In inherited connective tissue disorders that involve the cardiovascular system, for example, Marfan and Ehlers–Danlos syndromes, osteogenesis imperfecta, and pseudoxanthoma elasticum, impairment of the left ventricular systolic and diastolic function has been reported, and this does not appear to depend on secondary valvular regurgitation.Reference Kiotsekoglou, Saha and Moggridge 1 – Reference Nguyen, Terbah, Daudon and Martin 4 Such conditions are described as primary cardiomyopathy.Reference Alpendurada, Wong and Kiotsekoglou 5 The existence of cardiomyopathy in connective tissue diseases is still debatable; however, it is well known that the organisation and function of the myocardium is highly dependent on the cardiac extracellular matrix, which is comprised of fibrillar proteins and signalling molecules, such as transforming growth factor-β, and enzymes.Reference Pope, Sands, Smaill and LeGrice 6
Mitral valve prolapse is also a hereditary connective tissue disease with a sporadic or familial autosomal dominant inheritance – three loci have been identified, but, to date, no specific genes have been described – and X-linked inheritance – Filamin A was the first gene known to cause isolated non-syndromic mitral valve prolapse.Reference Murphy-Ryan, Psychogios and Lindor 7 – Reference Kyndt, Gueffet and Probst 9 Mitral valve prolapse refers to a systolic billowing of one or both mitral leaflets into the left atrium with or without mitral regurgitation. On using the current echocardiographic criteria for diagnosing mitral valve prolapse, the prevalence of this entity according to The Framingham Heart Study has been reported to be 2.4% of the population. On the basis of prior clinical and prognostic studies, patients with mitral valve prolapse classified as having classic, that is, a leaflet thickness ≥5 mm, or non-classic, that is, a leaflet thickness < 5 mm, prolapse.Reference Bonow, Carabello and Chatterjee 10 , Reference Freed, Benjamin and Levy 11
In patients with mitral valve prolapse, myxoid infiltration of the valve results in a remarkable excess thickened leaflet tissue with a destroyed three-layer leaflet architecture.Reference Anyanwu and Adams 12 However, little is known about the precise pathogenetic sequence that culminates in myxomatous mitral valve prolapse. Transforming growth factor-β plays an integral role in the development of endovascular cushions: the embryonic precursors to cardiac valves, such as filamin A, which serves as a positive regulator of transforming growth factor-β signalling, lead to an increased expression of numerous transforming growth factor-β-related genes that regulate cell proliferation and contribute to myxomatous valve disease.Reference Sasaki, Masuda and Ohta 13 , Reference Ng, Cheng and Myers 14 Moreover, overexpression of transforming growth factor-β in both classic and alternative pathways results in increased extracellular matrix protein synthesis – excess of extracellular matrix proteins in the myocardium defines myocardial fibrosis.Reference Khan and Sheppard 15 However, the impact of transforming growth factor-β on the left ventricular function in patients with mitral valve prolapse has not been studied previously.
Left ventricular contraction abnormalities in symptomatic mitral valve prolapse patients without severe mitral regurgitation but with ventricular arrhythmias have been described in some studies using cardiac tomography, radionuclide angiography, and single-photon emission computed tomography.Reference Delhomme, Casset-Senon and Babuty 16 – Reference Casset-Senon, Babuty and Philippe 18 Moreover, a marked reduction in the myocardial deformation indices has been demonstrated in patients with degenerative severe mitral regurgitation.Reference Marciniak, Sutherland and Marciniak 19 , Reference Lancellotti, Cosyns and Zacharakis 20 Two-dimensional speckle-tracking echocardiography is a relatively new technique used for evaluation of the myocardial function. Strain and strain rate analysis increases the sensitivity in detecting subclinical cardiac involvement in some conditions including cardiomyopathy and valvular cardiac diseases.Reference Mor-Avi, Lang and Badano 21 However, there are at present no data on the left ventricular systolic function in asymptomatic young patients with mitral valve prolapse.
Therefore, the goal of this study was to evaluate the left ventricular function in young adults with mitral valve prolapse without significant mitral regurgitation using two-dimensional speckle-tracking echocardiography and to determine the possible role of the transforming growth factor-β pathway in its deterioration.
