Introduction
The importance of eye contact in social interactions cannot be emphasized enough. A wealth of research exists showing the strong influence of eye contact on human attention, behaviour and cognition (see reviews: Kleinke, Reference Kleinke1986; Senju and Johnson, Reference Senju and Johnson2009). In fact, our sensitivity to eye contact and socially relevant information is innate, as evidenced by newborns’ responses to faces (Hains and Muir, Reference Hains and Muir1996; Farroni et al., Reference Farroni, Csibra, Simion and Johnson2002). This is further highlighted by the strong prior that we have for direct gaze (Mareschal et al., Reference Mareschal, Calder and Clifford2013). Thus, in situations of uncertainty, for example, at night, when the direction of eye gaze is unclear, humans have an expectation that others’ gaze is directed at them. In addition, there is also evidence showing that our sensitivity for direct gaze extends beyond our awareness. For example, faces with direct gaze break through continuous flash suppression faster compared with averted gaze (Stein et al., Reference Stein, Senju, Peelen and Sterzer2011; Chen and Yeh, Reference Chen and Yeh2012). Such a difference in participants’ reaction times to detect initially suppressed stimuli is often used as a measure of ‘access to awareness’ (Gayet et al., Reference Gayet, Van der Stigchel and Paffen2014; Stein and Sterzer, Reference Stein and Sterzer2014). In this case, it thus provides evidence for differences in processing direct and averted gaze. Interestingly, the difference in the access to awareness between direct and averted gaze is also directly reflected in the neural activity required to process these faces: Direct gaze requires lower levels of neural activity to reach awareness compared with averted gaze (Madipakkam et al., Reference Madipakkam, Rothkirch, Guggenmos, Heinz and Sterzer2015). More recently, a bias in eye movements to direct gaze faces that were suppressed from awareness showed unequivocal evidence for the unconscious processing of direct gaze, emphasizing the importance of this cue in human communication (Rothkirch et al., Reference Rothkirch, Madipakkam, Rehn and Sterzer2015). Given this central role for eye gaze in human social interactions and development, it is unsurprising that deficits in the processing of eye gaze information and atypical eye contact are primary hallmarks of psychiatric disorders characterized by impairments in social interactions like autism (Senju et al., Reference Senju, Tojo, Yaguchi and Hasegawa2005) and social phobia (Horley et al., Reference Horley, Williams, Gonsalvez and Gordon2003).
It has long been thought that the deficits observed in autism lie on a continuum of social-communication disability extending into the typical population (Wing, Reference Wing, Schopler and Mesibov1988; Baron-Cohen, Reference Baron-Cohen1997). In line with this continuum view, Baron-Cohen et al. designed the autism spectrum quotient (AQ), a self-report questionnaire, to measure the extent to which an adult with normal intelligence has ‘autistic traits’ (Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001). Accordingly, the variance in the general population's AQ scores is reflected in their performance in cognitive tasks in which individuals with autism spectrum disorder (ASD) are impaired. For example, differences in the strength of gaze cueing effects are correlated with typically developed (TD) participants’ AQ scores, with low AQ scorers showing stronger effects of gaze cueing and a more holistic processing of stimuli, while high AQ scorers (with more autistic-like traits) show a preference for the local processing of stimulus features (Bayliss and Tipper, Reference Bayliss and Tipper2005). Other gaze cueing tasks in individuals with high autistic traits have found similar weakened attentional responses to eye gaze (Hudson et al., Reference Hudson, Nijboer and Jellema2012; Zhao et al., Reference Zhao, Uono, Yoshimura and Toichi2015). Similarly, a high degree of autistic traits is related to low trustworthiness perception of others’ faces (Bayliss and Tipper, Reference Bayliss and Tipper2006). These results suggest that the cognitive style of autistic individuals is indeed reflected in a broader phenotype across the population.
Atypical sensitivity to eye gaze in autism is a well-established finding (Senju et al., Reference Senju, Tojo, Yaguchi and Hasegawa2005; Kliemann et al., Reference Kliemann, Dziobek, Hatri, Steimke and Heekeren2010). Accordingly, Akechi et al. (Reference Akechi, Stein, Senju, Kikuchi, Tojo, Osanai and Hasegawa2014) recently reported that the preferential access to awareness of direct gaze as observed in TD participants was absent in participants with ASD. Further evidence for an absence of an unconscious processing of direct gaze was recently found in ASD (Madipakkam et al., Reference Madipakkam, Rothkirch, Dziobek and Sterzer2017). However, categorical differences in the access to awareness between ASD and TD participants do not provide information about whether variations in subclinical AQ scores of participants are mirrored in differences in their access to awareness of direct and averted gaze. Such a relation would provide further evidence for a continuum view of ASD and may aid the early detection of participants at a high risk of ASD.
