Introduction
Cancer-related drowsiness (CRD), somnolence, or excessive daytime sleepiness is common (Davidson et al., Reference Davidson, MacLean and Brundage2002) and associated with significant distress in patients with advanced cancer (Degner & Sloan, Reference Degner and Sloan1995; Parker et al., Reference Parker, Bliwise and Ribeiro2008). Drowsiness was previously defined in noncancer settings as “excessive daytime sleepiness and an inability to remain awake which not relieved with sleep” (Atkinson & Davenne, Reference Atkinson and Davenne2007). Drowsiness may be either the result of abnormalities in “sleep drive” resulting from sleep deprivation/loss (Vgontzas, et al., Reference Vgontzas, Pejovic and Zoumakis2007) or sleep propensity (quickness of falling asleep) (Johns, Reference Johns2010). Drowsiness is associated with significantly decreased physical activity (McClain et al., Reference McClain, Lewin and Laposky2014), resulting in interference in daily activities. Prior studies suggest that drowsiness was associated with increased cardiometabolic risks (Atkinson & Davenne, Reference Atkinson and Davenne2007; Buxton & Marcelli, Reference Buxton and Marcelli2010; Patel & Hu, Reference Patel and Hu2008) and impairment in work-related abilities (Cleeland et al., Reference Cleeland, Mayer and Dreyer2014).
CRD is more common in older patients undergoing treatment (Cleeland et al., Reference Cleeland, Mayer and Dreyer2014; Sanford et al., Reference Sanford, Zhao and Salsman2014; Wochna Loerzel, Reference Wochna Loerzel2015) and its severity increases as the disease progresses (Hui et al., Reference Hui, Santos and Chisholm2015; Mercadante et al., Reference Mercadante, Casuccio and Fulfaro2000). This type of sleep disorder and its health consequences are often neglected in the advanced cancer patient (ACP) population. CRD is not routinely evaluated in routine cancer care. Routine evaluation of the severity of CRD with a validated tool may be important because it can provide insight into the possible etiologic factors associated with CRD and assist in monitoring the effects of CRD treatments. There are limited published data specifically regarding the frequency and characteristics of CRD in this patient population.
The primary aim of this study was to identify the frequency and factors associated with severity of CRD. The secondary aim was to determine the screening performance of the Edmonton Symptom Assessment Scale-drowsiness (ESAS-D) item using Epworth Sedation Scale (ESS) as a gold standard.
Methods
The University of Texas MD Anderson Cancer Center institutional review board approved this study.
Our current study is a secondary analysis of a previously published prospective survey (Yennurajalingam et al., Reference Yennurajalingam, Balachandran and Pedraza Cardozo2015) conducted from October 2012–June 2013. A total of 180 ACP admitted to MD Anderson Cancer Center inpatient service for at least 24 hours were screened for eligibility and possible enrollment. Eligibility criteria included a diagnosis of advanced cancer, normal cognition, and ability to read, write, and speak English.
After providing signed informed consent, the study participants underwent a single interview with the research coordinator, during which the study was explained. They were then asked to complete the demographic data and study questionnaires independently or with the assistance of the research staff for a single time of 25–30 minutes.
Assessment tools
ESAS is a valid and reliable tool for the assessment of the intensity of symptoms in cancer populations. The symptoms include pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, feelings of well-being, and “other symptom.” In this study, we used the sleep item as the “other symptom.” The severity of each symptom was rated from 0 to 10 on a numerical scale, with 0 meaning that symptom is absent and 10 meaning that it is of the worst possible severity (Bruera et al., Reference Bruera, Miller and Selmser1991).
ESS measures the general level of daytime sleepiness. It has eight questions and patients rate on a 4-point scale (0–3), their usual chances of falling asleep in eight situations or activities (Johns Reference Johns1991). ESS has been used in cancer and noncancer patients for the detection of sleep disorders such as narcolepsy, idiopathic hypersomnia, and excessive daytime sleepiness (Johns Reference Johns2000).
