Introduction
The birth of the first cloned mammal, Dolly the sheep, by somatic cell nuclear transfer (SCNT) was 2 decades ago (Wilmut et al., Reference Wilmut, Schnieke, McWhir, Kind and Campbell1997). During these past years, many different mammalian species have been cloned using SCNT [summarized by Wadman (Reference Wadman2007) and Check (Reference Check2007)]. Rescue of endangered animals can also be performed using SCNT, this process is called interspecies SCNT (iSCNT; Loi et al., Reference Loi, Ptak, Barboni, Fulka, Cappai and Clinton2001; Gomez et al., Reference Gomez, Pope, Giraldo, Lyons, Harris, King, Cole, Godke and Dresser2004, Reference Gomez, Pope, Ricks, Lyons, Dumas and Dresser2009). Although much effort has been devoted to improving cloning efficiency, the successful production of healthy cloned animals has not increased dramatically since Dolly. The complete outcome of animal cloning could be influenced by any step in the SCNT procedure (Campbell, Reference Campbell2007; Campbell et al., Reference Campbell, Fisher, Chen, Choi, Kelly, Lee and Xhu2007), including the selection of both recipient and donor cells, manipulation of enucleation and nuclear transfer (NT), methods of activation and even the culture conditions of cloned embryos. Hence, SCNT or iSCNT applications are impeded by the low efficiency of the production and development of cloned embryos.
As the most convincing application of SCNT is therapeutic cloning, i.e. the isolation of autologous embryonic stem (ES) cells from cloned blastocysts derived from patient’s somatic cells (Prelle et al., Reference Prelle, Zink and Wolf2002; Bongso et al., Reference Bongso, Fong and Gauthaman2008), several alternative methods have been developed to produce ES-like cells and to improve the NT system (reviewed by Hochedlinger and Jaenisch, Reference Hochedlinger and Jaenisch2006). Among these methods, the cell-free extract system (Byrne et al., Reference Byrne, Simonsson, Western and Gurdon2003), from amphibian and mammalian oocyte extracts, is advantageous for studying the molecular mechanisms of nuclear reprogramming, in addition to the production of de-differentiated cells (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004; Alberio et al., Reference Alberio, Johnson, Stick and Campbell2005; Tamada et al., Reference Tamada, Van Thuan, Reed, Nelson, Katoku-Kikyo, Wudel, Wakayama and Kikyo2006; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007; Miyamoto et al., Reference Miyamoto, Tsukiyama, Yang, Li, Minami, Yamada and Imai2009). According to previous reports, somatic cells can be reprogrammed by transferring their nuclear contents into oocytes (Wilmut et al., Reference Wilmut, Schnieke, McWhir, Kind and Campbell1997; McLean et al., Reference McLean, Wang, Babu, Edwards, Kasinathan, Robl and Sheppard2010) or by fusion with ES cells (Tada et al., Reference Tada, Takahama, Abe, Nakatsuji and Tada2001; Cowan et al., Reference Cowan, Atienza, Melton and Eggan2005), indicating that mammalian somatic cells can be reprogrammed by specific factors contained in unfertilized eggs and ES cells to regain totipotency or pluripotency (Takahashi and Yamanaka, Reference Takahashi and Yamanaka2006). The process occurring in eggs or oocytes includes: (1) nuclear morphological changes; (2) exchange of somatic proteins for oocyte proteins (e.g. chromosomal proteins); (3) histone and DNA modifications; and (4) transcriptional activation of a wide range of genes (Jullien et al., Reference Jullien, Astrand, Halley-Stott, Garrett and Gurdon2010, Reference Jullien, Pasque, Halley-Stott, Miyamoto and Gurdon2011). Therefore, transcription factors (TFs), such as Oct4, Sox2, and Nanog, have emerged to play a key role in determining the fate of undifferentiated or reprogrammed cells. When Oct4, Sox2, and Nanog are expressed, they can turn self-renewal genes ‘ON’ and differentiation genes ‘OFF’ (Chickarmane et al., Reference Chickarmane, Troein, Nuber, Sauro and Peterson2006; Pasque et al., Reference Pasque, Jullien, Miyamoto, Halley-Stott and Gurdon2011).
The quality and development of cloned blastocysts can be improved after pretreatment of donor cells with mature oocyte extracts (Tang et al., Reference Tang, Wang, Zhang, Gao, Ma, Yin, Sun, Liu and Zhang2009). From these studies, evidence showed that mature oocytes hold great reprogramming competence, but it was unknown if excess oocyte extract could assist in cloning efficiency. Several molecules, such as chromatin remodelling ATPase brahma-related gene 1 (BRG1), nucleoplasmin (Npm) and Tpt1, have been identified to be involved in nuclear reprogramming of mouse or human somatic cells using extracts from Xenopus laevis eggs or oocytes (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004; Tamada et al., Reference Tamada, Van Thuan, Reed, Nelson, Katoku-Kikyo, Wudel, Wakayama and Kikyo2006; Koziol et al., Reference Koziol, Garrett and Gurdon2007). BRG1 is the central catalytic subunit of many chromatin-modifying enzymatic complexes. It can alter the chromatin structure of target promoters by the energy derived from ATP hydrolysis and is a major co-regulator of transcription (Trotter and Archer, Reference Trotter and Archer2008).
Therefore, the aim of this study was to evaluate the reprogramming events of mouse somatic cells (testing in NIH/3T3 cells) under Xenopus egg extract treatment. The effect of BRG1 from Xenopus egg extracts on improving the development of cloned mouse embryos was studied.
Materials and methods
Preparation of Xenopus laevis egg extracts
Xenopus egg extracts were prepared according to previous studies (Alberio et al., Reference Alberio, Johnson, Stick and Campbell2005; Hansis, Reference Hansis2006; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007). Sexually mature female Xenopus were treated with 600 IU of human chorionic gonadotrophin (hCG; China Chemical and Pharmaceutical Co., Ltd) to obtain ovulated eggs. Eggs were collected in mare’s modified Ringers medium (MMR medium; 100 mM NaCl, 2 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 0.1 mM EDTA and 5 mM HEPES, pH 7.5). The jelly coat of the eggs was removed by incubation in dejellying solution [20 mM Tris–HCl pH 8.5, 1 mM dithiothreitol (DTT), and 110 mM NaCl] for 5 min. After dejellying, the eggs were rinsed three times with MMR medium, and eggs were activated by treatment with 4 μM calcium ionophore A23187 (Sigma-Aldrich, St. Louis, MO, USA) for 5 min. After activation, the eggs were rinsed with ice-cold extraction buffer [50 mM KCl, 5 mM MgCl2, 2 mM β-mercaptoethanol, 5 mM EGTA, 10 μg/ml cytochalasin B, protease inhibitor cocktail (Sigma-Aldrich) and 50 mM HEPES, pH 7.4] and transferred into centrifuge tubes. The eggs were centrifuged at 1500 g for 1 min at 4°C, the excess buffer was removed, and the eggs were centrifuged at 10,000 g for 25 min at 4°C to crush the eggs. A 1-ml syringe and 22-G needle were used to remove the middle layer, which was recentrifuged at 16,000 g for 15 min at 4°C. Then, the cytoplasmic layer was supplemented with 50 μg/ml cytochalasin B and recentrifuged at 20,000 g for 30 min at 4°C. The supernatant was supplemented with 2% glycerol to create the cleared cytoplasmic layer, which was snap frozen in liquid nitrogen and stored at −80°C until use.
