Published online by Cambridge University Press: 07 August 2001
The objective of this experiment was to detect a possible interaction between iron deficiency and intestinal nematode infections. We report on a 2×2 study where thirty-one 10-week-old pigs fed a low or a normal iron diet were infected with both Trichuris suis (4500 eggs) and Ascaris suum (1200 eggs). No significant difference was detected between diet groups with respect to parasitological parameters for A. suum or the total number of adult T. suis recovered at necropsy 10 weeks p.i. However, in the low iron group T. suis were located more proximally and the worms were increased in length. A higher proportion of pigs with initial faecal egg excretion at 6 weeks p.i. was observed in the low iron group, indicating a shortened pre-patency period. Worm fecundity and total faecal egg excretion were also highest in the low iron group. A significant correlation was found between female worm length and fecundity. The peripheral eosinophil counts were diminished in the low iron host groups. The infected low iron group experienced more severe pathophysiological changes in terms of hypoalbuminaemia and decreases in erythrocyte volumes. A significant inverse correlation existed between iron content in the bone-marrow and liver (body) store. In conclusion, iron deficiency increased the severity of T. suis infection in pigs.
Iron deficiency remains one of the major nutritional problems affecting 3.5 billion people worldwide (WHO, 1998). Even subclinical iron deficiency seems to have harmful effects on health status, productivity and cognitive performance (Stoltzfus & Dreyfuss, 1998). The course and severity of helminth infections in malnourished individuals has received surprisingly little attention despite the fact that iron is known to be of importance for the immune system (Dallman, 1987). A synergistic interaction could result whereby a pre-existing iron deficiency lowers host resistance to and increases the severity and duration of a subsequent infection with helminths such as Ascaris lumbricoides and Trichuris trichiura (Beisel, 1982; Bundy & Golden, 1987).
Pigs are used as host models for human nutrition because of similarities with respect to digestive anatomy and physiology (Miller & Ullrey, 1987). Furthermore, T. suis (Schrank, 1788) is very similar to the human whipworm T. trichiura with respect to life-cycle, morphology, location and interaction with the intestinal mucosa (Holland, 1987). Likewise, Ascaris suum is closely related to A. lumbricoides – the large roundworm of humans. Therefore, experimental T. suis and A. suum infections in the pig are considered to be good models for T. trichiura and A. lumbricoides in humans, parasites which infect at least 1000 and 1400 million people, respectively (Stephenson, 1987; Chan et al. 1994).
The aim of the present study was to investigate the interaction between iron deficiency and experimental T. suis and A. suum infections in pigs. During the course of infection pigs were followed with respect to clinical, haematological and parasitological parameters (including worm burdens) and these were related to body stores of iron.
Four specific-pathogen-free pregnant sows from a helminth-free experimental farm were included in the experiment. Sows and offspring were maintained in concrete floored pens until weaning. The Danish Landrace/Yorkshire/Duroc cross-bred pigs of both sexes were, after weaning at 4 weeks of age, first housed in a climate stable, hence in elevated pens with partly slatted floors and straw bedding in groups of 5–8 each. An experimental diet was fed to the pigs in amounts equal to approximately 75% of Danish feeding norms (Table 1) (Chwalibog, 1997); this ensured a realistic comparison with malnourished children. The diets were appropriately designed to supply all other essential nutrients, except iron, in sufficient amounts. Pigs were fed a weaner diet until week 10 and hereafter a grower diet. Divalent iron was supplied as ferrous sulphate in the mineral/vitamin mixture to one diet group but was omitted from the diet of the other group. The pigs had free access to water via drinking nipples. Pigs were weighed at farrowing (week 0) and weeks 9, 13, 17 and 20.
Two sows and their piglets were randomly divided into either a low (Fe−) or a normal iron (Fe+) group (Table 2). In turn, pigs in each of these diet groups were randomly divided into either an inoculated and control group, taking litter and sex into account, as follows: an inoculated group (Fe−/inf) fed a low iron diet (5 females and 6 castrates); an uninoculated control group (Fe−/con) fed a low iron diet (3 females and 5 castrates); an inoculated group (Fe+/inf) fed a normal iron diet (3 females and 3 castrates); and an uninoculated control group (Fe+/con) fed a normal iron diet (2 females and 4 castrates). In addition, some weak piglets were eliminated (Table 2). Four-day-old piglets in the normal iron groups (Fe+/inf and Fe+/con) were injected intramuscularly with 200 mg iron (Ferridex ® Vet 80 mg/ml, Rosco).