Pittsburgh Sleep Quality Index (PSQI) was used to measure the quality and patterns of sleep disturbance. Each item on a scale is graded 0–3. The sum of the seven component scores are used for the global sleep score (range 0–21). A global sleep score ≥ 5 was used to define SD (Buysse et al., Reference Buysse, Reynolds and Monk1989). PSQI has internal consistency (Cronbach α) of 0.83 overall.
Insomnia Severity Index (ISI). The ISI is a seven-item questionnaire designed to evaluate insomnia severity (Bastien et al., Reference Bastien, Vallières and Morin2001). Each item was rated using a 5-point scale ranging from 0 (not at all) to 4 (very much), for a total score ranging from 0 to 28.
STOP-Bang Scoring Model: The STOP-Bang test was used as a screening tool for obstructive sleep apnea (OSA; Chung et al., 2008) The questionnaire is short, easy to apply, and has sensitivity for moderate to severe OSA between 92.9% and 100% when compared with the gold standard of polysomnography (Chung et al., Reference Chung, Yang and Liao2013).
Restless leg syndrome questionnaire (RLS): This single item-screening questionnaire evaluates restless legs (Ferri et al., Reference Ferri, Lanuzza and Cosentino2007). The questions incorporate the clinical component used to make a diagnosis of RLS including characterization of symptoms, timing, and alleviating measures.
Hospital Anxiety and Depression Scale (HADS): The hospital anxiety and depression scale was developed in 1983 for screening of depression in medical patients. It has been studied and validated in cancer patients showing to be a useful screening instrument (Zigmond & Snaith, Reference Zigmond and Snaith1983).
Statistical considerations
We estimated the proportion of patients with CRD, SD, OSA, and RLS. We calculated correlation among CRD, ISI, PSQI, HADS, ESS, and other ESAS symptoms. To assess independent predictors, we initially conducted a univariate logistic regression analysis. Variables that were significant at the 20% level in univariate regressions were included in a multivariate model.
To determine the optimal cutoff score for clinically significant drowsiness as measured by ESAS-D. We used the ESS-D as a gold standard for subjective assessment of drowsiness. The clinically significant cutoff for the ESS is 10. Patients were randomly assigned to either a training dataset or a validation dataset. We used ESS from the training dataset to calculate sensitivity and specificity (along with 95% confidence intervals) for all possible cutoff values of the ESAS-D item, calculate area under the receiver operating characteristic curve, and chose the best clinical cutoff where the best clinical cutoff was defined as the cutoff which maximizes the sum of sensitivity and specificity subject to the constraint that sensitivity is at least 70%.
Results
A total of 1057 consecutive ACP were screened for participation of this study; 483 were not eligible. The reasons were as follows: (1) patients < 18 years (n = 4); (2) no advanced cancer (n = 8); (3) delirium (n = 270); (4) non-English speaking (n = 114); and sleep disturbance, 0/10 on ESAS (n = 72). Of the 589 patients who were eligible and approached, 180 were enrolled and 409 declined to participate. Reasons for refusal were: (1) symptom distress (n = 88) (2) not interested (n = 277) (3) clinician refused to allow the patient to be approached (n = 24), and (4) missing data (n = 2).
Of the180 patients assessed, clinically significant drowsiness was found in 50% ACP, median scores (IQR) were as follows: ESS 11 (7–14); ESAS- drowsiness item 5 (2–6); PSQI 8 (5–11); ISI 13 (5–19); Stop Bang Scoring 3 (2–4); and HADS-D 6 (3–10). Sleep apnea was found in 61% and RLS in 38% (Table 1).