The reprogramming mixture contained an ATP-regenerating system [1 mM ATP, 20 mM phosphocreatine, 20 U/ml creatine kinase, 1 mg/ml bovine serum albumin (BSA), 1 mM GTP, 100 mM dNTP and protease inhibitor cocktail] and two-thirds of the egg extract in the final volume.
Preparation of experiment cells
NIH/3T3 cells (mouse NIH Swiss embryo fibroblasts; Bioresource Collection and Research Center, BCRC 60008) were placed in 6-cm culture dishes and cultured in mouse 3T3 cell medium [m3T3M; Dulbecco’s modified Eagle’s medium (DMEM); Sigma] containing 10% bovine serum (BS; Gibco), penicillin and streptomycin at 37°C in 5% CO2 in air.
ES-D3 cells (mouse pluripotent ES cells; (BCRC 60205) were cultured on STO feeders in mouse ES cell medium (mESM; DMEM supplemented with 15% FBS, 0.1 mM β-mercaptoethanol, 1000 unit/ml mLIF, 0.5% non-essential amino acids, and 2 mM l-glutamine).
Permeabilization of cells and egg extract treatment
Cell membranes were permeabilized by adding 1 μl of streptolysin O (SLO; Sigma, 1 μg/ml stock) to cell suspensions (per 5×105 cells in 1 ml of Ca2+- and Mg2+-free PBS) to yield a final concentration of 500 ng/ml. Then, the cells were incubated for 50 min at 37°C with slow agitation. At the end of the incubation, the cells were diluted with PB1 buffer (110 mM KAc, 5 mM NaAc, 2 mM MgAc, 1 mM EGTA, 2 mM DTT, protease inhibitor cocktail and 20 mM HEPES, pH 7.3) to stop the SLO reaction and centrifuged at 2400 rpm for 5 min at 4°C. After centrifugation, the cells were mixed with Xenopus egg extracts at 22°C for 2 h. The cells were cultured at 37°C in mDMEM for the first 6 days and then mESM for the last 4 days after Xenopus egg extract treatment. This treatment was modified from previous studies (Alberio et al., Reference Alberio, Johnson, Stick and Campbell2005; Hansis, Reference Hansis2006; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007; Fig. 2).
Alkaline phosphatase (AP) staining
After 10 days of culture, extract-treated cell colonies were washed with PBS and fixed with 4% paraformaldehyde (pH 7.4) in PBS for 15 min. After removing the paraformaldehyde, the cells were washed with PBS three times. Then, AP staining solution (0.26 M Trizma maleate pH 9.0, 8 mM MgCl2, Fast-Red TR salt, and α-naphthyl phosphate; Sigma) was added and the mixture incubated at room temperature for 30 min until a red colour developed, and the cells were observed under a microscope.
Reagents and media
All inorganic and organic chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. Oocytes, reconstructed oocytes and embryos were cultured in KSOMAA (Chemicon, MR-106D) at 37°C in a humidified 5% CO2 in air incubator. CZB-based medium (Chatot et al., Reference Chatot, Ziomek, Bavister, Lewis and Torres1989) was used for all manipulations of oocytes/embryos. CZB containing 5.5 mM glucose (CZBG) was used for transient culture at 37°C during oocyte/embryo handling in HEPES-buffered CZBG (HCZBG) at room temperature. The pH and osmolarity of the medium applied in this study were adjusted to 7.4 and 275–285 mOsm/kg, respectively. The medium supplemented with 0.5% (w/v) fatty acid-free BSA was sterilized using a 0.22 µm filter and stored at 4°C for 2 weeks.
Collection of oocytes and zygotes
Sexually mature female C57BL/6 and male DBA/2 mice were purchased from BioLasco Taiwan Co. Ltd, and B6D2F1 (C57BL/6×DBA/2) mice were used as oocyte donors. The animals were maintained in an individually ventilated cage (IVC) system under conditions of 14 h light (07:00–21:00) and 10 h dark. Food and water were provided ad libitum. Female B6D2F1 mice (8–10 weeks old) were superovulated by intraperitoneal injections of 10 IU of pregnant mare serum gonadotrophin (PMSG; China Chemical and Pharmaceutical Co., Ltd) followed by a second intraperitoneal injection 47 h later of 10 IU of human chorionic gonadotropin (hCG, China Chemical and Pharmaceutical Co., Ltd).
Cumulus−oocyte complexes (COCs) were collected from the oviducts of superovulated B6D2F1 mice 13.5 h after hCG injection. The COCs were immersed in PBS containing 0.1% hyaluronidase for 5 min and pipetted to remove cumulus cells. The denuded oocytes were washed with HCZBG and observed under a dissecting microscope. Those oocytes with the first polar body and intact ooplasm were determined as mature oocytes (at the metaphase II stage, MII) and selected for experiments. After washing and selection, the MII oocytes were held in CZBG before activation or enucleation. To recover zygotes, female mice were caged with males individually on the day of hCG injection. Mating was verified by the presence of a vaginal plug on the following morning, which was designated as E0.5. The zygotes were flushed from the oviducts of pregnant females at E1.5.
Nuclear transfer
The reconstruction of embryos by SCNT was performed in accordance with a previously reported method with some modifications (Wakayama and Yanagimachi, Reference Wakayama and Yanagimachi1998; Markoulaki et al., Reference Markoulaki, Meissner and Jaenisch2008). Enucleation was performed using a Nikon inverted microscope (TE2000-U) equipped with a Narishige micromanipulator. Every 10 denuded mouse mature oocytes were placed in drops of H;’CZBG medium containing 5 mg/ml of CB (HCZBG-CB) for 3 min and enucleated by aspirating the MII spindle with a pipette that was driven by a Piezo-drill (PMM-150FU, Prime Tech Ltd, Ibaraki, Japan). After enucleation, the oocytes were washed and kept in CZBG medium at 37°C in a humidified 5% CO2 incubator before somatic cell injection. The donor cumulus cells harvested from the COCs of superovulated B6D2F1 mice were washed with HCZBG and resuspended in a mixture composed of HCZBG and 6% polyvinyl pyrrolidone (PVP) in PBS (1:1, v/v). The enucleated oocytes and some donor cells were transferred into drops of HCZBG-CB individually on a Petri dish for micromanipulation. In some experiments in this study, drops of Xenopus egg extracts were also placed on the Petri dish. One donor cell was drawn in and out of the injection pipette to break the cell membrane and then injected into the enucleated oocyte with the assistance of Piezo pulses. For Xenopus egg extract co-injection experiments, the membrane-broken donor cell was repeatedly pipetted in the drop of Xenopus egg extract, and some of the extract (approximately three times the volume of the donor cell) was drawn into the injection pipette with the donor cell before co-injection into enucleated oocytes. The reconstructed oocytes were washed and cultured in CZBG at 37°C in a humidified 5% CO2 incubator for 1 h to allow chromosome condensation before parthenogenetic activation.
