Introduction
Dementia with Lewy bodies (DLB) has been reported as being the second most common dementia subtype in older people following Alzheimer's disease (AD) (Geser et al. Reference Geser, Wenning, Poewe and McKeith2005; Zupancic et al. Reference Zupancic, Mahajan and Handa2011). Nevertheless, DLB remains under-diagnosed, with more than 50% of cases missed (Palmqvist et al. Reference Palmqvist, Hansson, Minthon and Londos2009). Accurate diagnosis is important for appropriate disease-specific management and for service development; for example, DLB has a different symptom profile and course compared to other dementias, a differential response to cholinesterase inhibitors and a greatly increased susceptibility to severe, adverse reactions to neuroleptic medication (Ballard et al. Reference Ballard, Grace, McKeith and Holmes1998; Wilcock, Reference Wilcock2003).
Those with DLB also suffer from higher mortality and use more resources than those with AD of similar severity (Williams et al. Reference Williams, Xiong, Morris and Galvin2006; Bostrom et al. Reference Bostrom, Jonsson, Minthon and Londos2007). This is a result of greater functional impairment and increased risk of falls due to a combination of cognitive impairment, extrapyramidal symptoms and autonomic dysfunction (McKeith et al. Reference McKeith, Rowan, Askew, Naidu, Allan, Barnett, Lett, Mosimann, Burn and O'Brien2006; Hanyu et al. Reference Hanyu, Sato, Hirao, Kanetaka, Sakurai and Iwamoto2009; Sonnesyn et al. Reference Sonnesyn, Nilsen, Rongve, Nore, Ballard, Tysnes and Aarsland2009). Caregiver burden and distress are also greater in DLB when compared to AD (Ricci et al. Reference Ricci, Guidoni, Sepe-Monti, Bomboi, Antonini, Blundo and Giubilei2009). This is probably due to worsening function and the combination of motor and cognitive problems, along with pronounced behavioural disturbances, hallucinations and sleeping difficulties, consequently leading to greater dependence earlier in the course of disease (Ferman et al. 2004; Galvin et al. Reference Galvin, Duda, Kaufer, Lippa, Taylor and Zarit2010). A clearer recognition of the number of patients with DLB is important to inform appropriate resource allocation by health-care bodies and assist with the delivery of local services.
The only previous systematic review of epidemiological studies identified just seven population prevalence and incidence studies and showed a wide variation in the prevalence of DLB of 0–30% of the dementia population (Zaccai et al. Reference Zaccai, McCracken and Brayne2005). However, since that review there has been considerably heightened awareness of the clinical syndrome of DLB, with more studies including this as a subtype of dementia to be assessed for, and International Consensus Criteria (ICC) for DLB have been revised in an attempt to improve case detection (McKeith et al. Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005). The previous distinction between probable and possible DLB is retained. Probable DLB refers to a clinical syndrome that has been well validated against autopsy and has a high positive predictive value (>75%) (Litvan et al. Reference Litvan, Bhatia, Burn, Goetz, Lang, McKeith, Quinn, Sethi, Shults and Wenning2003). Possible DLB is a more uncertain category, with the positive predictive value less clearly established but more likely to be ⩽50% (O'Brien et al. Reference O'Brien, McKeith, Walker, Tatsch, Booij, Darcourt, Marquardt, Reininger and Group2009). The aims of the current study were to (i) provide an updated review including all recently published prevalence and incidence studies; (ii) examine the impact of the introduction of the new DLB diagnostic criteria on prevalence rates; and (iii) compare prevalence estimates from population samples to those from clinical settings. Although the latter are susceptible to referral bias, further knowledge about referral pathways and frequency for those with DLB is important information when planning secondary care services.