In the present study, we performed two independent experiments where we investigated the extent to which differences in the degree of participants’ autistic traits are reflected in their processing of direct and averted gaze. In experiment 1, we tested both ASD and TD participants. All ASD participants were not taking psychotropic medication for at least 6 months prior to the day of testing. Experiment 2 consisted of only TD participants to specifically probe the effect of autistic-like traits on access to awareness in the typical population and to further corroborate the findings from experiment 1.
Materials and methods
Participants
Nineteen adults with ASD [12 males; mean age: 35.1 ± 2.1 (s.e.m.) years] and 22 TD controls [12 males; mean age: 34.5 ± 1.7 (s.e.m.) years] participated in experiment 1. The two groups did not differ in chronological age, gender, verbal intelligence as measured by a German vocabulary test [Mehrfachwahl–Wortschatz-Test MWT)] (Wittorf et al., Reference Wittorf, Wiedemann and Klingberg2014), and attention as measured by the d2 test (Bates and Lemay, Reference Bates and Lemay2004) (Table 1). The vocabulary test MWT assesses the crystallized intelligence level and is often used in studies with clinical populations (Kliemann et al., Reference Kliemann, Dziobek, Hatri, Steimke and Heekeren2010) due to its practical feasibility. The test takes 5 min to administer and consists of a row of four fictive words and one real word. The participants’ task is to identify the real word among the choice of fictive words. The test has a high reliability (Cronbach's α = 0.94) and high validity (r = 0.76–0.81) with other verbal intelligence tests like the HAWIE-R, the German version of the Wechsler Intelligence Test (Lehrl et al., Reference Lehrl, Triebig and Fischer1995; Satzger et al., Reference Satzger, Fessmann and Engel2002). All participants were invited if they were off psychotropic medication for at least 6 months prior to the day of testing. ASD diagnoses were confirmed by clinical experts according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for Asperger syndrome and autism without intellectual difficulties. Furthermore, for 12 of the 19 participants, diagnosis was substantiated by the Autism Diagnostic Observation Schedule [ADOS-G; mean: 11.2 ± 1.1 (s.e.m.), cut-off autism spectrum: 7] (Lord et al., Reference Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles and Rutter2000). In addition, the Structured Clinical Interview for Axis I Disorders (SCID-I) was carried out with all participants to control for comorbidities in the ASD group and to rule out other psychiatric disorders in the control group. ASD adults were recruited through the outpatient clinic for adults of the Charité – Universitätsmedizin Berlin, Germany, and through an online forum (Aspies e.V.). The control group was recruited by local advertisement.
Table 1. Participant characteristics

M ± s.d., mean ± standard deviation; TD, typically developed; ASD, autism spectrum disorder; IQ was assessed with a test for verbal intelligence [Mehrfachwahl–Wortschatz-Test (MWT); Lehrl et al., Reference Lehrl, Triebig and Fischer1995]; attention was assessed with the d2 test; AQ, autism spectrum quotient (Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001); ADOS, autism diagnostic observation schedule – generic (Lord et al., Reference Lord, Risi, Lambrecht, Cook, Leventhal, DiLavore, Pickles and Rutter2000).
In experiment 2, 21 participants who did not take part in experiment 1 were tested. Scores from the AQ questionnaire were unavailable for one participant who was therefore excluded from all analyses. The final sample consisted of 20 participants [six males; mean age: 25.3 ± 0.72 (s.e.m.) years] with no history of neurologic or psychiatric disorders.
Both experiments 1 and 2 were conducted in accordance with the 2008 World Medical Association Declaration of Helsinki and were approved by the local ethics committee of the Charité – Universitätsmedizin Berlin. All participants had normal or corrected-to-normal vision, were naïve to the purpose of the study and received payment for their participation. Written informed consent was obtained from all participants prior to the start of the experiment. A subset of participants from both experiments took part in two other studies involving different tasks. The data from participants from one of these studies were published in 2015 (Madipakkam et al., Reference Madipakkam, Rothkirch, Guggenmos, Heinz and Sterzer2015), while data from participants for the other study were published in 2017 (Madipakkam et al., Reference Madipakkam, Rothkirch, Dziobek and Sterzer2017).