Methods
A total of 78 consecutive asymptomatic young patients – 28% female and 72% male – with mitral valve prolapse were enrolled in our observational, prospective, single-centre study. Any other inherited connective tissue disorders that may cause secondary mitral valve prolapse, such as Marfan and Ehlers–Danlos syndromes, etc., were excluded. The mean age of the patients was 19.7 ± 1.6 years. Mitral valve prolapse patients did not receive any medical therapy, including β-blockers.
The control group consisted of 80 gender-matched and age-matched healthy individuals. All gave informed consent and the protocol was approved by the local ethics committee.
Echocardiography
Standard echocardiography extended with speckle-tracking echocardiography – strain rate and strain imaging – was performed in all individuals. All echocardiographic measurements were performed by an experienced echocardiographer using a Vivid 7 ultrasound system (by GE Healthcare, Milwaukee, United States of America) equipped with a harmonic 3.5 MHz phased array transducer.
Mitral valve prolapse was diagnosed by billowing one or both mitral leaflets >2 mm above the mitral annulus in the long-axis parasternal view. Prolapse was defined as classic if the maximal leaflet thickness was ≥5 mm, otherwise it was defined as non-classic.Reference Bonow, Carabello and Chatterjee 10 , Reference Freed, Benjamin and Levy 11 Mitral regurgitation was assessed according the European Association of Echocardiography recommendations. Vena contracta imaging of the mitral regurgitation jet and proximal isovelocity surface area imaging were performed.Reference Lancellotti, Moura and Pierard 22
The end-diastolic and end-systolic left ventricular diameters were measured using B-mode echocardiography. The left ventricular end-diastolic and end-systolic volumes and ejection fractions were calculated using a modified Simpson rule. The transmitral flow velocity was recorded from the apical four-chamber view. The mitral annular motion velocity was recorded at the septum and lateral wall using pulsed tissue Doppler imaging.
Two-dimensional speckle-tracking echocardiography
Longitudinal strain and strain rates were determined from three standard apical views using two-dimensional speckle-tracking echocardiography with a gray-scale frame rate of 50–85 fps.
At each plane, one cardiac cycle was acquired while the individuals held their breath and was stored. Image analysis was performed offline on an EchoPAC'08 workstation (GE Healthcare). The left ventricle was divided into 18 segments. Strain rate was determined as the maximal negative value during the ejection phase. Peak systolic strain was defined as the magnitude of strain at the aortic valve closure. The peak longitudinal early diastolic filling strain rate was also measured.
Laboratory methods
Peripheral venous blood was obtained from each patient with mitral valve prolapse and the sera were isolated and stored at −70°C. Biologically active transforming growth factor-β1 and β2 protein concentrations in serum were determined by enzyme-linked immunosorbent assays using the Human Platinum ELISA test system (Bender MedSystems Diagnostics GmbH, Vienna, Austria). The inter-assay and intra-assay variations were 8% and 6%, respectively. The sensitivity, that is, the minimum level of detection, was 5 pg/ml.
Statistical analysis
The variables are presented as mean ± standard deviation. The categorical variables are presented as percentages. All echocardiographic data and myocardial deformation indices were normally distributed. Differences between the groups were analysed using two-sided Student's t-test for continuous variables and the χ 2-test for categorical variables. The effect sizes for deformation indices were measured by Cohen's d using means and standard deviations. The relationship between pairs of continuous variables was expressed using the Pearson correlation. The myocardial deformation indices were tested as dependent variables using univariate linear regression analyses to explore the significance of possible influencing factors. The reproducibility was expressed by the coefficient of repeatability, interclass correlation coefficient, and the mean error (%). Statistical significance was set at p < 0.05. All statistical analyses were performed using the Statistica 8 software (StatSoft Inc., Tulsa, United States of America).