Method
A literature search was undertaken in April 2012 using the online medical database PubMed. PubMed was chosen as it provides free access to over 6000 journals containing more than 1 million research papers. Crucially this also includes e-articles prior to print, a function that is not offered by other commonly cited databases such as Scopus or Web of Science (Falagas et al. Reference Falagas, Pitsouni, Malietzis and Pappas2007). By sifting through the references of the articles that we read in full, we were confident that we were unlikely to miss studies that may not have been identified in our original search. Papers were limited to English language only with no time restrictions. The following combinations of keywords were used:
-
(1) ‘dementia’ AND ‘lewy’ AND [‘incidence’ OR ‘prevalence’] (543 articles)
-
(2) ‘dementia’ AND ‘subtypes’ AND [‘incidence’ OR ‘prevalence’] (336 articles)
-
(3) ‘dementia’ AND ‘door-to-door’ (75 articles)
From our extensive search of community-based prevalence and incidence studies we found that the term ‘door-to-door’, used to describe the method of data collection, was common in abstracts and titles and a good way of capturing epidemiological studies. Using this term in a PubMed search uncovered papers not identified by our original search terms.
All titles and abstracts were sieved independently by two authors for suitability for inclusion and a list of papers potentially meeting study inclusion criteria was created. Full papers were then read and those meeting study criteria were selected for inclusion. The bibliographies of selected papers were examined for evidence of references not identified by the aforementioned database search. Those that met the selection criteria were added to the final list. These papers form the basis of this review.
Inclusion criteria
Population-based studies
To warrant inclusion, papers were required to describe an original epidemiological study using a two-step approach to prevalence estimates for DLB or dementia subtypes including DLB. This meant first assessing all members of the population or randomly selected population sample for evidence of cognitive impairment and then following up positive results to classify their impairment into either mild cognitive impairment (MCI) or a dementia subtype. Incidence studies followed up the dementia-free cohort for evidence of emerging disease. A defined ‘at risk’ population was a requirement, that is over a certain age bracket (usually 65), and the study needed to involve either the total at-risk population or a non-biased, random sample.
Clinical (secondary care) studies
Studies involving consecutive referrals, which included all new cases of dementia and where the explicit aim was to identify the prevalence of dementia subtypes, were included. The use of imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI) or single-photon emission computed tomography (SPECT) was allowed but did not form part of the inclusion criteria. Retrospective studies were included providing diagnosis was made according to standardized clinical criteria.
Diagnostic criteria
Each included study required the use of a validated diagnostic tool, such as the Mini Mental State Examination (MMSE), in the first phase as part of the screening process to identify potential cognitive impairment. The MMSE is globally probably the most widely used tool for assessing cognition although it should be acknowledged that it is likely to miss early cases of dementia, especially non-amnestic presentations, which are common in DLB (Mitchell, Reference Mitchell2009), and the fluctuating course of DLB arguably makes it more likely to be missed than other dementias.
During the second stage, a formal diagnosis of dementia needed to be in accordance with DSM-III-R or DSM-IV following a thorough clinical history and examination by at least one recognized medical professional, usually a neurologist, psychiatrist or geriatrician.
To determine the type of dementia on a case-by-case basis, each study was required to have used recognized diagnostic criteria for each subtype and to explicitly mention the use of DLB diagnostic criteria (either the original 1996 ICC or the revised 2005 criteria; McKeith et al. Reference McKeith, Galasko, Kosaka, Perry, Dickson, Hansen, Salmon, Lowe, Mirra, Byrne, Lennox, Quinn, Edwardson, Ince, Bergeron, Burns, Miller, Lovestone, Collerton, Jansen, Ballard, deVos, Wilcock, Jellinger and Perry1996, Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005) for diagnosing or ruling out DLB as the final dementia subtype.
Exclusion criteria
Studies based entirely on autopsy collections were excluded.
Statistical analysis
Confidence intervals (CIs) were calculated using the Clopper–Pearson method.
Systematic review
The search for papers was conducted following guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to ensure quality in systematic reviews (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009). The PRISMA flow diagram summarizes the search (Fig. 1).

Fig. 1. Systematic review: the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Results
Of the 707 abstracts identified by our search, 46 individual papers were selected as a being potentially relevant. A further 28 papers were added following a thorough search of the references from these papers.