Stimuli
The stimulus set comprised three greyscale female face exemplars each with a direct and averted gaze that have been used in several previous studies investigating gaze processing (Stein et al., Reference Stein, Senju, Peelen and Sterzer2011; Rothkirch et al., Reference Rothkirch, Madipakkam, Rehn and Sterzer2015). The direct and averted gaze was formed by the pupil direction, which was either directed towards or away from the observer, respectively. Thus, the only difference between the two gaze directions was the shifted irises within the eye, avoiding low-level stimulus confounds. The faces were 3.8° × 4.5° (width × height) in size and equalized for global contrast (root mean square contrast of 0.05) and luminance. Visual stimuli were presented with Matlab (The MathWorks, Natick, MA, USA), using the Cogent 2000 toolbox (http://www.vislab.ucl.ak.uk/cogent.php). In experiment 1, stimuli were presented on a 19-inch CRT monitor (resolution: 1024 × 768 Px; refresh rate: 60 Hz) and participant's head was stabilized by a chin rest at a viewing distance of 60 cm. To achieve binocular fusion, participants viewed the screen through a mirror stereoscope.
Experiment 2 was an independent task performed in combination with a larger functional magnetic resonance imaging (fMRI) study that investigated the neural responses to gaze direction in dependence on awareness (Madipakkam et al., Reference Madipakkam, Rothkirch, Guggenmos, Heinz and Sterzer2015). This independent task (experiment 2 in the present study) took place before the main fMRI experiment while participants already lay in the scanner. In the previous study, the purpose of this task was to determine the participants’ dominant eye, information that was required for the subsequent main fMRI task. Participants performed this task while T1 anatomical brain scans for the main fMRI study were acquired. Dichoptic presentation of the stimuli in the scanner was achieved by using an fMRI compatible cardboard divider and a pair of prism lenses worn by the participants (Schurger, Reference Schurger2009). The stimuli were projected via an LCD projector (ProExtra Multiverse Projector, Sanyo Electric Co. Ltd, Osaka, Japan; refresh rate 60 Hz) onto a screen of size 24.9° × 18.6°.
Procedure
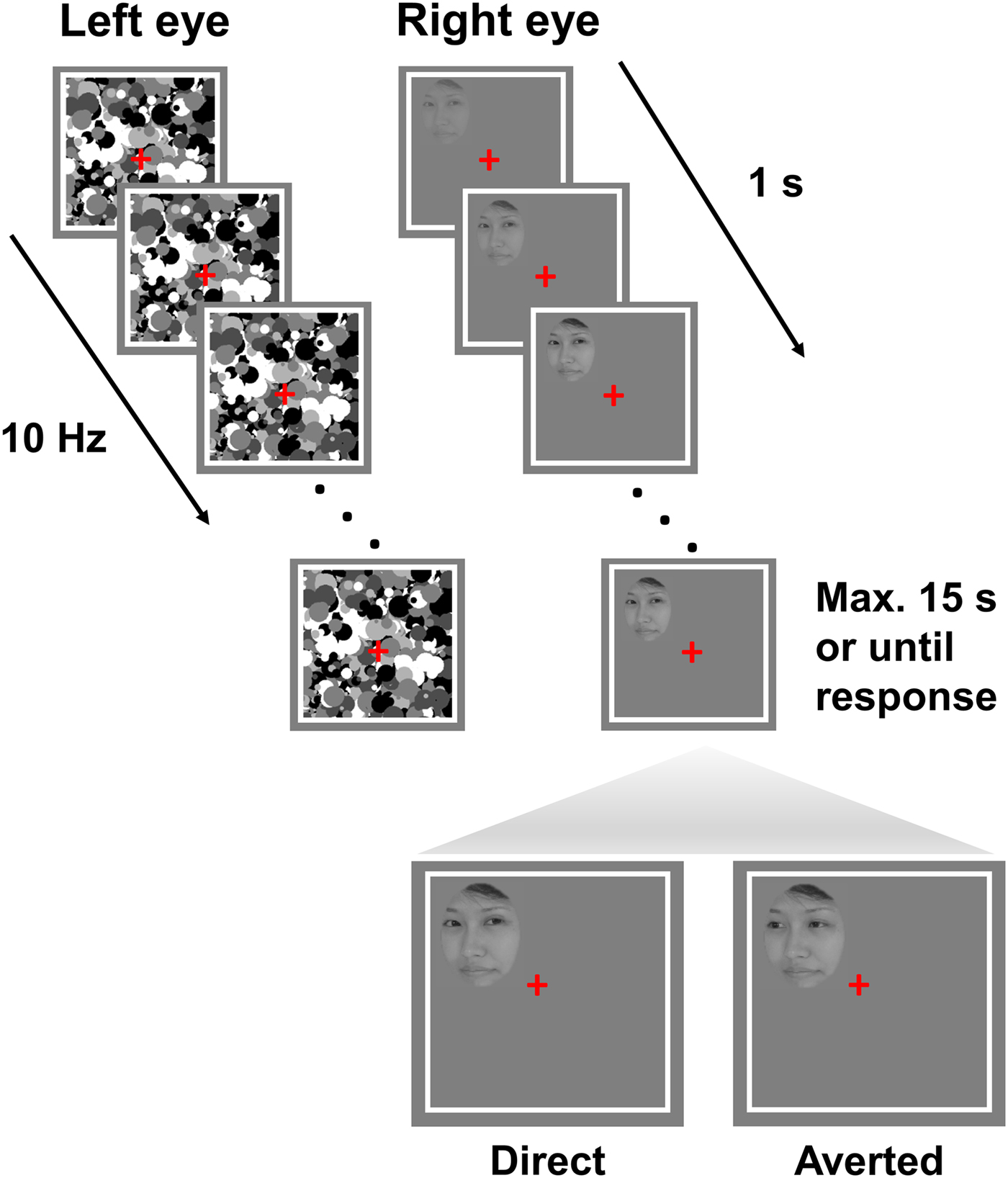
Each trial began with a 2 s presentation of white frames (12.0° × 12.0°) with a grey background and a red fixation cross (Fig. 1). Thereafter, high-contrast, grey scale, dynamic masks were flashed to a randomly selected eye at a frequency of 10 Hz, while simultaneously a face stimulus with either a direct or averted gaze was introduced to the other eye. The contrast of the face stimulus gradually increased from 0% to 100% within the first second from