Table 2. Experimental design (Four litters of piglets were randomly divided into 2 diet groups each consisting of 2 litters which were weaned when 4 weeks of age. In one of the diet groups piglets were injected intramuscularly with 200 mg iron when 4 days old and fed a normal iron-containing diet, while piglets in the other diet group remained uninjected and were fed a low iron diet. Half of the piglets in each diet group were randomly allocated to be infected when 10 weeks old (Fe−/inf and Fe+/inf) or remain as controls (Fe−/con and Fe+/con). Autopsy was made 10 weeks post-inoculation when pigs were 20 weeks old*.)

Infective T. suis eggs were originally isolated in 1996 from soil from an organic farm and subsequently passaged in helminth-naïve pigs. The eggs used in the present experiment were isolated from faeces of experimentally infected pigs, embryonated in vermiculite according to the method described by Burden & Hammet (1976) and subsequently stored at 10 °C for 2 years. The strain of A. suum eggs was originally isolated in 1993, also from an organic pig farm. Ten-week-old pigs (Fe−/inf and Fe+/inf) were inoculated orally via a stomach tube with 1500 and 400 infective T. suis and A. suum eggs, respectively, on 3 consecutive days. Faecal samples were collected from the rectum of each pig at the time of inoculation, 5 weeks p.i. and weekly hereafter. Egg counts were determined using a concentration McMaster Technique as described by Roepstorff & Nansen (1998), with a detection limit of 20 eggs per gram (e.p.g.). Blood samples were taken at weeks 4, 10, 14 and 19 from the jugular vein of each pig and haemoglobin concentration, packed cell volume (PCV), mean corpuscular volume (MCV), serum albumin concentration and peripheral eosinophil count were measured and analysed.
All pigs were slaughtered 10 weeks p.i. Feed was withheld on the day of necropsy and pigs were euthanized by electrical stunning followed by exsanguination. The unopened small intestine was emptied by passing lukewarm tap water through it twice. Intestinal contents were washed with a stream of water on a sieve to recover macroscopically visible A. suum. Trichuris suis worms were recovered according to the method described by Roepstorff & Murrell (1997). The large intestine was divided into 5 sections, designated as follows, starting at the caecum: (1) caecum; (2) 0–20% of the total length of colon; (3) 21–40%; (4) 41–60% and (5) 61–100% including rectum. The sections were opened with scissors and the intestinal wall was gently washed to liberate T. suis. Representative aliquots of 10% of the intestinal contents and intestinal wall washings were washed over a sieve of mesh size 212 μm. Retained samples were fixed in 15 °C iodine (80 g iodine and 400 g potassium iodide in 800 ml of distilled water) for later isolation of T. suis. Samples were transferred to a Petri dish held over a light table and decolourized with a 30% sodium thiosulphate solution. Worms visible with the naked eye were recovered, stored in 70% ethanol and differentiated according to developmental stage and sex (Beer, 1973a).
Body lengths of intact worms were measured by means of a stereo-microscope and a digital image analysis system (Microvision®, DTI, Denmark); for those pigs harbouring more than 10 worms of either sex, 10 worms were selected randomly. The proportion of female worms was calculated by dividing the number of female worms by the total number of adult worms. Percentage establishment was defined as the percentage of the infective eggs recovered as worms. For those pigs harbouring female worms, fecundity was estimated by dividing e.p.g. at the day of autopsy by the number of females recovered.
Livers and spleens were weighed, whereafter samples of liver (50 g), spleen (50 g) and bone-marrow (1–3 g) were taken and kept at −80 °C for later determination of the iron and zinc concentrations. Analysis was made by atomic absorption spectrophotometry after combustion at 550 °C and dissolution of the ash in HNO3 and HCl (Larsen & Sandström, 1992). In order to estimate the total body stores of iron and zinc, organ weights (liver, spleen) were multiplied by the tissue concentration. In the case of bone-marrow body weight was used as a relative weighing factor.
