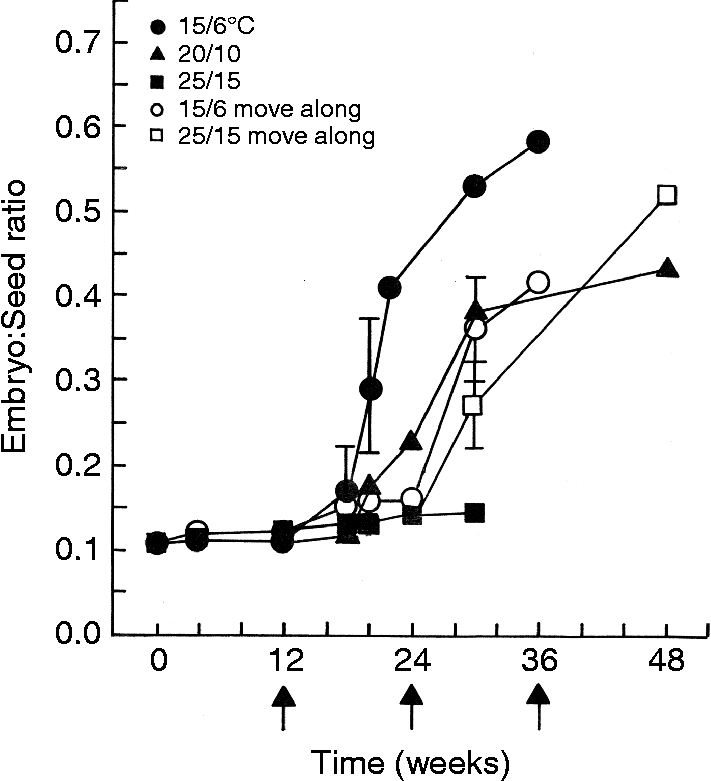
Figure 3 Cumulative embryo length:seed length (E:S) ratios (mean ± SE, if ≥ 0.05) of seeds of Cheirodendron trigynum collected on the Big Island in 2005 and incubated continuously at 15/6, 20/10 or 25/15°C, and subjected to a low to high (15/6 → 20/10 → 25/15 → 20/10 → 15/6°C) or to a high to low (25/15 → 20/10 → 15/6 → 20/10 → 25/15°C) sequence of temperature regimes. Arrows along the x-axis indicate the times when seeds were moved.
Discussion
Seeds of C. trigyrum collected in 2002 and 2005 exhibited a 525% and 454% increase in E:S ratio, respectively, before they germinated. Thus, the small embryo has MD and grows inside the seed before germination occurs. However, regardless of collection date and incubation temperatures, germination percentages were < 10% until ≥ 12 weeks of incubation. That is, regardless of incubation temperatures, embryo growth was not initiated until ≥ 12 weeks, after which embryos grew rapidly, especially at 15/6 and 20/10°C (Fig. 3). Based on the long delay before the beginning of embryo growth, we conclude that embryos in fresh seeds are physiologically dormant, i.e. the seeds have MPD.
There was a close correspondence between embryo growth and germination, and C. trigynum seeds germinated as soon as the embryo grew, as in the seeds of Chaerophyllum tainturieri (Baskin and Baskin, Reference Baskin and Baskin1990) and Thalictrum mirabile (Walck et al., Reference Walck, Baskin and Baskin1999). Thus, the temperature at which seeds germinate is in close agreement with the temperature requirement for embryo growth. For example, in the 2005 seeds incubated at 15/6°C, the mean E:S ratio after 12 weeks was 0.11 and no seeds had germinated; after 20 weeks the mean E:S ratio was 0.29 and 10% of the seeds had germinated; and after 32 weeks the mean E:S ratio was 0.41, and 74% of the seeds had germinated (Figs 1c, 3).
The nine levels of MPD are subdivided into two subclasses: simple and complex. In the simple levels of MPD, embryos grow at temperatures suitable for warm stratification ( ≥ 15°C), and some or all of the PD is broken before the embryo grows. In the complex levels of MPD, embryos grow at temperatures suitable for cold stratification (c. 0–10°C), and PD and MD can be broken simultaneously (Nikolaeva, Reference Nikolaeva1969; Baskin and Baskin, Reference Baskin and Baskin2014). The 2002 seeds incubated at 25/15°C germinated to 100% (Fig. 1a), which means embryo growth occurred at temperatures suitable for warm stratification and that seeds have a simple level of MPD. However, the 2003 and 2005 seeds did not germinate to high percentages when incubated continuously at 25/15°C (Fig. 1b, c), but they did so when moved from 25/15 to 20/10°C. The increase in germination at 20/10°C suggests that 25/15°C may have been above the optimum temperature for germination.
The temperature required for dormancy break and germination varied with the seed collection. Seeds collected on Oahu in 2002 and incubated continuously at 15/6, 20/10 or 25/15°C, moved from high to low or from low to high temperatures, germinated to 94–100%. For seeds collected on the Big Island in 2003, 15/6 and 20/10°C were clearly the optimum temperatures for germination, with only 30% of the seeds germinating at 25/15°C. In seeds moved from high → low temperature regimes, about 40% (of 68% total) germination occurred while seeds were at 25/15°C the second time. For seeds collected on the Big Island in 2005, 45% of the seeds germinated at 25/15°C, but they germinated to 96–99% at the other incubation conditions. Embryo growth in the move-along experiment for the 2005 seeds shows that the previous temperature to which seeds are exposed makes a difference to the optimum temperature for embryo growth. In the high → low temperatures regimes, the last two regimes (15/6 and 20/10°C) were best for embryo growth. In the low → high temperature regimes, the middle two regimes (20/10 and 25/15°C) were the best for embryo growth (Fig. 3).