the start of the trial and remained at maximum contrast until a response was made or for a maximum of 15 s. The stimuli could be presented in one of the four quadrants within the white frame (3.4° horizontal displacement from the fixation cross and 3° vertical displacement). Participants completed a total of 48 trials. In experiment 1, participants used the keys F, J, V and N to indicate the quadrant in which the face appeared. For example, the key F corresponded to the upper left quadrant. In experiment 2, participants were provided with a button box with four buttons, each corresponding to a particular quadrant to indicate the location of the face. They were instructed to maintain fixation, to respond as fast and accurately as possible and to respond as soon as any part of the face became visible. Importantly, in both experiments, participants’ task (i.e. location discrimination) was orthogonal to the condition of interest (i.e. gaze direction of the presented faces). Participants were therefore unaware of the existence of two different gaze directions, which was irrelevant to the task. The eye to which the face stimulus and masks were presented were randomized and counterbalanced.
Fig. 1. Trial structure. Face stimuli with either direct or averted gaze were presented at random to one of the two eyes at one of the four quadrants of the white square. Participants fixated on the red cross and were instructed to respond as fast and accurately as possible as soon as they localized the stimulus. The contrast of the stimulus increased from 0% to 100% over the first second of the trial and remained at maximum either till participants made a response or for 15 s. Note that the stimuli are not drawn to scale.

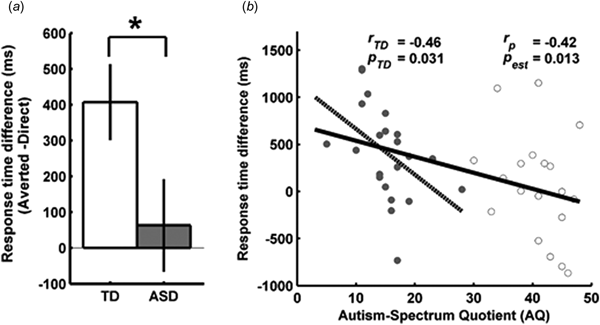
Fig. 2. Suppression time of direct and averted gaze faces and the relationship to autistic traits. (a) Access to awareness of direct gaze was significantly faster in the TD group than in the ASD group [t (39) = 2.09, p = 0.04], corroborating previous research. Error bars represent standard errors of the mean (Cousineau, Reference Cousineau2005). (b) There was a significant negative correlation between the AQ scores and the access to awareness. That is, participants with a higher sensitivity to direct gaze scored less on the AQ questionnaire. r TD and p TD and the dotted line refer to the values obtained and the regression line, respectively, when the correlation was performed only for the TD group. r p and p est refer to the values obtained from the linear regression model across groups for the factor AQ score. Filled circles represent the TD participants, while the unfilled circles represent the ASD participant. There was no overlap in the AQ scores between the two groups.