Immunodepletion and western blotting analysis
BRG1 in Xenopus egg extracts was immunoprecipitated by the Catch and Release v2.0 kit (MP Biomedicals) according to the manufacturer’s instructions. Briefly, after washing the resin packed in the spin column with 1× Catch and Release Wash Buffer, 100 μg of Xenopus egg extract, 4 μg of anti-BRG1 antibody (Santa Cruz Biotechnology) and 10 μl of Antibody Capture Affinity Ligand were added into the spin column and incubated on a rotator at 4°C for 6 h. The spin column was then inserted into a capture tube and centrifuged at 5000 rpm for 60 s to collect the flow through. The flow through containing no BRG1 was designated as Extract−, transferred into microcentrifuge tubes and stored at −80°C until use. The eluate was collected by adding 70 μl of 1× Denaturing Elution Buffer to the spin column and centrifuging at 5000 rpm for 60 s. The eluate containing Brg1 was stored at −80°C for western blot analysis.
Xenopus egg extracts containing BRG1 or no BRG1 were designated as Extract+ and Extract−, respectively. The eluate containing BRG1 was boiled for 5 min in sodium dodecyl sulfate (SDS) sample buffer, run on a 13.3% SDS-polyacrylamide gel electrophoresis (PAGE) gel, and transferred to a nitrocellulose membrane (NC membrane) in transfer buffer (25 mM Tris–HCl, pH 8.3, 192 mM glycine, 10% methanol) using an ATTO tank transfer system (ATTO Corp.) at a constant 80 V for 60 min. The NC membrane was washed in TTBS (200 mM Tris–HCl, 5 M NaCl, 0.05% Tween-20, pH 7.5) several times and blocked in 5% skimmed milk in TTBS (w/v/) overnight at 4°C with gentle rotation. After several 10 min washes with TTBS, the NC membrane was probed with an anti-BRG1 antibody (1:400, Santa Cruz Biotechnology) in TTBS at 4°C for 6 h and then washed thoroughly with TTBS several times. The NC membrane was then incubated with horseradish peroxidase-conjugated donkey anti-goat IgG (1:2000; Santa Cruz Biotechnology) in TTBS at room temperature for 1 h. After washing with TTBS, the NC membrane was visualized using the ChemiLucent ECL detection system (Chemicon, 2600) according to the manufacturer’s instructions.
Immunocytochemical staining
Expanded blastocysts derived from reconstructed embryos and fertilized embryos were washed twice in PBS containing 0.1% polyvinyl alcohol (w/v, PBS−PVA) and fixed with 3.5% paraformaldehyde in PBS−PVA for 30 min. The fixed blastocysts were then washed twice in PBS−PVA and permeabilized in PBS supplemented with 3% BSA and 0.1% Triton X-100 at 4°C overnight. After permeabilization, the samples were incubated with mouse anti-Cdx2 (1:100; BioGenex, CDX2–88), goat anti-Oct4 (1:100; Santa Cruz Biotechnology, SC-8628), rabbit anti-Nanog (1:100; Chemicon, AB5731) or rabbit anti-Sox2 (1:100; Chemicon, AB5603) at room temperature for 90 min and then washed with PBS−PVA three times. FITC-conjugated anti-goat (Sigma, A5420) and anti-mouse (Santa Cruz Biotechnology, SC-3738) antibodies were diluted 1:300 and applied to the appropriate samples at room temperature for 30 min. After three 15-min washes, DNA was stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) contained in mounting medium at room temperature for 5 min. The embryos were washed thoroughly, mounted on slides and sealed with clear fingernail polish.
Statistical analysis
All experiments were replicated at least three times. The results were evaluated using chi-squared analysis in SAS software, and a value of P<0.05 was considered statistically significant.
Results
Functional Xenopus egg extracts in somatic cell reprogramming
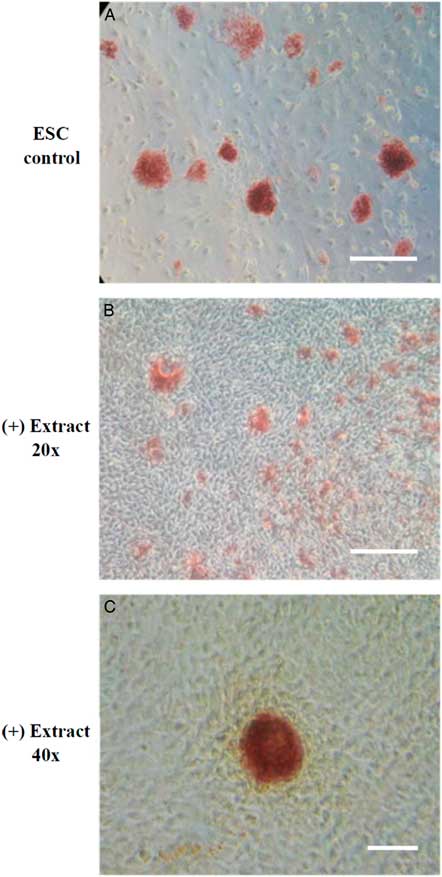
The experiment design was modified from Takahashi and Yamanaka (Reference Takahashi and Yamanaka2006). The somatic cells treated with Xenopus egg extracts were cultured in mDMEM for the first 4 days and changed to mESM afterwards. In our study, the extract-treated cells (ETCs) proliferated as typical fibroblast-like cells at the early stage of culture and began to form a colony-like morphology after 4 days of culture. Further analysis of AP staining, which is one of the first pluripotency markers to be expressed in cells undergoing reprogramming (Duinsbergen et al., Reference Duinsbergen, Eriksson, ’t Hoen, Frisén and Mikkers2008), demonstrated that the AP-positive colonies were observed in ETCs after at least 10 days (Fig. 1).