Table 1. Patient demographics
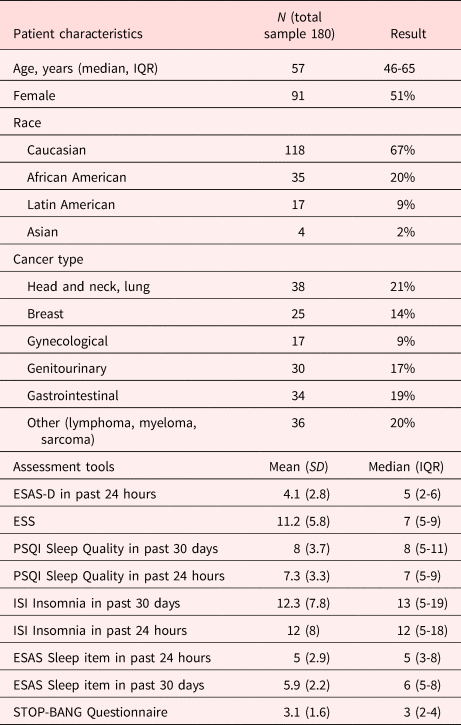
ESAS-D, Edmonton Symptom Assessment System-drowsiness; ISI, Insomnia Severity Index; PSQI, Pittsburg Sleep Quality Index; STOP-Bang Questionnaire, screening tool for obstructive sleep apnea.
ESAS-D was associated with other ESAS items (r, p) sleep (0.38, <0.0001); pain (0.3, <0.0001); fatigue (0.51, <0.0001); depression (0.39, <0.0001); anxiety (0.44, <0.0001); shortness of breath (0.32, <0.0001); anorexia (0.36, <0.0001), feeling of well-being (0.41, <0.0001), ESS (0.24, 0.001), and opioid daily dose (0.19, 0.01).
Multivariate analysis showed that insomnia ISI (OR = 2.35; 0.036), ESAS Fatigue (OR = 9.08; <0.0001), ESAS Anxiety (OR = 3.0; 0.009), and ESAS feeling of well-being (OR = 2.27; p = 0.04) were associated with CRD.
An ESAS-D cutoff score ≥3 (of 10) resulted in a sensitivity and specificity of 78% and 33% and of 75% and 40% in the training and validation samples, respectively (Table 2 and Figure 1).

Fig. 1. Receiver operating characteristic (ROC) curve to determine the cutoff values for the Edmonton Symptom Assessment Scale-drowsiness item using Epworth Sedation Scale as a gold standard.
Table 2. Distribution of possible cutoff values of ESAS-D item for clinically significant drowsiness

ESAS, Edmonton Symptom Assessment System.
Discussion
In our study, we found 50% of ACPs had clinically significant drowsiness. The results of our study suggest that CRD was significantly associated with severity of fatigue, anxiety, insomnia, and worse feeling of wellbeing scores. Our preliminary analysis also suggest that a cutoff score of ≥3/10 on ESAS-D item was sensitive (81%) to screen patients with clinically significant drowsiness.
Prior studies suggest the frequency of drowsiness ranges from 25% to 36% (Schwartz et al., Reference Schwartz, Roth and Hirshkowitz2009) in primary care population. CRD has seldom been studied in cancer patients. In one cross-sectional survey study of 982 mixed-type cancer patients by Davidson et al. (Reference Davidson, MacLean and Brundage2002), CRD was reported in 28% of cancer patients. In our study, we found 50% of ACPs had CRD as assessed by the ESS. These findings suggests that its frequency is higher in ACPs than in general population. In our study, we used a more validated questionnaire (ESS) compared with a simple survey used in previous studies. Clinically significant drowsiness is frequently underdiagnosed because of a lack of routine screening. The ESS is a frequently used questionnaire to screen drowsiness. This tool has been validated in previous studies (Johns, Reference Johns1991); however, it is not always convenient to use ESS in routine clinical practice, especially when assessing other cancer-related symptoms at the same time. Commonly used assessment tools in routine cancer care such as ESAS (Bruera et al., Reference Bruera, Miller and Selmser1991; Chang et al., Reference Chang, Hwang and Feuerman2000; Paiva et al., Reference Paiva, Manfredini and Paiva2015) do assess drowsiness simultaneously with other cancer-related symptoms; however, there are limited data to suggest a cutoff for the diagnosis of clinically significant CRD. In this study, we found the screening performance of ESAS drowsiness item to be ≥3/10; however, receiver operating characteristic analysis suggests low specificity. In view of lack of any literature in regard to screening performance of ESAS-D, the information obtained from our study would be very useful to the practicing clinicians as a benchmark to identify and monitor clinically significant CRD in routine cancer care.