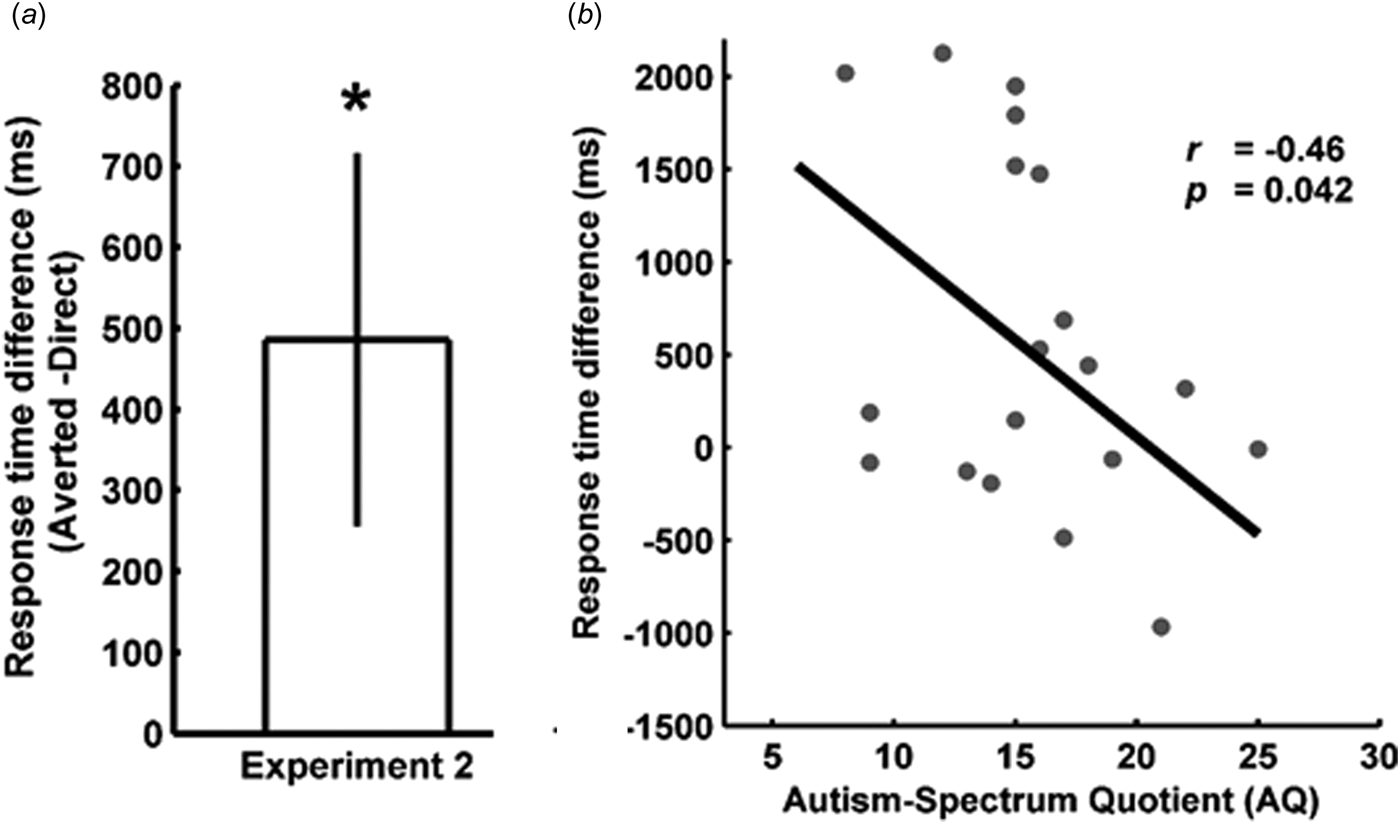
Fig. 3. Results of experiment 2. (a) In line with the literature and results of experiment 1, the response time difference was significantly >0 in experiment 2, indicating a preferential access to awareness of direct gaze in the typically developed (TD) participants. (b) Negative correlation between the sensitivity to direct gaze and the score on the autism questionnaire in an independent sample of TD participants, replicating the results of experiment 1.
At the end of the experiments, participants filled out the AQ questionnaire (Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001), which is a 50-item self-report questionnaire with 10 questions targeting each of five subdimensions comprising social skills, attention to detail, attention switching, communication and imagination. Participants indicated how strongly they agree or disagree based on a four-point rating scale. Depending on participants’ response, items were scored with a 1 or a 0 resulting in a score between 0 and 50. While the AQ itself is not employed as a diagnostic tool, it is used as an instrument to identify the extent of autistic traits in adults with typical intelligence and has good clinical validity (Woodbury-Smith et al., Reference Woodbury-Smith, Robinson, Wheelwright and Baron-Cohen2005). As such, a cut-off of 32 is used for identifying individuals with clinically significant levels of autistic traits (Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001).
Data analyses
The mean response time (RT), which indicates the access to awareness of the face stimuli, was computed for all correct responses. Trials in which participants failed to respond within 15 s were regarded as incorrect and excluded from analyses. In a first step, a 2 × 2 analysis of variance (ANOVA) with the factors gaze direction and group was performed on the raw RTs to test for significant differences in access to awareness between groups. In a second step, RTs were used to compute a RT difference, i.e. the difference between the RT to localize the face with averted gaze and direct gaze. Positive differences indicate a faster localization of the face with direct gaze, while zero would suggest no difference in localization speed.