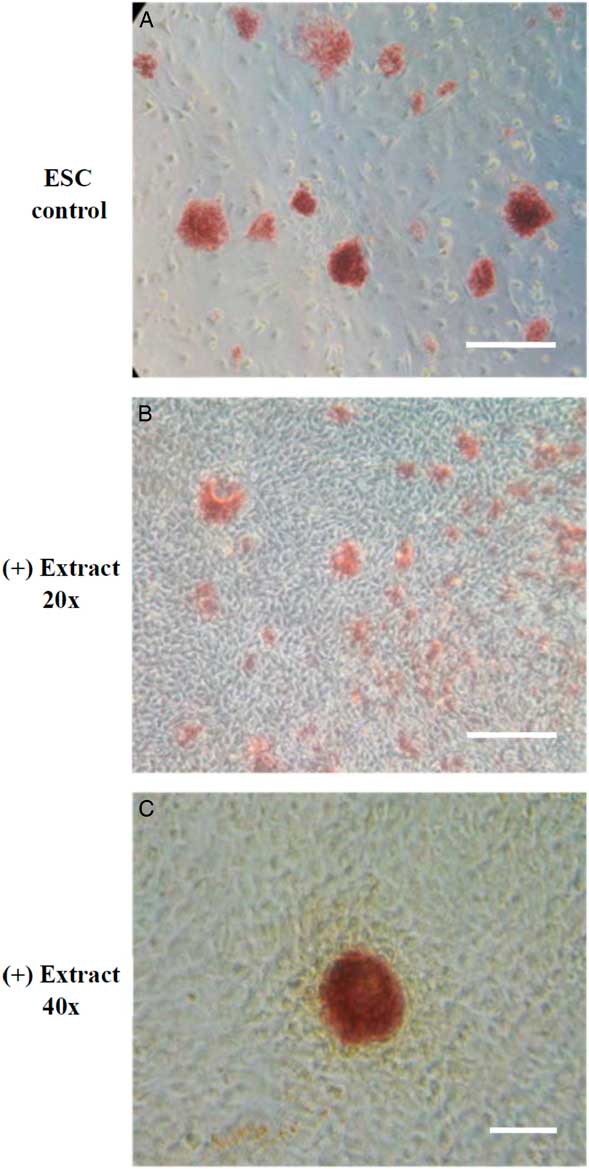
Figure 1 The expression of alkaline phosphatase (AP) in permeabilized NIH/3T3 cells treated with Xenopus egg extracts. NIH/3T3 cells permeabilized by SLO were treated with Xenopus egg extracts, cultured in m3T3M for the first 4 days and then cultured in mESM from day 5. On day 10, cell colonies were identified and examined for AP activity. (A) positive control of mouse ES cell colonies. (B, C) ETCs with AP-positive colonies. Scale bar: A, B: 100 µm; C: 150 µm.
Reactivation of Oct4 and pluripotent markers in ETCs
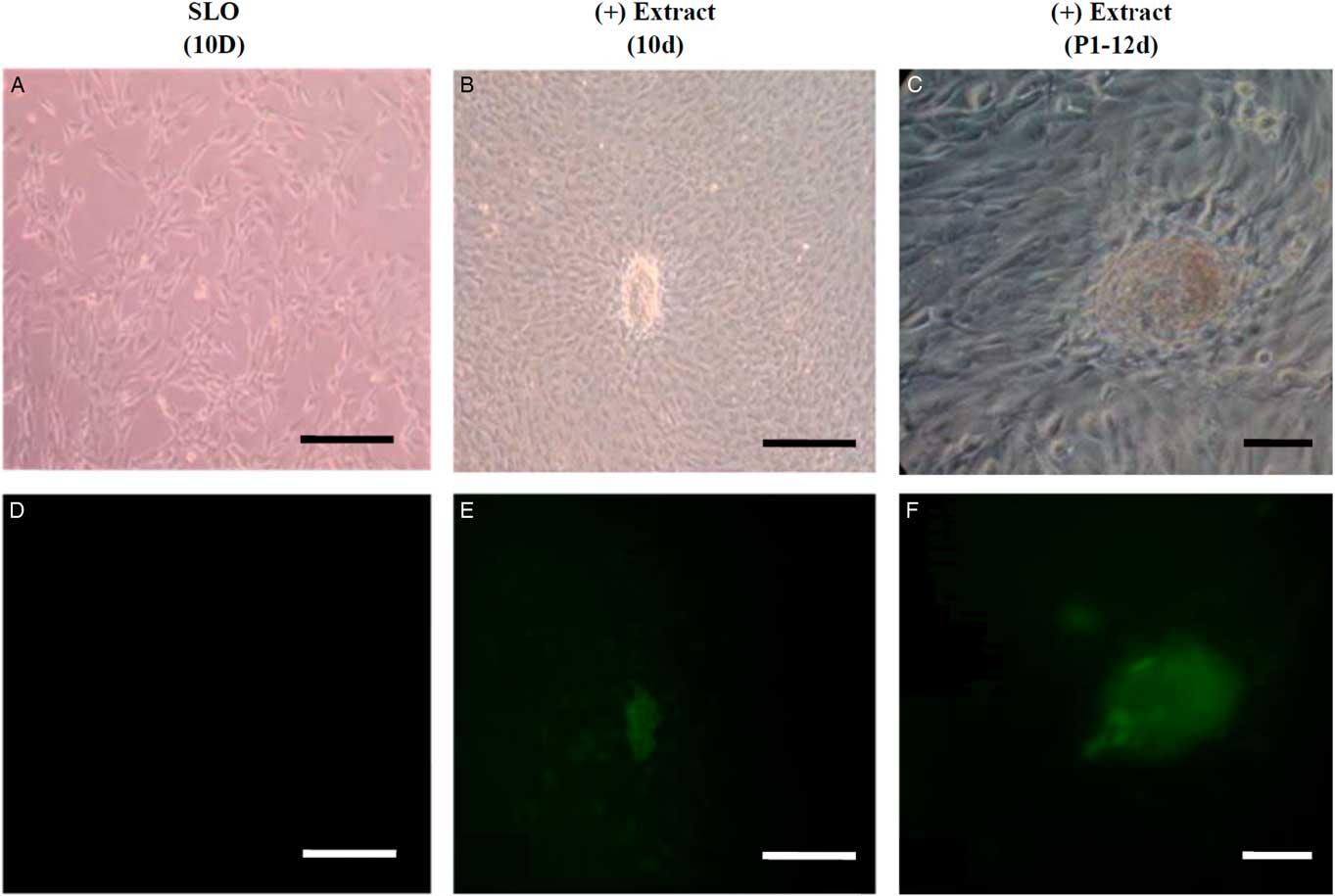
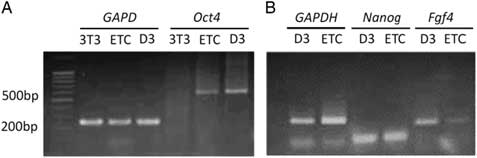
Different analysis strategies of egg extract treatments were used to improve somatic cell reprogramming efficiency by Xenopus egg extracts. We constructed and transfected an Oct4−EGFP reporter vector into mouse NIH/3T3 cells, which were selected for at least six passages to establish an Oct4−EGFP NIH/3T3 cell line. The Oct4−EGFP NIH/3T3 cells were a convenient and efficient extract reprogramming platform and were treated with Xenopus egg extracts. After 10 days, we observed green fluorescent-positive colonies with Oct4 expression. Our results demonstrated that the method of reversible permeabilization could introduce reprogramming molecules from Xenopus egg extracts to mammalian somatic cells and induce pluripotent Oct4 gene reactivation in ETCs cells in vitro (Fig. 2). In addition, we analyzed the expression of ES cell marker genes in D3 (ES cell line), Oct4−EGFP−NIH/3T3 cells and ETCs by RT-PCR. The results showed that Oct4−EGFP−NIH/3T3 cells after Xenopus egg extract treatment became ETCs, and the RNA expression of oct4, nanog and fgf4 was detected in ETCs (Fig. 3). Therefore, the reprogramming function of Xenopus egg extract was validated.