A total of 18 population prevalence and three population incidence papers were identified. Of these, seven had been included in the review by Zaccai et al. (Reference Zaccai, McCracken and Brayne2005). In addition, 10 clinical prevalence studies were found to meet the inclusion criteria.
The main reason for non-inclusion of studies was the absence of DLB criteria in identifying different dementia subtypes. A significant number of papers using solely autopsy collections were rejected because they used neuropathology as the sole determinant in making a diagnosis, giving rise to the problem of selection bias of autopsy collections. One paper was rejected for failing to use a standardized screening tool for identifying cognitive impairment (Shaji et al. Reference Shaji, Iype and Anandan2002).
Diagnostic criteria
Diagnostic criteria used in population- and clinic-based prevalence studies are shown in Table 1, along with diagnostic criteria used in population incidence studies.
Table 1. Diagnostic criteria used in (a) population- and clinic-based prevalence studies and (b) population incidence studies

Population prevalence
The mean prevalence of DLB in the whole population over 65 was 0.36% or one in 270 people, with a wide range from zero to 21.9% (Table 2). Of those with dementia, 4.2% were found to have DLB. This equates to one in 24 cases. There was a wide variation in prevalence rates across studies. Among the more recent (>2005) studies ,the prevalence of DLB in the over-65 population ranged from zero to 1.2% and from zero to 9.7% of those with dementia. This is a narrower range than the previously reported prevalence of 0–5% of the general population (over 65 years of age) and 0–22.8% of those with dementia (Zaccai et al. Reference Zaccai, McCracken and Brayne2005). Only two studies included possible and also probable DLB cases (Stevens et al. Reference Stevens, Livingston, Kitchen, Manela, Walker and Katona2002; Gascon-Bayarri et al. Reference Gascon-Bayarri, Rene, Del Barrio, De Pedro-Cuesta, Ramon, Manubens, Sanchez, Hernandez, Estela, Juncadella and Rubio2007); these reported an increase in DLB diagnoses from 9.7% to 30.5% and from 9.1% to 12.7% of all dementias respectively, once possible cases were added to probable ones.
Table 2. Population-based prevalence studies
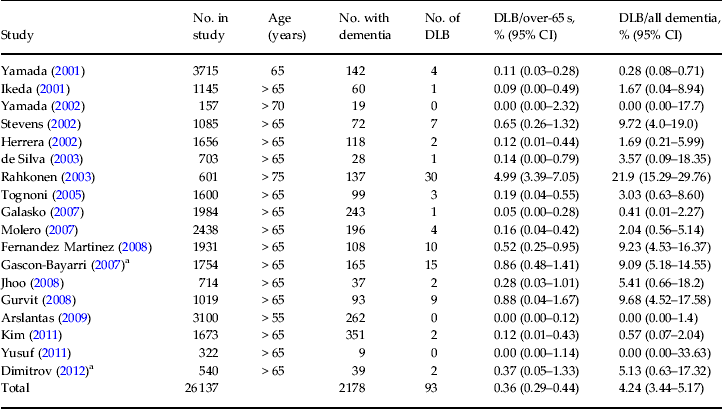
DLB, Dementia with Lewy bodies; CI, confidence interval.
a Used the revised 2005 criteria.
Population incidence
Annual incidence rates for DLB were found to be 3.8% (range 3.2–4.5%) of new dementia diagnoses and 0.87 (range 0.57–1.4) cases/1000 person-years (Table 3).
Table 3. Population-based incidence studies

DLB, Dementia with Lewy bodies.
a Used the revised 2005 criteria.
Clinical prevalence
The mean prevalence of DLB in clinical (secondary care) populations from the 10 included studies was 7.5%, or one in 13 patients with dementia (Table 4). Prevalence varied from 2.2% to 24.7% of all dementia cases and was overall significantly higher than the proportion of cases identified in population studies (χ 1 2 = 23.38, p < 0.001). This represents a 47–106% (95% CI) increase in the proportion of dementia cases with DLB in secondary care compared to the general population.