Critically, the RT difference was correlated against the AQ scores to investigate whether interindividual differences in autistic traits provided by the self-report questionnaire are reflected in the localization of direct gaze. To this end, we performed a linear regression with the RT differences as the dependent variable and group and AQ scores as independent variables.
In experiment 2, mean RTs from all correct responses were again computed for direct and averted gaze and the RT difference was tested against 0 in a one-sample t test. In a second step, a linear regression was performed between the RT difference and the AQ scores of the TD participants.
Results
Participant characteristics
Detailed participant characteristics from experiment 1 are listed in Table 1. While participants from the two groups in experiment 1 did not differ regarding gender, age, verbal intelligence and attention, the mean AQ score of the ASD participants (40.8 ± 5.0 s.d.) was well above the cut-off of 32, while the TD participants had a mean AQ score of 15.3 ± 4.7 s.d.
In experiment 2, the mean AQ score of the TD group was 15.9 ± 1.0 s.e.m. Thus, in both experiments of the present study, the mean AQ score of the TD group was well below the cut-off of 32. There was no overlap in AQ scores between the ASD and TD participants in experiment 1, further underlining the discriminative ability of the questionnaire.
Behavioural data
In experiment 1, there was no significant difference in the proportion of correct trials between the TD (80.5 ± 3.2% s.e.m.) and the ASD group [71.9 ± 4.2% s.e.m.; (t (39) = 1.73, p = 0.09)].
This proportion of correct trials excludes both incorrect responses (TD: 4.4% ± 1.3 s.e.m. and ASD: 6.7% ± 1.6 s.e.m.) and missed responses (TD: 15.1% ± 3.3 s.e.m. and ASD: 21.4% ± 3.6 s.e.m.).
A 2 × 2 ANOVA with the factors gaze direction and group revealed significant main effects of gaze direction [F (1,39) = 8.05, p = 0.007] and group [F (1,39) = 4.83, p = 0.034]. The main effect of gaze direction was due to overall shorter RTs for direct (M = 4264.56 ms ± 323.38 s.e.m.) v. averted gaze (M = 4512.48 ms ± 327.26 s.e.m.), while the main effect of group resulted from overall faster RTs in the TD (M = 3759.44 ms ± 428.77 s.e.m.) compared with the ASD group (M = 5116.93 ms ± 441.99 s.e.m.). Importantly, there was a significant interaction effect between gaze and group [F (1,39) = 4.32, p = 0.04]. The TD group had a significant positive RT difference [M = 407.62 ms ± 105.96 s.e.m.; one sample t test against 0: t (21) = 3.85, p = 0.001] indicating that direct gaze facilitates localization during interocular suppression, a well-established effect (Stein et al., Reference Stein, Senju, Peelen and Sterzer2011; Chen and Yeh, Reference Chen and Yeh2012). In contrast, the ASD group did not show such a facilitated localization response for direct gaze [M = 62.99 ms ± 129.65 s.e.m.; one sample t test against 0: t (18) = 0.49, p = 0.63]. Such an absence of a preferential processing of direct gaze and a general reduced speed in the processing of face stimuli in autism is in line with the previous research (McPartland et al., Reference McPartland, Dawson, Webb, Panagiotides and Carver2004; Akechi et al., Reference Akechi, Stein, Senju, Kikuchi, Tojo, Osanai and Hasegawa2014).
Interestingly, a linear regression with the RT difference as the dependent variable and AQ scores and group as independent variables was significant [F (2,38) = 5.82, p = 0.006; R 2 = 0.24]. AQ scores significantly predicted the RT difference (β = −42.15, t = −2.59, p = 0.014), but group did not (t = 1.65, p = 0.11). To further explore this relation in participants without a clinical diagnosis of autism, we performed the correlation analysis for the control participants alone. This analysis still revealed a significant negative correlation between the AQ scores and the access to awareness of direct gaze (r = −0.46, t = −2.32, p = 0.031). There was no correlation between the RT difference and AQ scores in the ASD group (r = −0.32, t = −1.38, p = 0.19). This suggests that already at the subclinical level, differences in AQ scores are reflected in the localization of direct gaze. Participants with lower AQ scores have a greater sensitivity to direct gaze.
In order to corroborate the results of the correlation in a subclinical population, we repeated the experiment with an independent sample of 20 participants (experiment 2). Participants responded correctly in 88.9 ± 2.8% s.e.m. of all trials. Again, the RT difference was significantly >0 [M = 485.88 ms ± 230.42 s.e.m.; one sample t test against 0: t (19) = 2.10, p = 0.048], replicating the results of experiment 1 as well as previous studies (Stein et al., Reference Stein, Senju, Peelen and Sterzer2011; Chen and Yeh, Reference Chen and Yeh2012). Importantly, there was also a significant negative correlation between the access to awareness of direct gaze and the AQ scores (r = −0.46, t = −2.2, p = 0.042), confirming the results of experiment 1.