In our study, we found a significant association between CRD and fatigue. Prior studies also show a strong positive correlation between fatigue and various sleep parameters, including CRD (Roscoe et al., Reference Roscoe, Kaufman and Matteson-Rusby2007). Using a multidimensional model, Hwang et al. (Reference Hwang, Chang and Rue2003) showed a significant correlation between drowsiness and fatigue. CRD usually clusters with other symptoms such as fatigue, anxiety, nausea, decreased appetite, dyspnea, and poor sense of well-being (Cheung et al., Reference Cheung, Le and Zimmermann2009; Fan et al., Reference Fan, Hadi and Chow2007); therefore, targeted interventions of associated symptoms, such as fatigue, may positively affect the CRD (Roscoe et al., Reference Roscoe, Kaufman and Matteson-Rusby2007). Similarly, management of CRD could potentially improve fatigue, anxiety, nausea, decreased appetite, dyspnea, poor sense of well-being, and overall quality of life (Baldwin et al., Reference Baldwin, Griffith and Nieto2001).
The management of CRD is complex because of its multifactorial nature in ACP patients. CRD may be attributed to the cancer itself, effects of cancer-related symptoms such as pain, cancer-related treatments such as chemotherapy, opioid analgesics (Ripamonti & Bruera, Reference Ripamonti and Bruera1997), or antiemetic agents (Glare et al., Reference Glare, Miller and Nikolova2011; Rao & Faso, Reference Rao and Faso2012). The most effective initial step in management is therefore to identify the causative mechanism. If the “primary” cause cannot be treated, a trial of pharmacological and nonpharmacological approaches should be explored. Management of drowsiness therefore may include treatment of CRD using psychostimulants drugs such as methylphenidate (Bruera et al., Reference Bruera, Chadwick and Brenneis1987; Wilwerding et al., Reference Wilwerding, Loprinzi and Mailliard1995) modafinil, or armodafinil (Ballon & Feifel, Reference Ballon and Feifel2006; Valentino & Foldvary-Schaefer, Reference Valentino and Foldvary-Schaefer2007). Nonpharmacological management includes eliminating polypharmacy and using behavioral therapy such as counseling focused on sleep hygiene. This management strategy may not only alleviate CRD but also improve fatigue and sleep disturbance. Further randomized controlled studies are needed to determine the best strategy to manage CRD in ACPs.
Inflammation has been associated with common symptoms and clinical correlates of cancer including CRD. Prior studies suggested significant association between inflammatory cytokines and CRD (Vgontzas et al., Reference Vgontzas, Papanicolaou and Bixler1997). Exogenous administration of interleukin-6 (IL-6) in patients with cancer (Mastorakos et al., Reference Mastorakos, Chrousos and Weber1993) and increased production of endogenous IL-6 (Papanicolaou et al., Reference Papanicolaou, Tsigos and Oldfield1996) were associated with increased CRD and fatigue, suggesting that IL-6 may be associated with CRD. High IL-6 levels also correlate with high cancer symptom burden in stem cell transplant patients. Further studies are needed to understand the mediating role of inflammatory cytokines, specifically IL-6, in the causation of drowsiness and its associated symptom clusters.
Future studies should focus on the operational definition of CRD; moreover, important future studies are required to determine the causation, subtypes, and objective measures that can be used in routine clinical care to develop personalized management strategies.
Conclusion
CRD is frequent and often neglected in ACP. Our study confirms the association of CRD with severity of fatigue, anxiety, insomnia, and worse feeling of well-being. An ESAS-D score ≥3 has moderate sensitivity and low specificity. Because of a lack of adequate literature regarding ESAS-D screening performance, this information is likely to screen most of the ACP with significant CRD. There is a need for future studies to confirm the finding of this study .This may help in creating personalized management strategies to reduce CRD in ACP by addressing underlying causative mechanisms.
Acknowledgments
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award number R21NR016737.S.Y. has full control of all primary data and agrees to allow the journal to review the data if requested.
Conflicts of interest
Genentech and Helsinn provided research funding to S.Y.’s institution.
Supplementary Material
To view supplementary materials for this article, please visit https://doi.org/10.1017/S1478951518000779.