Table 4. Clinic-based prevalence studies

DLB, Dementia with Lewy bodies; n.a., not available.
a Used the revised 2005 criteria.
Probable versus possible DLB
The mean prevalence for all included studies is a reflection of probable DLB cases only. However, four papers also provided prevalence rates for possible DLB (Stevens et al. Reference Stevens, Livingston, Kitchen, Manela, Walker and Katona2002; Yokota et al. Reference Yokota, Sasaki, Fujisawa, Takahashi, Terada, Ishihara, Nakashima, Kugo, Ata, Ishizu and Kuroda2005; Gascon-Bayarri et al. Reference Gascon-Bayarri, Rene, Del Barrio, De Pedro-Cuesta, Ramon, Manubens, Sanchez, Hernandez, Estela, Juncadella and Rubio2007; Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008). Not surprisingly, all reported a substantial increase in cases when possible cases were also included in the DLB subcategory (Fig. 2). Only one of these papers used the revised 2005 criteria (Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008). It is important for all future studies to provide data separately for possible and probable cases to facilitate cross-study comparison.

Fig. 2. Probable versus probable + possible dementia with Lewy bodies (DLB) in the same study.
Comparing the revised 2005 ICC with the original 1996 criteria
Population prevalence studies that used the 2005 criteria reported a mean prevalence of 8.2% compared to 3.7% among those who used the 1996 criteria, a finding that was statistically significant (χ 1 2 = 5.96, p = 0.01). Clinical prevalence studies using the 2005 diagnostic criteria reported an average prevalence of 11.4% versus 6.7% in those who had used the 1996 criteria. Again this was a significant difference (χ 1 2 = 14.76, p = 0.001).
Consistent with these findings, the only study that directly compared and contrasted the 1996 and 2005 criteria on the same sample found a 25% increase in probable DLB cases identified (Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008). The two most recent incidence studies both used the 2005 criteria (Matsui et al. Reference Matsui, Tanizaki, Arima, Yonemoto, Doi, Ninomiya, Sasaki, Iida, Iwaki, Kanba and Kiyohara2009; Perez et al. Reference Perez, Helmer, Dartigues, Auriacombe and Tison2010) and found a 35–40% increase in DLB compared to the only previous incidence study (1996 criteria), although this was not statistically significant (χ 1 2 = 0.47, p = 0.49).
Gender differences
Eight prevalence studies included the gender of those diagnosed with DLB. Of these, five reported disproportionately more females with the disease when controlling for the gender of the sample population (Rahkonen et al. Reference Rahkonen, Eloniemi-Sulkava, Rissanen, Vatanen, Viramo and Sulkava2003; Yokota et al. Reference Yokota, Sasaki, Fujisawa, Takahashi, Terada, Ishihara, Nakashima, Kugo, Ata, Ishizu and Kuroda2005; Gascon-Bayarri et al. Reference Gascon-Bayarri, Rene, Del Barrio, De Pedro-Cuesta, Ramon, Manubens, Sanchez, Hernandez, Estela, Juncadella and Rubio2007; Jhoo et al. Reference Jhoo, Kim, Huh, Lee, Park, Lee, Choi, Han, Choo, Youn, Lee and Woo2008; Yoshida et al. Reference Yoshida, Terada, Honda, Ata, Takeda, Kishimoto, Oshima, Ishihara and Kuroda2011) and three reported disproportionately more males (Yamada et al. Reference Yamada, Hattori, Miura, Tanabe and Yamori2001; Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008; Alladi et al. Reference Alladi, Mekala, Chadalawada, Jala, Mridula and Kaul2011) (Fig. 3). The only incidence study to report on gender found 58.6% of DLB cases to be female but did not report the gender distribution of the original sample (Perez et al. Reference Perez, Helmer, Dartigues, Auriacombe and Tison2010).

Fig. 3. Gender distribution of dementia with Lewy bodies (DLB). The figure shows the proportion of the population who are female compared to the proportion of DLB cases that are female.