Finally, as the tasks in experiments 1 and 2 were the same, to increase statistical power, we pooled the data from the neurotypical controls across the two experiments. Study number was included as a covariate as the participants significantly differed in age between the two studies [t (40) = 4.9, p < 0.001]. The 2 × 2 ANOVA with the factors gaze direction and study still revealed a main effect of gaze direction [F (1,40) = 13.2, p = 0.001]. There was no significant interaction between gaze and study [F (1,40) = 0.10, p = 0.75]. Critically however, the linear regression with the RT difference as the dependent variable and AQ scores and study number as independent variables was significant [F (2,39) = 4.47, p = 0.018; R 2 = 0.19]. AQ scores significantly predicted the RT difference (β = −74.01, t = −3.0, p = 0.005), but study number did not (t = 0.55, p = 0.58), confirming that in individuals with subclinical levels of autistic traits, an increasing level of autistic traits is directly related to a decrease in the sensitivity to eye contact.
Discussion
We performed two independent experiments to investigate the extent to which individual levels of autistic traits in TD adults of normal intelligence are related to the awareness of others’ faces with direct v. averted gaze. In both experiments, we consistently observed significant negative correlations between participants’ sensitivity to direct gaze and their scores on the AQ questionnaire. This supports the continuum view of autism and provides the first evidence that subclinical differences in the level of autistic traits in TD adults are reflected in their sensitivity to direct gaze: with an increasing level of autistic traits, sensitivity to eye contact with other people decreases.
The notion of a ‘broad autism phenotype’ posits that the symptomatology related to ASD occurs to some extent in the general population and is especially prominent in the social domain (Constantino and Todd, Reference Constantino and Todd2003). In experiment 1 of our study, both clinically diagnosed participants with ASD and a control group of TD participants were required to detect faces with direct or averted gaze. In line with the literature on gaze processing, faces initially suppressed from awareness were detected faster when they were looking at the observer in the TD group (Stein et al., Reference Stein, Senju, Peelen and Sterzer2011; Chen and Yeh, Reference Chen and Yeh2012), but not in the ASD group (Akechi et al., Reference Akechi, Stein, Senju, Kikuchi, Tojo, Osanai and Hasegawa2014). We further observed a linear relationship between the preferential access to awareness of direct gaze, i.e. the difference between the time to detect a face with direct v. averted gaze, and autistic traits across the whole group. This relationship was predicted by the factor AQ scores and not group. In addition, within the sample of TD participants, there was a significant negative correlation between the access to awareness of direct gaze and autistic traits. This suggests that the responsiveness or sensitivity to direct gaze is already reduced in individuals showing subclinical levels of autistic symptoms. Critically, this linear relationship between the levels of autistic traits and the sensitivity to direct gaze was replicated in an independent sample of TD participants in experiment 2. In line with the notion of a ‘broad autism phenotype’, our results thus show that the reduced sensitivity to direct gaze in autism extends to the general population. It is important to note that the relationship to AQ scores is based on the reaction time differences for detecting a direct v. averted gaze direction. Therefore, it is difficult to disambiguate on the basis of our current data whether autistic traits are associated with reduced processing of direct gaze or whether there is also a component of facilitated processing of averted gaze. This exact influence of autistic traits on gaze processing remains an open question for future research.
The recognition of others’ gaze direction is highly relevant in social contexts, as it indicates others’ focus of attention. While individuals with autism exhibit atypical responses to others’ eye gaze, especially reduced responses to direct gaze (Senju and Johnson, Reference Senju and Johnson2009), individuals with high, but subclinical levels of autistic traits show similar impairments in their responsiveness to others’ gaze direction. For instance, individuals with high autistic traits have a diminished tendency to reciprocate direct gaze in comparison with individuals with low autistic traits (Chen and Yoon, Reference Chen and Yoon2011). Moreover, attentional biases triggered by the eye gaze of other people's faces are less strong in individuals with high autistic traits (Bayliss and Tipper, Reference Bayliss and Tipper2005; Hudson et al., Reference Hudson, Nijboer and Jellema2012; Zhao et al., Reference Zhao, Uono, Yoshimura and Toichi2015). In the present study, we show that autistic traits are already related to an earlier stage in the processing of gaze directions, namely to the time point when the stimulus reaches visual awareness. The preferential access to the awareness of direct gaze usually observed in TD individuals implies an early differentiation between direct and averted gaze, presumably because direct gaze is potentially more relevant as it signals that someone's attention is directed to oneself. The reduction of this preference for direct gaze that we observed in participants with high autistic traits could thus underlie the aforementioned decreased attentional responses to eye gaze in this group of individuals. Importantly, in the present study, participants performed a localization task, in which the eye gaze direction was irrelevant. Thus, the observed results are unlikely due to top-down-related processes, for example, participants intentionally looking for direct gaze or avoiding it. Moreover, the ability to rapidly detect and discriminate others’ eye gaze may even form the basis for more complex decisions in social contexts, since inferences about mental states of others rely on simple facial cues such as eye gaze (Frith and Frith, Reference Frith and Frith2006). In line with this notion, individuals with high autistic traits show less pro-social behaviour (Jameel et al., Reference Jameel, Vyas, Bellesi, Roberts and Channon2014) and experience less enjoyment from social rewards (Foulkes et al., Reference Foulkes, Bird, Gökçen, McCrory and Viding2015). Future studies could thus investigate the degree to which the awareness of eye gaze predicts complex social behaviour to further elucidate the role of autistic traits in social interactions.