Results
The mitral valve prolapse and control groups did not differ in terms of most demographic and clinical characteristics, such as age (19.7 ± 1.6 versus 19.9 ± 1.5 years, respectively; p = 0.42), gender proportion (male: 72% versus 60%, χ 2=2.44, respectively; p = 0.11), weight (61.6 ± 7.9 versus 60.5 ± 9.5 kg, respectively; p = 0.43), heart rate (76.8 ± 14.3 versus 74.2 ± 15.7 b.p.m., respectively; p = 0.28), and blood pressure (systolic: 115.6 ± 8.5 versus 117.8 ± 9.4 mmHg, respectively; p = 0.13; diastolic: 69.8 ± 7.4 versus 71.3 ± 8.9 mmHg, respectively; p = 0.25). However, patients with mitral valve prolapse were taller (1.86 ± 0.11 versus 1.79 ± 0.09 m, respectively; p < 0.0001) and had a larger body surface area (1.88 ± 0.08 versus 1.78 ± 0.16 m2, respectively; p < 0.0001), which is common for young adults with this disease.
In the mitral valve prolapse group, 29 patients were identified as having classic prolapse – leaflet thickness ≥5 mm – and 49 patients were diagnosed as having non-classic prolapse – leaflet thickness <5 mm. These two groups also did not differ in terms of anthropometric data and age and gender proportion.
Standard echocardiographic parameters of the studied patients are presented in Table 1. There were no significant differences in left ventricular dimensions and volumes or in the global systolic function between the non-classic prolapse and control groups. However, in patients with classic prolapse, the left ventricular systolic and diastolic dimensions, in agreement with previous studies, were significantly higher than those in the non-classic prolapse and control groups.Reference Yiginer, Keser and Ozmen 23 Other chamber dimensions and volumes – right ventricular end-diastolic dimension and left atrial volume index – did not differ between the groups. The global diastolic left ventricular function, evaluated by transmitral and tissue Doppler, also did not vary between both mitral valve prolapse groups and healthy individuals.
Table 1 Echocardiographic data in the mitral valve prolapse and control groups.

e′ – peak early diastolic mitral annular motion velocity; E/e′ – ratio of transmitral and annular early diastolic velocities; Z-score – aortic root diameter, standardised to body surface area
*Significance of differences between the classic and non-classic prolapse groups
†Significance of differences between the classic prolapse and control groups
‡Significance of differences between the non-classic prolapse and control groups
Classic and non-classic prolapse patients, when compared with the control group, had larger aortic root dimensions at the sinus level of the Valsalva, but the Z-score was the same in both groups because of the higher body surface area in prolapse patients. In contrast, the pulmonary artery diameter was similar in all groups.
Patients with mitral valve prolapse, both classic and non-classic, not surprisingly had significantly longer and thicker mitral valve leaflets and a larger mitral annulus diameter than did the healthy individuals. Mitral regurgitation was none-to-mild in all groups and was mostly late systolic in the mitral valve prolapse patients.
Myocardial deformation indices
From 2844 segments, only 2019 (71%), because of tracking difficulties, reverberations, or poor image quality, were accepted for deformation analysis.
In the classic prolapse group, we observed a significant reduction in global longitudinal systolic strain (Table 2, Fig 1) as compared with the control group (−15.5 ± 2.9% versus −19.6 ± 3.4%, respectively; p = 0.00001) and the non-classic prolapse group (−15.5 ± 2.9% versus −18.7 ± 3.8%, respectively; p = 0.0002). Similar differences were observed for all left ventricular walls. The systolic longitudinal global strain rate (Table 2) was also reduced when compared with the control group (−1.03 ± 0.2 versus −1.22 ± 0.18/second, respectively; p = 0.00001) and the non-classic prolapse group (−1.03 ± 0.2 versus −1.17 ± 0.16/second, respectively; p = 0.001). Similarly, the longitudinal early diastolic strain rates were lower in the classic prolapse group as compared with the control group (1.3 ± 0.25 versus 1.62 ± 0.25/second, respectively; p = 0.00001) and the non-classic prolapse group (1.3 ± 0.25 versus 1.56 ± 0.26/second, respectively; p = 0.0001), although we found no differences between the groups in tissue Doppler parameters such as e′ and E/e′.
Table 2 Global and local longitudinal strain (%) and strain rate (1/second) in the mitral valve prolapse and control groups.

*Significance of differences between the classic and non-classic prolapse groups
†Significance of differences between the classic prolapse and control groups
‡Significance of differences between the non-classic prolapse and control groups

Figure 1 An example of low global strain in a patient with classic mitral valve prolapse (two-dimensional speckle-tracking echocardiography).