Worm numbers, mean intestinal location, worm distribution in sections, percentage establishment, proportion of female T. suis, worm length, faecal egg counts and worm fecundity were compared using the Student's t-test. When necessary data were log10 (x+10)-transformed. Where appropriate the non-parametric Wilcoxon–Mann–Whitney test was used. Mean intestinal location of T. suis in each pig was estimated by assuming that all worms in each section were localized in the middle of that particular section. The percentage of worms in each section of individual pigs was calculated, allowing relative distributions of T. suis in sections of the large intestine to be compared between groups.
A split plot analysis of variance (ANOVA) model with litter as a random factor, diet and infection as fixed factors and sex as a covariate was used to analyse serum albumin, haemoglobin, PCV, MCV, peripheral eosinophil count, tissue iron and zinc concentrations and body weight.
Univariate repeated measurements ANOVA was used for longitudinal analyses for body weight and blood parameters with sex as a covariate. Analysis was made for 2 separate time-periods delimited by the time of inoculation. Longitudinal analysis of faecal egg excretion was performed by estimating the area under the e.p.g. curve of individual pigs from 6 to 10 weeks p.i. It was assumed that the counts obtained weekly represented mean daily counts for the whole week.
For those pigs harbouring female T. suis at slaughter Spearman's correlation coefficient was used to relate the faecal egg excretion to the female worm burden, while Pearson's correlation coefficient was used to relate worm fecundity to female worm length as well as the iron content in bone marrow/liver and spleen/liver. Before analysis fecundity and lengths were logarithmically transformed (log10 (x+10)). The prevalences of worm-positive pigs at necropsy and egg-excreting pigs 6 weeks p.i. were compared between groups using Fisher's Exact test. The parameter of the negative binomial distribution, k, was estimated as k = mean2/(variance-mean); k decreases as overdispersion increases. Mean and variance were calculated on total worm burdens. The SAS 6.12® programme was used for analysis and the level of significance for all tests was α = 0.05.
The numbers of T. suis worms recovered from individual pigs are presented in Table 3, representing a mean establishment of 13% in both experimental groups. The mean number (±S.D.) of worms was not significantly different between experimental pigs on low (Fe−/inf) or normal (Fe+/inf) iron diet, respectively (590±589 versus 605±534; P = 0.96). Nine pigs (82%) in Fe−/inf and 5 pigs (83%) in Fe+/inf harboured worms but these prevalences were not significantly different (P = 1.0). Mean intestinal section location (±S.D.) was significantly (P = 0.047) more proximal in Fe−/inf compared with Fe+/inf, 1.9±0.3 versus 2.4±0.4, respectively. The relative distributions of worms in the 5 sections of the large intestine for the 2 groups are shown in Fig. 1. No significant differences were detected between groups in relation to percentage of worms recovered in the 5 intestinal sections (P>0.05).
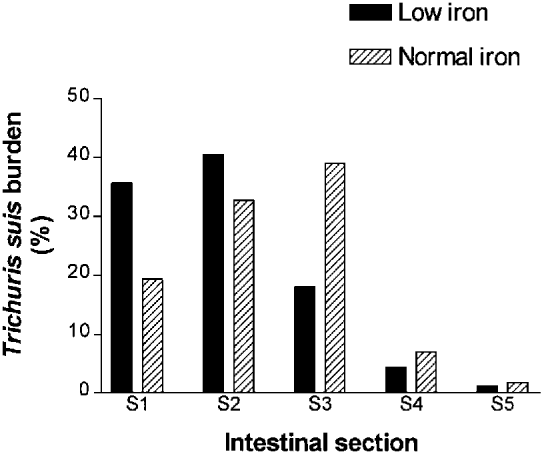
Fig. 1. Comparison of the relative distribution (%) of Trichuris suis in 5 intestinal sections of pigs from the low iron (Fe−/inf) and normal iron (Fe+/inf) group, respectively. S1, caecum; S2, 0–20% of the total length of colon; S3, 21–40%; S4, 41–60%; S5, 61–100% including rectum. Mean (±S.D.) intestinal location: 1.9±0.3 versus 2.4±0.4; P = 0.047.