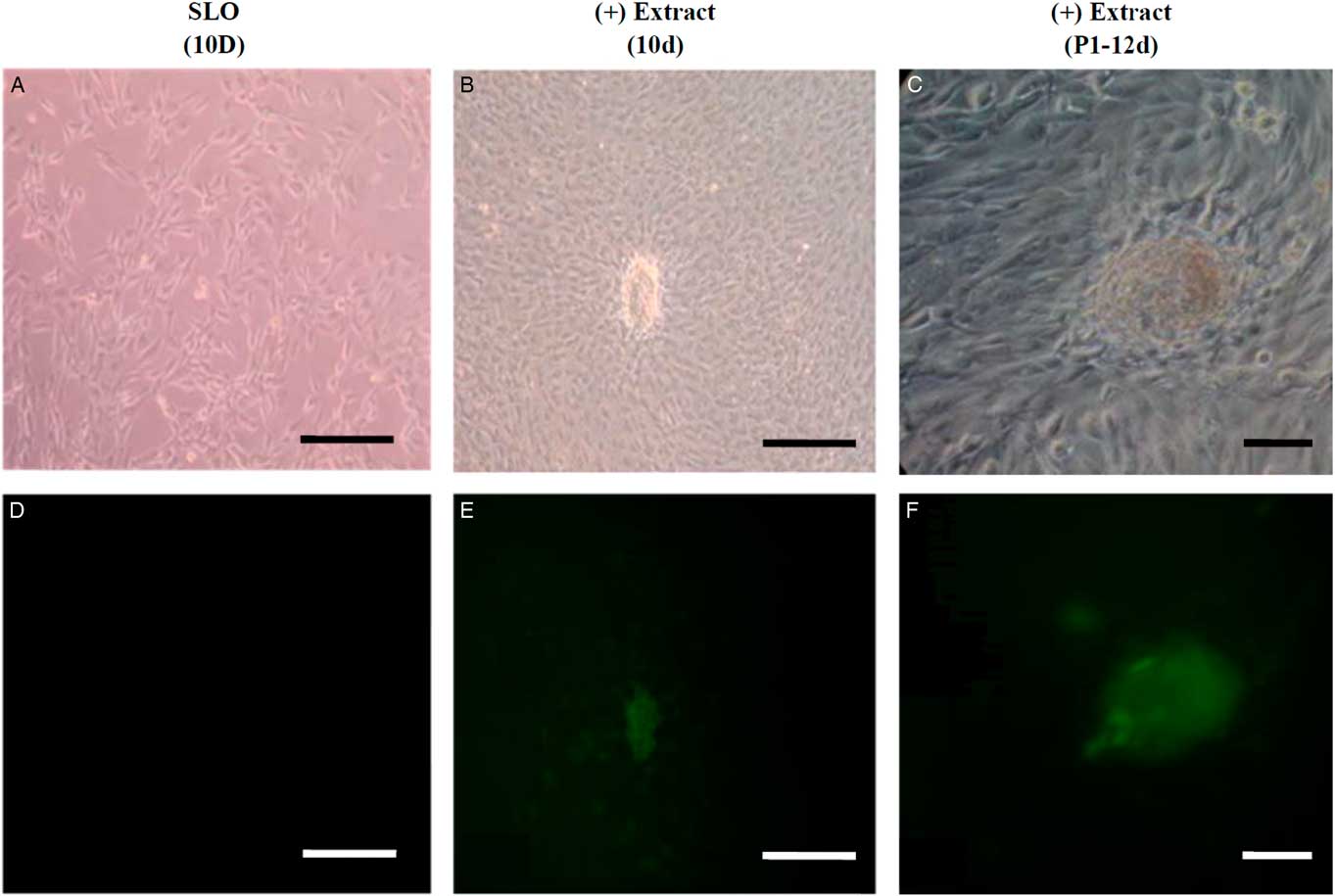
Figure 2 Oct4 activation of green fluorescence in permeabilized Oct4-EGFP-NIH/3T3 cells treated with Xenopus egg extracts. (A, B) Oct4-EGFP-NIH/3T3 cells permeabilized by SLO after 10 days, which were the treatment control. (C, D) Oct4-EGFP-NIH/3T3 cells permeabilized by SLO were treated with Xenopus egg extracts and cultured in vitro. On day 10, cell colonies were found and examined for morphology and fluorescence. (E, F) Cell colonies (ETCs) derived from Oct4-EGFP-NIH/3T3 cells treated with Xenopus egg extracts were selected and reseeded on STO feeder cells in mESM on day 12. The cell morphology was detected under light (A, C, E) and green fluorescence (B, D, F). Scale bars: 100 µm.
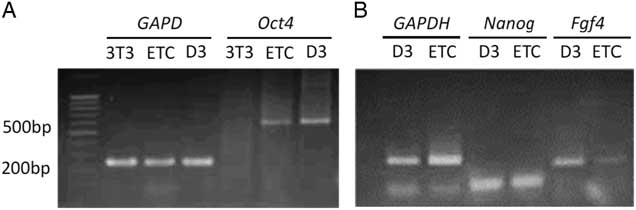
Figure 3 Analyses of the expressions of ES cell marker genes in D3, extract-treated cells (ETCs) and Oct4–EGFP–NIH/3T3 cells by RT-PCR. Total RNAs extracted from D3, ETCs and Oct4–EGFP–NIH/3T3 cells were reverse transcribed into cDNAs and analyzed for the expression of Oct4 (A), Nanog and Fgf4 (B). GAPDH is the internal control. 3T3: Oct4–EGFP–NIH/3T3 cells; ETC: Oct4–EGFP–NIH/3T3 cells treated with Xenopus egg extracts; D3: mouse embryonic stem cells.
The effect of Xenopus egg extracts on the development of cloned mouse embryos
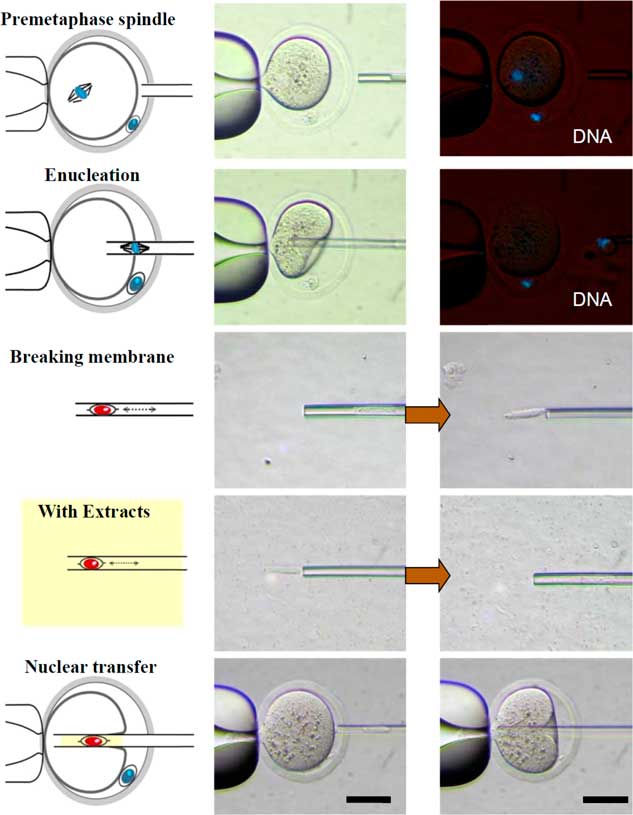
The procedure of reconstructed oocyte production is illustrated in Fig. 4. The donor cell membrane was broken by repeated pipetting before injection into the enucleated oocyte (NT group). In the experimental group (Extract group), the broken donor cell was drawn in and out of the injection pipette. Some Xenopus egg extracts, which were approximately three times the volume of the donor cell, were co-injected into the enucleated oocytes. The reconstructed oocytes were activated 19 h after hCG injection, based on results from a previous experiment.