Age and DLB
Of the 11 population samples that included an average age of participants, there was a positive correlation between age and the proportion of DLB compared to all dementia, although this was not found to be significant (R 2 of 0.25, p = 0.45) (Fig. 4). There was no correlation between age and prevalence in clinical studies (R 2 = −0.0004, p = 0.9) (Fig. 5).

Fig. 4. Population prevalence of dementia with Lewy bodies (DLB) among people with dementia. Relationship to mean sample age (Stevens et al. Reference Stevens, Livingston, Kitchen, Manela, Walker and Katona2002; de Silva et al. Reference de Silva, Gunatilake and Smith2003; Tognoni et al. Reference Tognoni, Ceravolo, Nucciarone, Bianchi, Dell'Agnello, Ghicopulos, Siciliano and Murri2005; Galasko et al. Reference Galasko, Salmon, Gamst, Olichney, Thal, Silbert, Kaye, Brooks, Adonay, Craig, Schellenberg and Borenstein2007; Gascon-Bayarri et al. Reference Gascon-Bayarri, Rene, Del Barrio, De Pedro-Cuesta, Ramon, Manubens, Sanchez, Hernandez, Estela, Juncadella and Rubio2007; Molero et al. Reference Molero, Pino-Ramirez and Maestre2007; Fernandez Martinez et al. Reference Fernandez, Castro, Perez de las, Mandaluniz, Gordejuela and Zarranz2008; Gurvit et al. Reference Gurvit, Emre, Tinaz, Bilgic, Hanagasi, Sahin, Gurol, Kvaloy and Harmanci2008; Jhoo et al. Reference Jhoo, Kim, Huh, Lee, Park, Lee, Choi, Han, Choo, Youn, Lee and Woo2008; Yusuf et al. Reference Yusuf, Baiyewu, Sheikh and Shehu2011; Dimitrov et al. Reference Dimitrov, Tzourio, Milanov, Deleva and Traykov2012).

Fig. 5. Clinical prevalence of dementia with Lewy bodies (DLB) among people with dementia. Relationship to mean sample age (Chan et al. Reference Chan, Chiu, Lam and Leung2002; Harvey et al. Reference Harvey, Skelton-Robinson and Rossor2003; Sambrook et al. Reference Sambrook, Herrmann, Hebert, McCracken, Robillard, Luong and Yu2004; Yokota et al. Reference Yokota, Sasaki, Fujisawa, Takahashi, Terada, Ishihara, Nakashima, Kugo, Ata, Ishizu and Kuroda2005; Shinagawa et al. Reference Shinagawa, Ikeda, Toyota, Matsumoto, Matsumoto, Mori, Ishikawa, Fukuhara, Komori, Hokoishi and Tanabe2007; Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008; Alladi et al. Reference Alladi, Mekala, Chadalawada, Jala, Mridula and Kaul2011; Yoshida et al. Reference Yoshida, Terada, Honda, Ata, Takeda, Kishimoto, Oshima, Ishihara and Kuroda2011).
Discussion
The impact of the revised (2005) ICC for diagnosis
Both population-based and clinical studies conducted using the revised 2005 diagnostic criteria for DLB reported significantly higher prevalence rates than those using the original 1996 criteria. This increase in diagnoses was also seen in incidence studies, although this was not statistically significant, most probably because of the much smaller number of incidence studies that have been reported.
There may be alternative explanations for the higher prevalence rates in studies using the revised criteria. DLB has received greater recognition in recent years and this increase in awareness arguably makes case identification more likely in the more recent studies, which used the revised criteria. However, we found no evidence to support this explanation as several of those studies continued to use the original criteria yet showed no link between recentness and greater detection rates. It could also be argued that the methodology was better designed to detect DLB in more recent papers using the revised 2005 criteria, although in the absence of universally detailed descriptions of the thoroughness of the diagnostic process, this is difficult to compare. The statistical and empirical evidence that the 2005 guidelines improve detection rates suggests that the revised criteria are indeed more sensitive and that it would be preferable for all future epidemiological studies to use the revised criteria to improve the accuracy of estimates.