The neural processing of faces and eye gaze in particular is associated with activations in a widespread brain network. Higher levels of autistic traits are related to functional as well as structural alterations in this network. For instance, altered neural responses to eye gaze have been observed in the superior temporal sulcus (STS), amygdala and intraparietal sulcus in individuals with high autistic traits (Nummenmaa et al., Reference Nummenmaa, Engell, von dem Hagen, Henson and Calder2012). Similarly, unaffected siblings of individuals with autism show reduced responses in the fusiform face area to face stimuli (Dalton et al., Reference Dalton, Nacewicz, Alexander and Davidson2007). Moreover, autistic traits are negatively correlated with white matter volume in the amygdala and STS (Dalton et al., Reference Dalton, Nacewicz, Alexander and Davidson2007; von dem Hagen et al., Reference von dem Hagen, Nummenmaa, Yu, Engell, Ewbank and Calder2011). Interestingly, these brain regions are critically involved in the awareness of eye gaze (Madipakkam et al., Reference Madipakkam, Rothkirch, Guggenmos, Heinz and Sterzer2015). It is thus conceivable that in these regions the initial processing of eye gaze before it enters awareness is less efficient in individuals with higher autistic traits, which eventually weakens the priority of direct gaze. It has been proposed that especially the STS may adopt an important role for the interpretation of communicative signals and the development of social skills (Redcay, Reference Redcay2008). Impairments in the ability to detect or respond to social signals as a component of autistic traits may thus especially be related to a dysfunction of the STS.
The results of the current study could aid the search for biomarkers for the early diagnosis of autism. In infants, attentional biases to faces as well as the neural sensitivity to eye gaze have already proved to be indicative of a later diagnosis of autism (Katarzyna et al., Reference Katarzyna, Fred and Ami2010; Pierce et al., Reference Pierce, Conant, Hazin, Stoner and Desmond2011; Elsabbagh et al., Reference Elsabbagh, Mercure, Hudry, Chandler, Pasco, Charman, Pickles, Baron-Cohen, Bolton and Johnson2012). In a similar vein, the current findings could help in the early detection of individuals who are at a risk for the disorder given its high heritability (Kanner, Reference Kanner1943; Folstein and Rutter, Reference Folstein and Rutter1977). In addition to the previous reports, the task employed in the present study was also sensitive to subclinical levels of autistic traits. To what extent this pronounced sensitivity to the whole spectrum of autistic characteristics can contribute to a further improvement of the early detection of autism remains an area for future research. In psychiatry, the notion that psychotic symptoms lie on a continuum is now a widely accepted concept (David, Reference David2010). In the field of autism, this is a more recent development. The continuum view helps to shift the diagnosis of the disorder from a categorical to a more dimensional approach. The current results highlight the fact that covert differences in gaze processing could be present before the more overt difficulties in social interactions in individuals who might later be diagnosed with ASD. The design of the current study has several advantages that could enable its administration in a young population. Firstly, the task is extremely simple and does not require verbal skills enabling pre-literate participants to perform it. Secondly, the task can be successfully administered in <10 min as the differences in the processing of gaze direction seem to be robust. Finally, the task does not require a very complicated set-up to be administered. While the current study used a mirror stereoscope for dichoptic stimulus presentation and Matlab as presentation software, other methods like red–green anaglyph glasses (Tong et al., Reference Tong, Meng and Blake2016) and other software can be used. An earlier diagnosis and intervention could help in the treatment outcomes of these individuals who show subtle impairments in social functioning before a full clinical diagnosis.
Acknowledgements
This work was supported by the German Research Foundation [(DFG) to P.S. & A.R.M, grant number STE 1430/7-1, R.O., grant number RO 4836/2-1]; Cluster of Excellence Neurocure to A.R.M. (EXC 257) and the Berlin School of Mind and Brain to I.D.
Conflict of interest
None.