For the 2003 and 2005 seeds, germination was best at 15/6 and 20/10°C. Since seeds incubated at 15/6 and 20/10°C received some temperatures suitable for cold stratification each day, i.e. 6 and 10°C are cold-stratifying temperatures (see Stokes, Reference Stokes and Ruhland1965; Nikolaeva, Reference Nikolaeva1969), we need to ask: is there a benefit of the low temperature phase of the cycles (cold stratification) on dormancy break and germination? For the 2003 seeds cold-stratified at 5°C prior to incubation at 20/10°C, there was a detrimental effect of cold stratification in terms of time required for seeds to germinate (Table 1). On the other hand, seeds warm-stratified (25/15°C) prior to being incubated at 20/10°C exhibited a decrease in time to germination. Thus, the 2003 and 2005 seeds may have germinated to higher percentages at 15/6 and 20/10°C than at continuous 25/15°C, because 25°C is above the optimum for germination. Further, we suggest that 15/6 and 20/10°C promote germination because the 15 and 20°C high daily temperatures of the two regimes are suitable for warm stratification.
What level of simple MPD do the seeds have? The various levels of simple MPD are non-deep, intermediate, deep, deep epicotyl, non-deep epicotyl and deep double (Baskin and Baskin, Reference Baskin and Baskin2014). Since shoot emergence is not delayed after the radicle emerges from seeds of C. trigynum, epicotyl and double simple MPD can be ruled out. In deep simple and in intermediate simple MPD, part of the PD is broken, the embryo grows and then the remainder of the PD must be broken before the seeds can germinate (Nikolaeva, Reference Nikolaeva2001; Phartyal et al., Reference Phartyal, Kondo, Baskin and Baskin2009). Seeds of C. trigynum did not need any additional treatments such as warm (or cold) stratification after embryo growth in order for seeds to germinate. Thus, we conclude that seeds from both Oahu and the Big Island have non-deep simple MPD.
The general formula for non-deep simple MPD is C1Bb, where C1 is non-deep PD and Bb an underdeveloped embryo (B) that grows at warm temperatures (subscript b) (Baskin and Baskin, Reference Baskin and Baskin2014). Baskin and Baskin (Reference Baskin and Baskin2014) recognize two types of the non-deep simple level of MPD: Type 1, C1bBb; and Type 2, C1aBb. In Type 1, non-deep PD is broken by warm temperatures (subscript b of C1b) and in Type 2 by cold temperatures (subscript a of C1a). In both types, the underdeveloped embryo grows at warm temperatures. More specifically, then, the dormancy formula for C. trigynum is C1bBb.
Depending on the island where seeds of C. trigynum were collected, the germination responses of seeds at 25/15°C ranged from 100% (Oahu in 2002) to 31% (Big Island in 2003). However, given enough time, the response of seeds from the two islands was the same at 15/6 and 20/10°C, i.e. seeds eventually germinated to 96–100%. The reason for the differences in how seeds from the two islands responded to 25/15°C is not known and could be due to genetics, environmental parental effects or a combination of the two. Seeds collected from the same location on the Big Island in 2003 and in 2005 differed in how quickly they reached 50% germination at 20/10°C (42 vs. 28 weeks), but not at 15/6°C, suggesting that the environment during seed development could play a role in determining speed of germination at increased temperatures (e.g. 20/10°C) (for a review, see Baskin and Baskin, Reference Baskin and Baskin2014).
The ability of the 2002 seeds from Oahu to germinate at 15/6, 20/10 and 25/15°C indicates that they could germinate over a range of habitat temperatures. Further, temperature shifts did not improve germination of 2002 seeds. On the other hand, temperature shifts improved germination for the 2003 and 2005 seeds from the Big Island compared to continuous incubation at 25/15°C. Starting incubation at a cool regime promoted germination at a later warm regime, while starting at a warm regime promoted germination at a later cool regime. Thus, a shift in temperature promoted germination regardless of the direction of the shift.
Seed dormancy has been studied in various species of Araliaceae, and different levels of MPD have been found, including intermediate simple, e.g. Aralia mandshurica (Nikolaeva, Reference Nikolaeva and Khan1977); deep simple, e.g. Kalopanax pictus and Panax japonica (Nikolaeva et al., Reference Nikolaeva, Rasumova, Gladkova and Danilova1985); intermediate complex, e.g. Aralia cordia (Nikolaeva et al., Reference Nikolaeva, Rasumova, Gladkova and Danilova1985); and deep complex, e.g. Aralia spinosa (Nikolaeva et al., Reference Nikolaeva, Rasumova, Gladkova and Danilova1985). Thus, non-deep simple MPD in seeds of C. trigynum is the first report of this level of MPD in the Araliaceae.
Acknpwledgements
We thank Aileen Yeh for collecting seeds on the Big Island in 2003 and 2005.
Financial support
The Hawaii Conservation Alliance and HATCH Project Accession No. 0210780 are thanked for financial support.
Conflicts of interest
None.