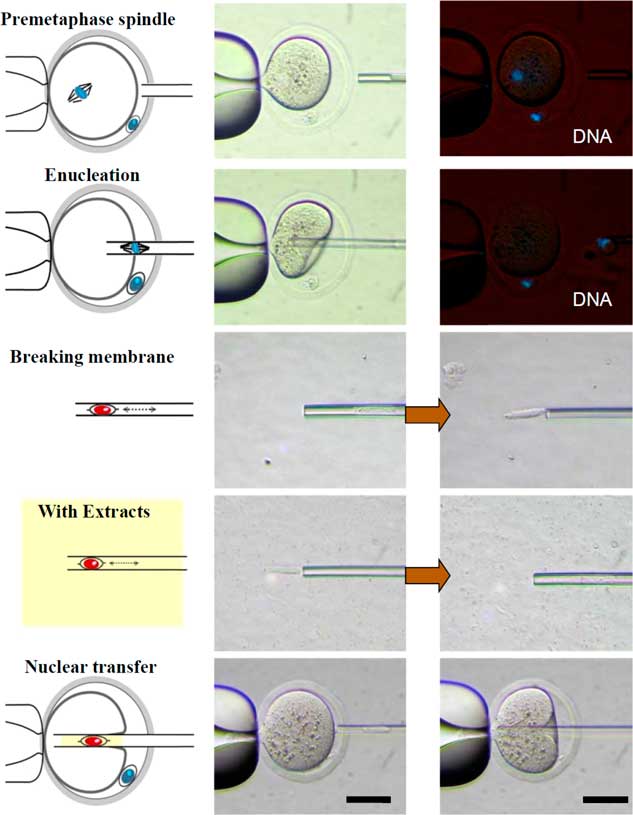
Figure 4 Schematic diagram of the experimental design for examining the effect of Xenopus egg extracts on the development of cloned mouse embryos. The cumulus cells (donor cells) were injected alone or co-injected with Xenopus egg extracts into enucleated oocytes. DNA was counterstained with Hoechst 33342 (blue). Scale bars: 50 µm.
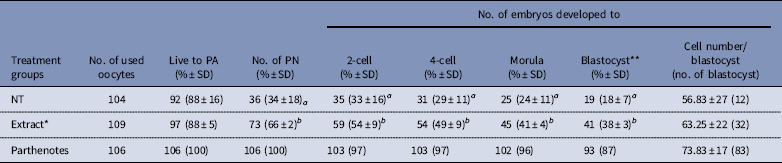
Preimplantation development of reconstructed oocytes in vitro is shown in Table 1. The percentages of cloned embryos at the pronuclear stage 6 h after activation were significantly higher in the Extract group than percentages in the NT group (66% vs 34%; P<0.01). Additionally, rates of cleavage stages and morula/blastocyst stages were significantly different between the Extract and NT groups. Development was improved in the reconstructed embryos with co-injection of Xenopus egg extracts (66–38% vs 34–18%, P<0.01). However, for cloned blastocysts derived from cumulus cells, there was no difference between NT alone or co-injected with cumulus cell and egg extracts. Some embryos developed to the expanded blastocyst stage and even could hatch from the zona pellucida. Although the cell number per blastocyst was slightly higher in the Extract group, there was no significant difference compared with the NT group (Table 1).
Table 1 Effect of Xenopus egg extract on the development of cloned mouse embryos in vitro
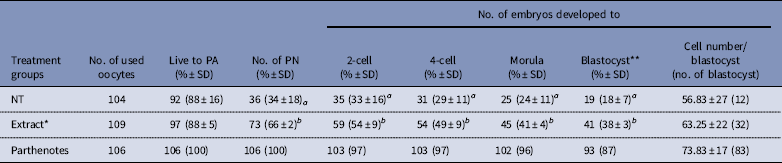
*Extract: Xenopus egg extracts (three-time volume of a cumulus cell) were co-injected with single cumulus cells.
**Number of blastocysts was evaluated 120 h after activation.
NT: reconstructed embryos with cumulus cell only; PA: parthenogenetic activation; PN: the pronuclear stage.
a,b Numbers with different superscripts in the same column differ significantly (P<0.01). Four replicates.
The effect of BRG1 contained in Xenopus egg extracts on the development of cloned mouse embryos
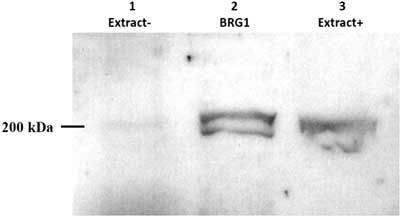
Xenopus egg extracts with (designated Extract+) or without (designated Extract−) BRG1 protein, depleted by immunoprecipitation, were tested using anti-BRG1 antibody. Immunoblotting results are shown in Fig. 5. A band at 200 kDa was found in Extract+ samples and the eluate. After removal of the BRG1 protein from egg extracts by immunoprecipitation, no BRG1 protein was detected.
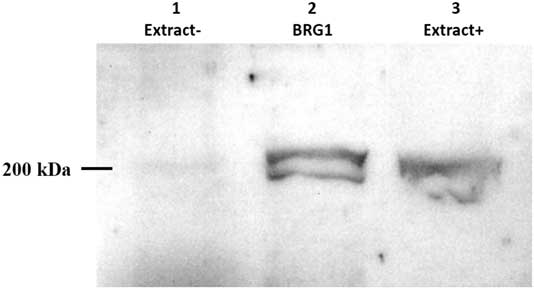
Figure 5 Analysis of BRG1 protein in Xenopus egg extracts by western blotting. Xenopus egg extracts were immunodepleted for BRG1 by immunoprecipitation and then separated by 13.3% SDS-PAGE. The expression of BRG1 protein was analyzed by western blotting. Lane 1: BRG1-depleted Xenopus egg extract (Extract−); total loaded protein, 50 µg. Lane 2: BRG1 collected by immunoprecipitation; total loaded protein, 1 µg. Lane 3: Untreated Xenopus egg extract (Extract+); total loaded protein, 50 µg.