Table 3. Number of Trichuris suis recovered from the large intestine (based on 10% samples) of individual pigs inoculated with 4500 eggs and fed a low (Fe−/inf) or normal iron (Fe+/inf) containing diet, respectively (S1, section 1 (caecum); S2, section 2 (0–20% of the total length of colon); S3, section 3 (21–40%); S4, section 4 (41–60%); S5, section 5 (61–100% including rectum). Total: all 5 sections. Test for difference in total worm burden between groups: P = 0.96.)

Only adult T. suis were recovered and they ranged from 16 to 40 mm in length. Overall, total mean worm lengths (mm) (±S.D.) were significantly higher in Fe−/inf compared with Fe+/inf (29.7±2.2 versus 26.5±3.2; P = 0.044). Total mean lengths of females were significantly higher in Fe−/inf than in Fe+/inf (30.6±2.4 versus 25.7±1.4; P = 0.003), while total mean lengths of males did not differ significantly (28.7±2.6 versus 26.1±3.3; P = 0.11). The median (interquartile range) proportion of female T. suis was not significantly different between Fe−/inf and Fe+/inf, i.e. 0.49 (0.43–0.55) versus 0.43 (0.40–0.43) (P = 0.23). The parameter k of the negative binomial distribution indicated some degree of aggregation of the adult T. suis populations in the 2 experimental groups (Fe−/inf: k = 1.0 and Fe+/inf: k = 1.3).
Faecal egg excretion in some of the inoculated pigs started 6 weeks p.i. A significantly higher prevalence of pigs excreted T. suis eggs in Fe−/inf (92%) compared with Fe+/inf (29%) at week 6 p.i. (P = 0.0096). The fact that a significantly higher median faecal egg excretion occurred in Fe−/inf compared with Fe+/inf at week 6 p.i. (380 versus 0 e.p.g.; P = 0.008) indicates a shortened prepatency period in the low iron group (Fig. 2). The highest mean e.p.g. levels were observed at week 7 p.i. in Fe−/inf (mean: 9910) and at week 8 p.i. in Fe+/inf (mean: 2573). Between weeks 6 and 10 p.i. the median e.p.g. was 380–11050 (max. week 7) in Fe−/inf and 0–2030 (max. week 8) in Fe+/inf. Longitudinal analysis showed that a significantly higher mean (±S.D.) total egg excretion occurred in Fe−/inf compared with Fe+/inf (33918±24347 versus 6993±5281; P = 0.004).
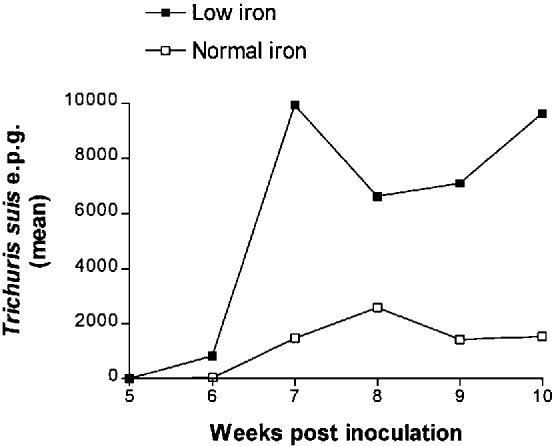
Fig. 2. Mean Trichuris suis eggs per gram faeces (e.p.g.) in the low iron (Fe−/inf) and normal iron (Fe+/inf) group, respectively, from 5 to 10 weeks post-inoculation. Mean (±S.D.) total faecal egg excretion: 33918±24347 versus 6993±5281; P = 0.004.
Comparison of groups revealed a significantly higher mean (min.–max.) fecundity of female T. suis in Fe−/inf compared with Fe+/inf (35 (16–100) versus 5 (3–7); P = 0.001). A significant correlation was detected between worm fecundity and female T. suis length (r = 0.63, n = 13, P = 0.02) (Fig. 3). For pigs harbouring female T. suis, a borderline significant correlation existed between number of female worms recovered at necropsy and concurrent egg output (r = 0.54, n = 13, P = 0.058), confirming that e.p.g. was a reliable measure of the intensity of infection.