Methodological issues: DLB as the focus of the study
The majority of papers aimed to identify dementia prevalence and then categorized this by subtype. Only in four prevalence papers (three clinic-based, one population-based) was the identification of DLB prevalence the primary aim of the study (Londos et al. Reference Londos, Passant, Brun and Gustafson2000; Chan et al. Reference Chan, Chiu, Lam and Leung2002; Rahkonen et al. Reference Rahkonen, Eloniemi-Sulkava, Rissanen, Vatanen, Viramo and Sulkava2003; Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008). Of these, three provided a detailed description of the neurological examination including explicit mention of extrapyramidal signs (Londos et al. Reference Londos, Passant, Brun and Gustafson2000; Rahkonen et al. Reference Rahkonen, Eloniemi-Sulkava, Rissanen, Vatanen, Viramo and Sulkava2003; Aarsland et al. Reference Aarsland, Rongve, Nore, Skogseth, Skulstad, Ehrt, Hoprekstad and Ballard2008). Prevalence rates in these three studies were high with a narrow range of 19.9–24.9%, suggesting that looking for specific evidence of DLB greatly increases the diagnostic yield. The guidelines for DLB require a thorough examination for extrapyramidal signs and accurate reporting of fluctuations as a minimum. Few papers provided this level of detail in their methods.
Sample age
The mean age of onset of DLB is reported to be 75 years (Barber et al. Reference Barber, Panikkar and McKeith2001), which may increase the likelihood of underestimating DLB when only younger samples are studied. One large study (n = 3100) that did not identify any cases of DLB reported that 50.7% of dementia cases were vascular and 48% AD. These atypical findings may reflect the sample age because 73% were under 70 and over 50% were under 65 (Arslantas et al. Reference Arslantas, Oezbabalik, Metintas, Oezkan, Kalyoncu, Oezdemir and Arslantas2009). Our findings showed a positive correlation between sample age and proportion of the dementia population with DLB. Although this was not found to be statistically significant, it should be further explored. Average age should be a mandatory reporting requirement in future studies and stratifying prevalence by age group would also be useful for more accurately establishing age-related patterns of disease.
Clinic versus population studies
The mean prevalence of DLB in secondary care is 47–105% higher than in the community. This finding may give us some insight into referral patterns. It would seem that individuals with DLB are more likely to be referred to secondary care than those suffering from other dementias. This may be due to the increased burden of symptoms, and greater resource use seen by those with DLB (Ballard et al. Reference Ballard, Grace, McKeith and Holmes1998; Wilcock, Reference Wilcock2003). However, this difference may also be explained by the difficulty of accurately differentiating between dementias in large community studies. Detecting clinical differences between AD, DLB and pervasive developmental disorders (PDD) is a challenge for physicians, but clinical differentiation is possible with careful assessment of attention, episodic memory and executive function (Kim et al. Reference Kim, Park, Kim, Kim, Kim, Kim, Kim, Moon, Bae, Woo, Ryu, Yoon, Lee, Lee, Lee, Lee, Lee, Lee, Lee, Chang, Jhoo and Cho2011). This is more realistically achievable in the clinic than in the community (Chan et al. Reference Chan, Chiu, Lam and Leung2002). Despite vulnerability to referral bias, the use of clinical studies is therefore still of benefit in estimating population prevalence and should be encouraged. Furthermore, population studies need to be large enough to represent the population but not too large that thorough assessment is not practicable (Barker & Foltynie, Reference Barker and Foltynie2003). We initially used the Kish population prevalence sample size calculation to suggest a sample size for future studies (Kish, Reference Kish1965). Assuming a prevalence of 0.36% in the population and a 95% CI, we calculated the necessary sample size to be greater than 4000. This is beyond the scope of most studies and we would therefore favour the more feasible approach of the 10/66 study group, which recommends that dementia prevalence studies involve a sample of between 1000 and 3000 people (Prince et al. Reference Prince, Ferri, Acosta, Albanese, Arizaga, Dewey, Gavrilova, Guerra, Huang, Jacob, Krishnamoorthy, McKeigue, Rodriguez, Salas, Sosa, Sousa, Stewart and Uwakwe2007).