The effect sizes measured by Cohen's d, when compared among the classic and non-classic mitral valve prolapse groups, were large (0.95) for longitudinal strain (0.95) and medium (0.77) for the longitudinal strain rate. The classic prolapse and control groups had large effect sizes for both longitudinal strain (1.3) and the strain rate (0.99).
There were no significant differences in the global longitudinal peak systolic strain (−18.7 ± 3.8% versus −19.6 ± 3.4%, respectively; p = 0.17) and strain rate (−1.17 ± 0.16 versus −1.22 ± 0.18/second, respectively; p = 0.11) between the non-classic prolapse and control groups. However, we found a significant decrease in terms of septal longitudinal strain and the strain rate in mitral valve prolapse patients, when compared with the control group (Table 2). In other segments, there were no significant differences in the longitudinal deformation indices.
Global myocardial indices in the classic mitral valve prolapse group correlated positively with the aortic root diameter (longitudinal strain: r = 0.46, p = 0.001). The same correlation was found in the septal segment deformation indices (inferoseptal longitudinal strain: r = 0.49, p = 0.0009) in the non-classic prolapse group. None of the deformation indices correlated with the maximal depth of mitral leaflet prolapse.
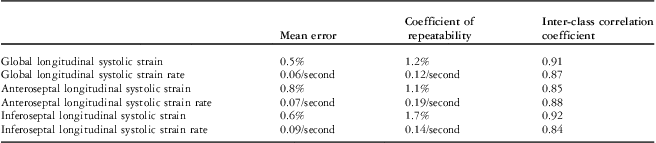
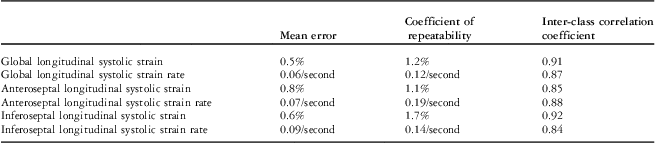
The inter-observer mean error, coefficient of repeatability, and interclass correlation coefficient for global and local septal myocardial deformation indices are shown in Table 3.
Table 3 Inter-observer reproducibility in assessment of the left ventricular global and local myocardial deformation indices.
Transforming growth factor-β serum concentration
Transforming growth factor-β1 and β2 serum levels were elevated in patients with classic prolapse as compared with the control group and the non-classic prolapse group (Table 4). In contrast to transforming growth factor-β1 levels, there were no differences in the levels of transforming growth factor-β2 between the non-classic prolapse and control groups.
Table 4 Transforming growth factor-β1 and β2 serum levels in the mitral valve prolapse and control groups.

*Significance of differences between the classic and non-classic prolapse groups
†Significance of differences between the classic prolapse and control groups
‡Significance of differences between the non-classic prolapse and control groups
In the classic mitral valve prolapse group, a high transforming growth factor-β1 serum level (>14.75 ng/ml) was detected in five patients, transforming growth factor-β2 (>2.0 ng/ml) in 11 patients, and both transforming growth factor-β1/β2 in one patient. In the non-classic prolapse group, a high transforming growth factor-β1 serum level was observed in one patient and a high transforming growth factor-β2 level was detected in 10 patients. Only two individuals from the control group had an elevated level of transforming growth factor-β2. Thus, high transforming growth factor-β1 and/or β2 levels were detected in 17 (59%) patients in the classic prolapse group and in only 11 patients (22%) in the non-classic group (χ 2 = 10.4; p < 0.002).
In addition, we identified the negative correlations between the serum level of transforming growth factor-β2 and systolic longitudinal strain (r = 0.40, p = 0.01) and the strain rate (r = 0.67, p = 0.001) in the classic prolapse group (Fig 2). Moreover, in the univariate linear regression analyses, in the classic prolapse group, longitudinal deformation indices were affected by age (p = 0.01), sex (p = 0.02), and transforming growth factor-β2 serum levels (p = 0.01).

Figure 2 Correlation between the longitudinal systolic strain rate and serum level of transforming growth factor-β2 in the classic mitral valve prolapse group (r = 0.67, p = 0.001).