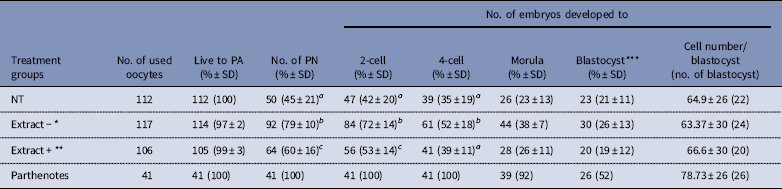
To evaluate the specificity of the BRG1 protein for reprogramming mouse cloned embryos, Extract− or Extract+ Xenopus egg extracts were co-injected into enucleated oocytes with cumulus cells. The percentages of pronuclear and 2-cell stage embryos were higher in the Extract+ group than in the NT group (60% vs 45%; 53% vs 42%; P<0.05). Furthermore, formation of pronuclei and early cleavage stage embryos was significantly higher in the Extract− group than in the Extract+ and NT groups (79% vs 45–60%; 52–72% vs 35–53%, P<0.05). The development of morulae/blastocysts and the cell numbers per blastocyst were not significantly different among the three groups, but the Extract− group results were still higher than in the Extract+ and NT groups (Table 2).
Table 2 Effect of BRG1 protein contained in Xenopus egg extracts on the development of mouse cloned embryos
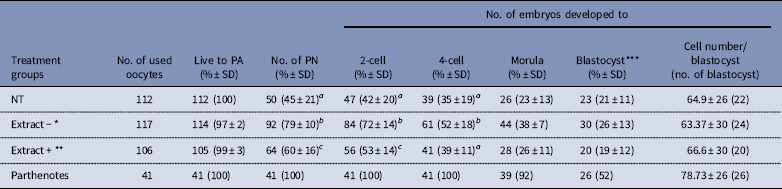
*Extract−: BRG1-depleted Xenopus egg extracts were co-injected with the cumulus cells.
**Extract+: Untreated Xenopus egg extracts were co-injected with the cumulus cells.
***Number of blastocysts was evaluated at 144 h after activation.
NT: reconstructed embryos with cumulus cell only; PA: parthenogenetic activation; PN: pronuclear stage.
a,b,cNumbers with different superscripts in the same column differ significantly (P<0.05). Five replicates.
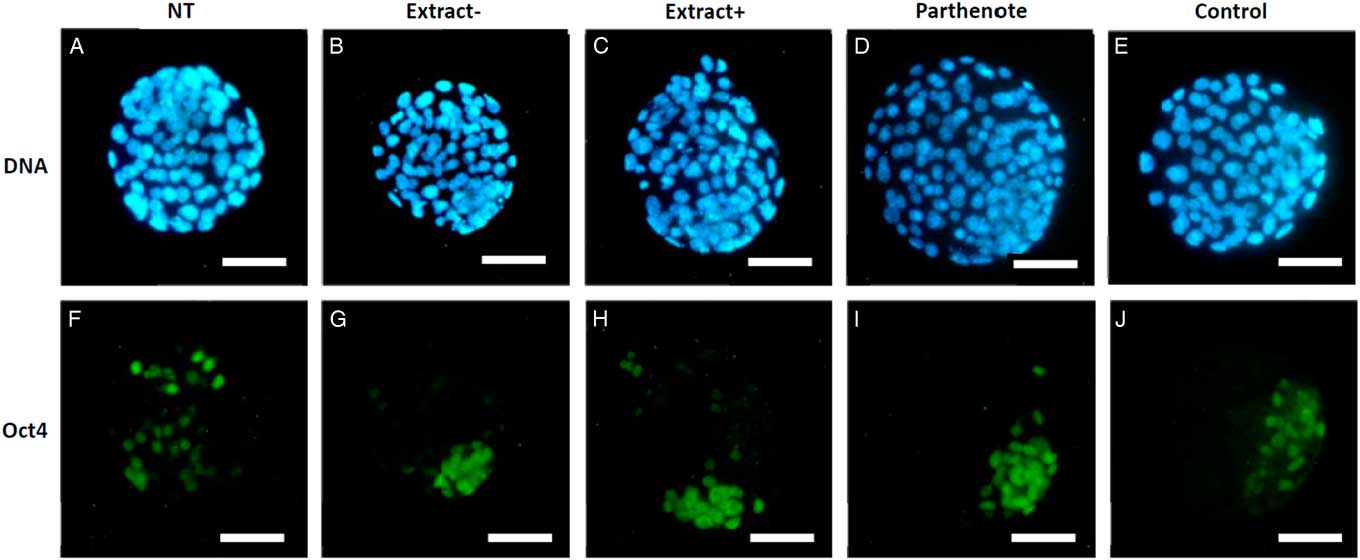
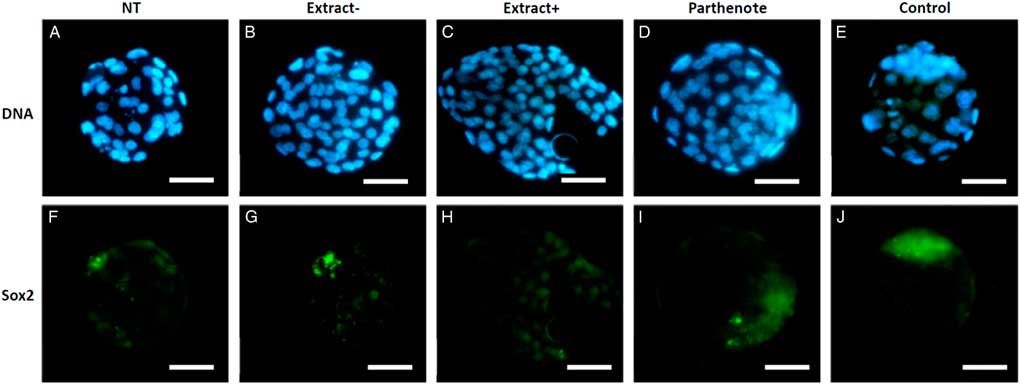
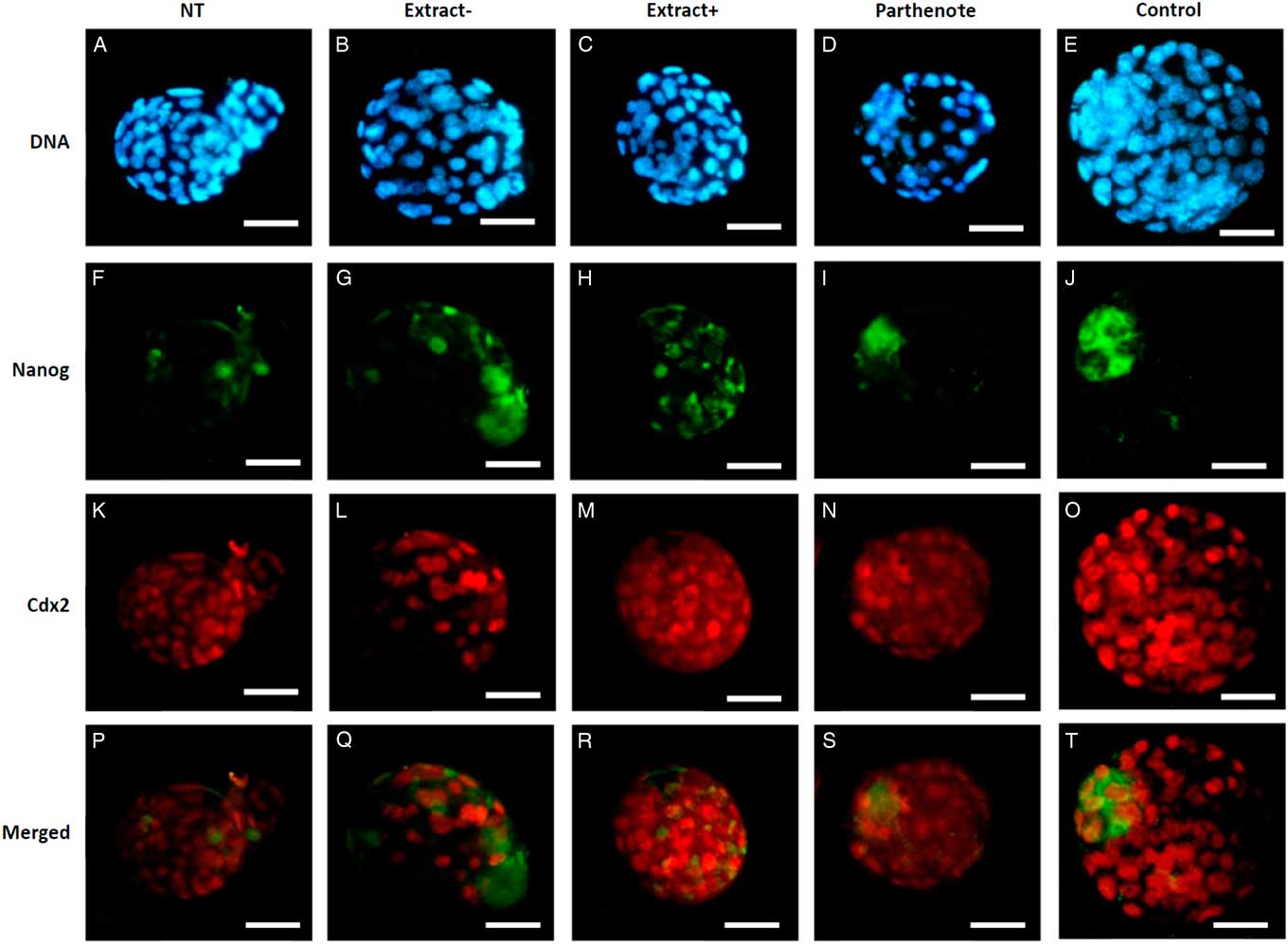
As shown in Fig. 6, the expanded blastocysts derived from Extract−, Extract+, NT, Parthenote and Control (normal fertilized blastocyst) groups showed various expression patterns for Oct4. By contrast with random expression of Oct4 in the trophectoderm (TE) and inner cell mass (ICM) in all