Fig. 3. Correlation between fecundity (e.p.g./number of female worms) and mean length of female Trichuris suis recovered from the 2 inoculated groups (Fe−/inf and Fe+/inf) (r = 0.63, n = 13, P = 0.02).
Ascaris suum worms were recovered from 4 (36%) and 2 (33%) pigs in Fe−/inf and Fe+/inf, respectively. The numbers of A. suum worms recovered were as follows: Fe−/inf (6, 7, 20, 28) and Fe+/inf (17, 39). No significant difference in either prevalence of A. suum-positive pigs (P = 1.0) or worm numbers (P = 0.95) was detected between Fe−/inf and Fe+/inf. Ascaris suum egg excretion started at week 6 p.i. and reached an individual maximum of 17440 e.p.g. It peaked at week 10 p.i. in Fe−/inf (median: 140) and Fe+/inf (median: 350). Longitudinal analysis revealed no significant difference in median (interquartile range) total egg excretion between groups (340 (0–3220) versus 630 (220–11800); P = 0.34).
Compared to Fe−/inf the other groups had a 25–34% higher content of iron in the livers; however, this was not significant due to comprehensive variation among pigs (Table 4). A borderline significant correlation was detected between concomitant iron in spleen and liver, indicating the validity of these organs as measures of body stores (r = 0.35, n = 30, P = 0.061). Infection was accompanied by the level of iron in the bone-marrow being significantly higher in Fe−/inf compared with the control groups (P = 0.033) (Table 4). A significant inverse correlation was found between iron content in bone-marrow and liver (r =−0.38, n = 31, P = 0.035). Besides, increased zinc levels in the livers of pigs were detected in the low iron groups (P = 0.033) (Table 4).
Table 4. Total iron (Fe) and zinc (Zn) stores (mg) (±S.D.) in livers and spleens for low iron/infected (Fe−/inf), low iron/control (Fe−/con), normal iron/infected (Fe+/inf) and normal iron/control (Fe+/con) pigs where the mean iron concentration (±S.D.) (mg/kg) of bone-marrow (BM) was adjusted according to body weight at the day of autopsy. (P-values for differences between groups in relation to the factors dietary iron and infection. *Significance at the α = 0.05 level.)

Haemoglobin concentrations, PCV's and MCV's were significantly higher in the normal iron groups compared with the low iron groups only at week 4 (P<0.001) (Fig. 4A–C). However, at week 14 diet was of borderline significance, haemoglobin levels being higher in Fe−/inf compared with Fe+/con (P = 0.051), while PCV levels were higher in Fe−/con than Fe+/con (P = 0.074). Longitudinal analysis revealed that diet was significantly affecting haemoglobin levels and PCV's before the time of inoculation (P<0.001). At week 19 an interaction was detected between diet and infection (P = 0.043), i.e. MCV levels were higher in Fe−/con compared with Fe−/inf. Sex also turned out to be a significant covariate being higher for female pigs compared with males (P = 0.03). Longitudinal analysis revealed that diet was a significant factor before inoculation (P<0.001), while in the period hereafter infection was accompanied by the MCV levels being significantly decreased in the low iron group (P = 0.02).

Fig. 4. (A) Mean haemoglobin (mmol/l), (B) mean packed cell volume (l/l) and (C) mean corpuscular volume (fl) from weeks 4 to 19 post-partum. Groups: Fe−/inf (low iron/infected), Fe−/con (low iron/control), Fe+/inf (normal iron/infected) and Fe+/con (normal iron/control). The arrow marks the time of infection.
Serum albumin levels were significantly higher in the low iron compared with the normal iron groups at week 4 (P = 0.01) (Fig. 5A). Furthermore, at week 14 sex was a significant covariate, values being higher for males than females (P = 0.04). At week 19 both diet (P = 0.038) and infection (P = 0.006) significantly affected albumin levels, i.e. Fe−/con was higher compared with the other groups. Longitudinal analysis showed a significant influence of diet (P = 0.004) before the time of inoculation.