Discussion
Increased activation of the transforming growth factor-β pathway contributes to the pathogenesis of valve myxomatous in mitral valve prolapse.Reference Ng, Cheng and Myers 14 , Reference Rizzo, Carturan and Gerosa 24 , Reference Hulin, Deroanne and Lambert 25 In the heart, transforming growth factor-β appears to be one of several factors that cause disease by inducing cardiac fibrosis. Transforming growth factor-β as a profibrotic cytokine stimulates the production of extracellular matrix proteins in a number of different organ systems and in overexpression results in tissue fibrosis and organ dysfunction.Reference Khan and Sheppard 15 The increased presence of extracellular matrix proteins within the myocardium results in an alteration of ventricular properties that causes both systolic and diastolic dysfunction.Reference Klein, Schaefer and Hilfiker-Kleiner 26 , Reference Meyer, Wang and Qu 27 In our study, we showed a significant elevation in transforming growth factor-β1 and β2 serum levels in patients with myxomatous mitral valve prolapse as compared with the control group and the non-classic prolapse group, which can contribute to subclinical myocardial involvement – a reduction in the global left ventricular systolic function estimated by speckle-tracking echocardiography. The left ventricular enlargement and systolic dysfunction in myxomatous mitral valve prolapse patients revealed in our study meets the definition of cardiomyopathy as part of generalised systemic disorders in the American Heart Association Scientific Statement.Reference Maron, Towbin and Thiene 28
The present study also detected a low longitudinal deformation in septal segments in young adults with non-classic mitral valve prolapse. In some genetic diseases such as Friedreich's ataxia, Fabry disease, or Duchenne cardiomyopathy, the first regional deformation changes occur in the inferolateral segment: as fibrosis develops first in this area, the reduction in the local strain rates becomes apparent.Reference Bijnens, Cikes, Claus and Sutherland 29 We suggest that the local reduction in the myocardial indices – identified in asymptomatic non-classic mitral valve prolapse patients – is associated with septal fibrosis. Although the presence of myocardial fibrosis in patients with mitral valve prolapse has been reported previously, further studies using magnetic resonance imaging or integrated backscatter assessment are needed.Reference Matos-Souza, Fernandes-Santos and Hoehne 30 However, these changes in deformations may be the first signs of future deterioration of the left ventricular systolic function by mitral valve prolapse progression.Reference Delhomme, Casset-Senon and Babuty 16 – Reference Casset-Senon, Babuty and Philippe 18
Moreover, we found aortic root enlargement in young patients with prolapse. Mitral valve prolapse associated with aortic root enlargement is an independent predictor of greater aortic size not only in patients with systemic connective tissue disorders such as Marfan syndrome, but also in the general population.Reference Matt, Schoenhoff and Habashi 31 It is well known that circulating transforming growth factor-β levels correlate with aortic root diameters and that elevated transforming growth factor-β signalling may contribute to the formation and progression of thoracic aortic aneurysms.Reference Jones, Spinale and Ikonomidis 32 , Reference Han, Peters and Salton 33 However, there is no information on the correlations between longitudinal systolic strain and strain rate and aortic root size in mitral valve prolapse, both of which can be affected by increased circulating transforming growth factor-β concentrations.
In summary, our results indicate a link between myxomatous mitral valve prolapse and myocardial dysfunction, which can be described as primary cardiomyopathy and may be explained by an overexpression of transforming growth factor-β.
Study limitations
Several limitations should be addressed in this study. The major results of the present study have been obtained from a relatively small group of young adults with myxomatous mitral valve prolapse. Moreover, there was a greater proportion of males compared with females in both the mitral valve prolapse group and the control group; this difference could affect the magnitude of the myocardial deformation indices, which are normally higher in women. Myxomatous changes in mitral leaflets were identified in our study only by echocardiography and have not been confirmed by histological examinations.
Conclusion
Global myocardial deformation indices are reduced in young individuals with myxomatous mitral valve prolapse. These changes in deformation may be the first signs of deterioration of the left ventricular systolic function and the existence of primary cardiomyopathy in asymptomatic young individuals with mitral valve prolapse. High levels of transforming growth factor-β may explain the occurrence of systolic dysfunction in these patients. However, further studies using magnetic resonance imaging are needed to assess the severity and extent of cardiac fibrosis in mitral valve prolapse.
Acknowledgements
None.
Conflict of Interest
None.