Gender differences
AD is more prevalent in women with dementia than men (67% v. 55%) whereas vascular dementia and mixed dementias are more common in men (31% v. 25%) (Knapp et al. Reference Knapp, Prince, Albanese, Banerjee, Dhanasiri, Fernandez, Ferri, McCrone, Snell and Stewart2007). Parkinson's disease dementia is more common in men (Mayeux et al. Reference Mayeux, Denaro, Hemenegildo, Marder, Tang, Cote and Stem1992) and, to date, it has generally been accepted that DLB is also more common in males than females (Klatka et al. Reference Klatka, Louis and Schiffer1996). The reason for these gender differences is still unclear but it is noteworthy that a previously reported male predominance in DLB was not supported by many of the studies reported here. This may reflect a lack of sex difference or the differences between cohort studies, where the male predominance had been identified, and more representative samples. These findings challenge the gender stereotype and should help to increase the likelihood of accurate diagnosis on a case-by-case basis. Future studies should report the gender of those with DLB.
Conclusions
From published studies, we found the prevalence of DLB to be 4.2% of all dementia cases in the community, with significantly higher rates (approximately 50% more) seen in clinical (secondary care) samples, either reflecting the increased morbidity and carer burden on the DLB syndrome compared to other dementias or more accurate diagnosis in secondary care settings. There was evidence that use of the revised DLB diagnostic criteria do indeed identify a greater number of clinical cases, although ultimately external validation of the diagnostic accuracy of cases (e.g. through an independent consensus panel or autopsy evidence) will be needed to confirm this. The sex distribution is uncertain, with several studies challenging the widely held view that DLB is more common in males. We recommend that future studies investigating prevalence and incidence of DLB use the revised ICC for diagnoses, always report on sex distribution, report separate estimates for possible and probable DLB cases and always include standardized measures of psychiatric symptoms, fluctuation and motor symptoms as provided in the 2005 consensus criteria (McKeith et al. Reference McKeith, Dickson, Lowe, Emre, O'Brien, Feldman, Cummings, Duda, Lippa, Perry, Aarsland, Arai, Ballard, Boeve, Burn, Costa, Del Ser, Dubois, Galasko, Gauthier, Goetz, Gomez-Tortosa, Halliday, Hansen, Hardy, Iwatsubo, Kalaria, Kaufer, Kenny, Korczyn, Kosaka, Lee, Lees, Litvan, Londos, Lopez, Minoshima, Mizuno, Molina, Mukaetova-Ladinska, Pasquier, Perry, Schulz, Trojanowski and Yamada2005). Examples of these include the Neuropsychiatric Inventory (Cummings et al. Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein1994), the Clinical Assessment of Fluctuation Scale (Walker et al. Reference Walker, Ayre, Cummings, Wesnes, McKeigh, O'Brien and Ballard2000), and the Unified Parkinson's Disease Rating Scale modified for those with dementia (Ballard et al. Reference Ballard, McKeith, Burn, Harrison, O'Brien, Lowery, Campbell, Perry and Ince1997). Future studies also need to strike a balance between sample size and the ability to perform thorough clinical histories and physical examinations on every participant. Until this optimum number has been agreed, it will continue to be useful to support population prevalences with those from clinically referred samples.
Acknowledgements
This research was supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre and the Biomedical Research Unit in Lewy Body Dementia based at Newcastle upon Tyne Hospitals National Health Service (NHS) Foundation Trust and Newcastle University and the NIHR Biomedical Research Centre and Biomedical Research Unit in Dementia based at Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declaration of Interest
J. O'Brien has acted as a consultant for GE Healthcare and Bayer Healthcare.