Fig. 5. Mean (A) serum albumin (g/l) and (B) peripheral eosinophil counts (bio/l) from weeks 4 to 19 post-partum. Groups: Fe−/inf (low iron/infected), Fe−/con (low iron/control), Fe+/inf (normal iron/infected) and Fe+/con (normal iron/control). The arrow marks the time of infection.
At week 4 the peripheral eosinophil counts were significantly lower in the low iron groups compared with the normal iron groups (P<0.001) (Fig. 5B). At week 14 infection was a significant factor (P = 0.002), i.e. Fe+/inf was higher than the control groups. Diet was a significant factor at week 19 (P = 0.021): Fe+/inf was higher compared with Fe−/con and Fe−/inf. Longitudinal analysis revealed that diet both before (P = 0.0001) and after infection (P = 0.045) was accompanied by the peripheral eosinophil count being significantly diminished in the low iron groups.
During the study no significant differences in body weights were detected between groups at any time (P>0.05). Pigs weighed 16.2±3.8 kg and 50.6±9.6 kg (mean±S.D.) at the time of inoculation and necropsy, respectively. Longitudinal analysis also revealed no difference in weight gains over time (P>0.05). The restrictive feeding caused some fighting-induced injuries among pigs which was the main reason for the exclusion of animals in this experiment. Pigs in the low iron groups were more inactive and lethargic. This may explain why most excluded pigs belonged to the normal iron groups. Diarrhoea occasionally occurred in a number of infected pigs. A pig with pneumonia was treated with penicillin.
The present experimental diets were designed with low iron contents to imitate the cereal-based diet of many humans (Allen & Ahluwalia, 1997). Iron from cereal grains has a low bioavailability compared with haem iron. Therefore, as animal protein decreases in the human diet, the prevalence of nutritional iron deficiency rises (Fleming, 1982). In the current study piglets were raised in an environment devoid of exogenous iron sources. As a consequence piglets on the low iron diet which were not parenterally supplied with iron became anaemic due to their low body stores, rapid growth rates and the natural low iron levels in sows milk (Miller & Ullrey, 1987). The low MCV levels detected were characteristic of microcytic anaemia, which is an outcome of iron deficiency (Sherwood, Pippard & Peters, 1998). However, when pigs in the low iron groups were allowed to consume considerable quantities of solid feed their haemoglobin and PCV levels became normal before the time of infection. Nevertheless, infection was accompanied by MCV levels that were significantly decreased in the low iron group, which was not observed in the normal iron group. Reduction in MCV levels is reported to occur only in severe iron deficiency (Sherwood et al. 1998), which is evidence that pigs in the low iron group were already subclinically iron deficient at the time of infection. The experimental infection results confirm that infected pigs in the low iron group became increasingly iron depleted due to T. suis infection. Beer, Sansom & Taylor (1974) reported an average erythrocyte loss rate which was approximately proportional to the infective dose of eggs. The mechanism of red cell loss has been described as a leakage of erythrocytes into the intestinal lumen from dilated blood capillaries in the lamina propria and from eroded blood vessels in the damaged mucosal surface forming petechial haemorrhages (Beer et al. 1974). If the faecal loss of erythrocytes and corresponding drain on iron reserves persists under conditions of low dietary iron then iron deficiency anaemia can be exacerbated by Trichuris infection (Holland, 1987).
An inverse linear relationship occurred between increased bone-marrow concentration and decreasing body stores of iron. The reduced content of iron in livers and spleens of pigs from the infected low iron group, contrasted with the increased iron levels in the bone-marrow, reflecting an accelerating erythropoiesis. It is clear that these pigs suffered more severely from T. suis infection in comparison to the normal iron group. The increased zinc levels in livers of pigs from the low iron groups probably reflects the competition between different cations in the phase of intestinal absorption (Whittaker, 1998).
Trichuris suis infection was also accompanied by significantly diminished serum albumin levels in the low iron group, which did not occur in the normal iron group. A decrease in serum albumin concentration following infection is reported to be associated with T. suis larvae re-emergence to the mucosal surface of the intestine to complete development (Batte et al. 1977). Nevertheless, the somewhat delayed appearance of hypoalbuminaemia in the present study suggests that both early and late stages of T. suis worms are damaging to the intestinal mucosa. Apart from dose size the main determinant for the presence of clinical signs seems to be whether or not secondary microbial invasion occurs (Beer, 1973b).
In our study low dietary iron caused, directly or indirectly, T. suis worms to occupy a more proximal position in the large intestine. A low dietary iron supply has formerly been shown to spread the helminth distribution in the small intestine of rats (Bolin et al. 1977). Trichuris suis is reported to have a predilection for the caecum and the upper third of the colon, although the entire colon including rectum is a potential habitat (Powers, Todd & McNutt, 1960). Worms recovered from the low iron group were also longer than those from the normal iron group. To our knowledge this has not been reported previously. The mean lengths of adult T. suis recorded in this study were less (27–30 mm) than those (48–50 mm) reported by Beer (1973b). This is likely to be due to reduced feeding and the fact that T. suis worms continue to grow after reaching the adult stage.
The fecundity of female T. suis was higher in the low iron group compared with the normal iron group (Bundy & Golden, 1987). Assuming a daily faecal egg excretion amounting to 0.8 kg this would correspond to a mean (min.–max.) of 28000 (12800–80000) and 4000 (2400–5600) eggs produced per female per day in the low and normal iron group, respectively, indicating that T. suis is not necessarily such a non-prolific egg layer as suggested by Beer et al. (1971). Moreover, a highly significant correlation was detected for the first time between female worm length and fecundity. The pre-patency period was also found to be shorter in the low iron group compared with the normal iron group, based on faecal samples taken 42–44 days p.i. Consequently, the total egg excretion was markedly higher in this group. Low dietary iron resulted in compromised ability of the host to react to worms, resulting in an intestinal environment favouring reproduction.
The more productive T. suis infection in the low iron group may be, at least partially, due to lowered oxidative stress in the intestinal lumen as a result of a decreased dietary iron level (Miller & Britigan, 1997). Reactive oxygen species (i.e. hydroxyl radical, superoxide anion, hydrogen peroxide) exert a biotoxic effect and are potentially harmful to T. suis. It may be that physiological processes with a high metabolic rate such as growth and reproduction (egg production) are the most vulnerable to a reducing environment. Reduced reactive oxygen species resulting from low dietary iron may provide more optimal growth conditions for T. suis, thereby increasing the severity of infection, increasing worm size as well as higher female worm fecundity and total faecal egg excretion, and a more proximal intestinal location.
Another possible explanation for the modulation of T. suis infection seen in the low iron group is impaired immunity (Bundy & Golden, 1987). Abnormalities in cell-mediated immunity under iron deficient conditions is well-documented (Dallman, 1987). Previously, it has been reported that worm expulsion following a primary infection is delayed (Bolin et al. 1977) in iron-deficient rats infected with Nippostrongylus brasiliensis. Further, no difference in initial worm establishment was observed, which is in accordance with the results of the current study. This may reflect the fact that initial ‘acquisition’ of immunity in helminth-naïve pigs is given high priority at a time when the scarce body store of iron is in demand for different physiological processes. In contrast, the ‘expression’ phase of the immune response seems may be suppressed in cases of moderate malnutrition (Duncombe, Bolin & Davis, 1979; Coop & Kyriazakis, 1999). In this study pigs which were supplied with adequate iron had a significantly higher base level and mobilizable pool of circulating eosinophils. This suggests that active cell-mediated immunity in the low iron group was down-regulated. In the present study A. suum infection most likely caused the eosinophilia. A lack of peripheral eosinophilia due to T. suis infection has been reported formerly, although eosinophils have been described to be prominent in the colonic mucosa of infected pigs (Powers et al. 1960; Beer & Lean, 1973). During periods of high nutrient demand such as rapid growth the requirements of immune function may be given lower priority than the ability of the host to maintain the functions of growth and to stabilise other physiological processes with a higher priority for survival (Coop & Kyriazakis, 1999).
Iron-deficient rats have impaired acquired resistance to reinfection with Nippostrongylus brasiliensis (Duncombe et al. 1979). However, the condition is reversible upon nutritional rehabilitation. Similarly, it has recently been documented in human studies that adults given supplemental iron have significantly lower reinfection rates of T. trichiura, A. lumbricoides and Schistosoma mansoni (Olsen, Nawiri & Friis, 2000).
In conclusion, the results of the current study demonstrated that a low dietary iron intake results in a shorter T. suis pre-patency period, more proximal location of worms, increased female worm length, and higher female fecundity (egg excretion). Even subclinical iron deficiency lowered host resistance to a moderate T. suis infection resulting in more severe pathophysiological consequences (hypoalbuminaemia and relative microcytic erythrocytosis) from the tissue damage and nutrient losses compared with the pigs supplied with sufficient amounts of iron. Bearing in mind the widespread occurrence of mild to moderate malnutrition in humans in the world, this experiment offers important insights into the potential importance of iron nutrition level in parasite infections in malnourished human populations.
The help of Torben Larsen with tissue analyses is gratefully appreciated. The Danish National Research Foundation is acknowledged for financial support.
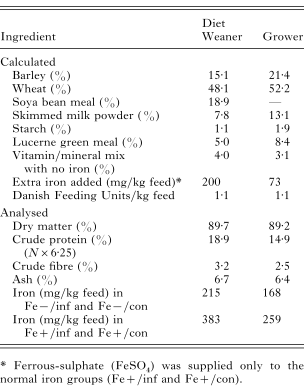
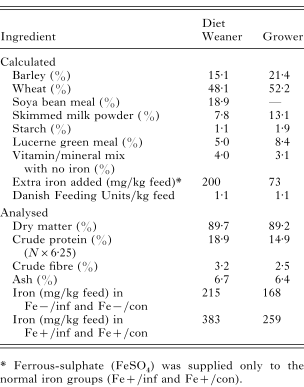
Table 1. Composition and chemical analysis of the experimental diets

Table 2. Experimental design

Fig. 1. Comparison of the relative distribution (%) of Trichuris suis in 5 intestinal sections of pigs from the low iron (Fe−/inf) and normal iron (Fe+/inf) group, respectively. S1, caecum; S2, 0–20% of the total length of colon; S3, 21–40%; S4, 41–60%; S5, 61–100% including rectum. Mean (±S.D.) intestinal location: 1.9±0.3 versus 2.4±0.4; P = 0.047.

Table 3. Number of Trichuris suis recovered from the large intestine (based on 10% samples) of individual pigs inoculated with 4500 eggs and fed a low (Fe−/inf) or normal iron (Fe+/inf) containing diet, respectively

Fig. 2. Mean Trichuris suis eggs per gram faeces (e.p.g.) in the low iron (Fe−/inf) and normal iron (Fe+/inf) group, respectively, from 5 to 10 weeks post-inoculation. Mean (±S.D.) total faecal egg excretion: 33918±24347 versus 6993±5281; P = 0.004.

Fig. 3. Correlation between fecundity (e.p.g./number of female worms) and mean length of female Trichuris suis recovered from the 2 inoculated groups (Fe−/inf and Fe+/inf) (r = 0.63, n = 13, P = 0.02).

Table 4. Total iron (Fe) and zinc (Zn) stores (mg) (±S.D.) in livers and spleens for low iron/infected (Fe−/inf), low iron/control (Fe−/con), normal iron/infected (Fe+/inf) and normal iron/control (Fe+/con) pigs where the mean iron concentration (±S.D.) (mg/kg) of bone-marrow (BM) was adjusted according to body weight at the day of autopsy.

Fig. 4. (A) Mean haemoglobin (mmol/l), (B) mean packed cell volume (l/l) and (C) mean corpuscular volume (fl) from weeks 4 to 19 post-partum. Groups: Fe−/inf (low iron/infected), Fe−/con (low iron/control), Fe+/inf (normal iron/infected) and Fe+/con (normal iron/control). The arrow marks the time of infection.

Fig. 5. Mean (A) serum albumin (g/l) and (B) peripheral eosinophil counts (bio/l) from weeks 4 to 19 post-partum. Groups: Fe−/inf (low iron/infected), Fe−/con (low iron/control), Fe+/inf (normal iron/infected) and Fe+/con (normal iron/control). The arrow marks the time of infection.