examined blastocysts derived from the NT groups (n=12, Fig. 6A , F), Oct4 was expressed strictly in the ICM in the blastocysts derived from the Parthenote (n=12, Fig. 6D , I) and Control groups (n=6, Fig. 6E , J). More confined expression of Oct4 in the ICM was observed in the blastocysts derived from the Extract− (five out of seven, Fig. 6B , G) and Extract+ (seven out of eight, Fig. 6C , H) groups. Similarly, the expression of Sox2 (Fig. 7I , J) and Nanog (Fig. 8I , J) was contracted in the ICM in the Parthenote (n=5 and n=6, respectively) and Control (n=5 and n=9, respectively) groups. However, low or no expression of Sox2 was found in blastocysts from the NT (n=3), Extract− (n=8) and Extract+ (n=4) groups, although some positive signals were noticed in the degenerating cells (Fig. 7F−H). In other words, no conclusive pattern for Sox2 was detected in these experimental groups. To distinguish between ICM and TE, blastocysts were co-stained with an antibody for the transcription factor Cdx2 (red), a trophoblast-specific marker. We found low expression of Nanog in the blastocysts derived from the NT (n=13) group. Conversely, a random, higher expression pattern of Nanog in TE and ICM was found in the Extract+ (n=12) group (Fig. 8H ). However, the Extract− (n=6) group (Fig. 8G ) had more condensed Nanog expression in the ICM.
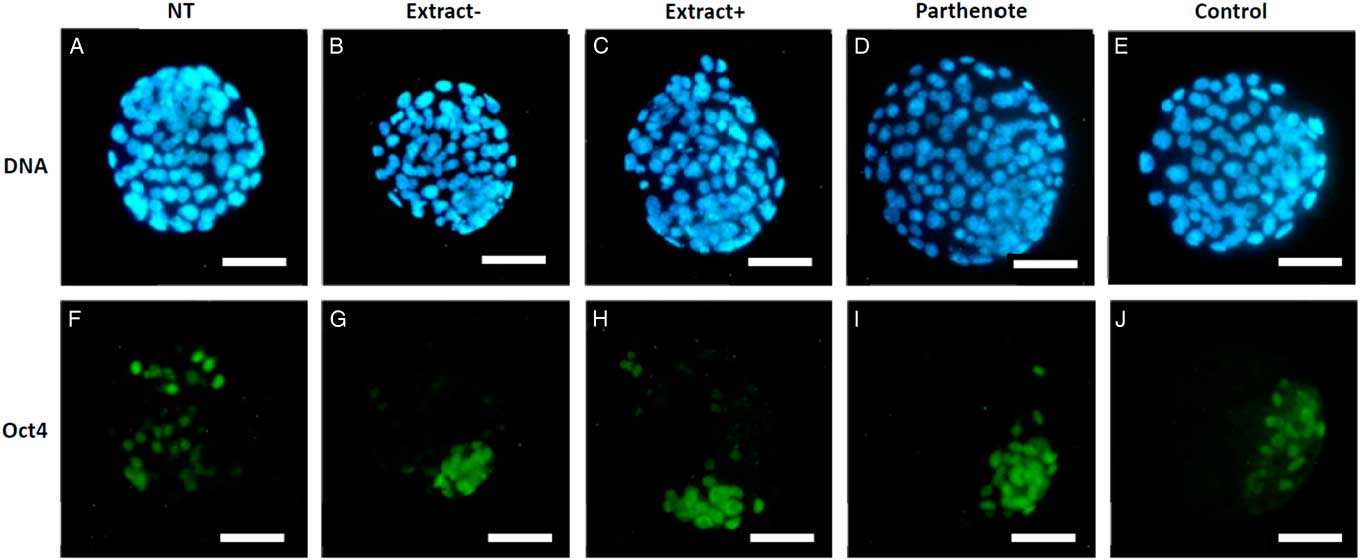
Figure 6 The expression of Oct4 in mouse blastocysts. Blastocysts derived from nuclear transfer (NT), NT with Xenopus egg extract (Extract+), NT with BRG1-depleted Xenopus egg extract (Extract−), parthenogenetically activated (Parthenote) and normal fertilized (Control) embryos were stained by immunocytochemistry to detect the expression of Oct4 (green, A–E). DNA was counterstained with DAPI (blue, F–J). Scale bars: 50 µm.
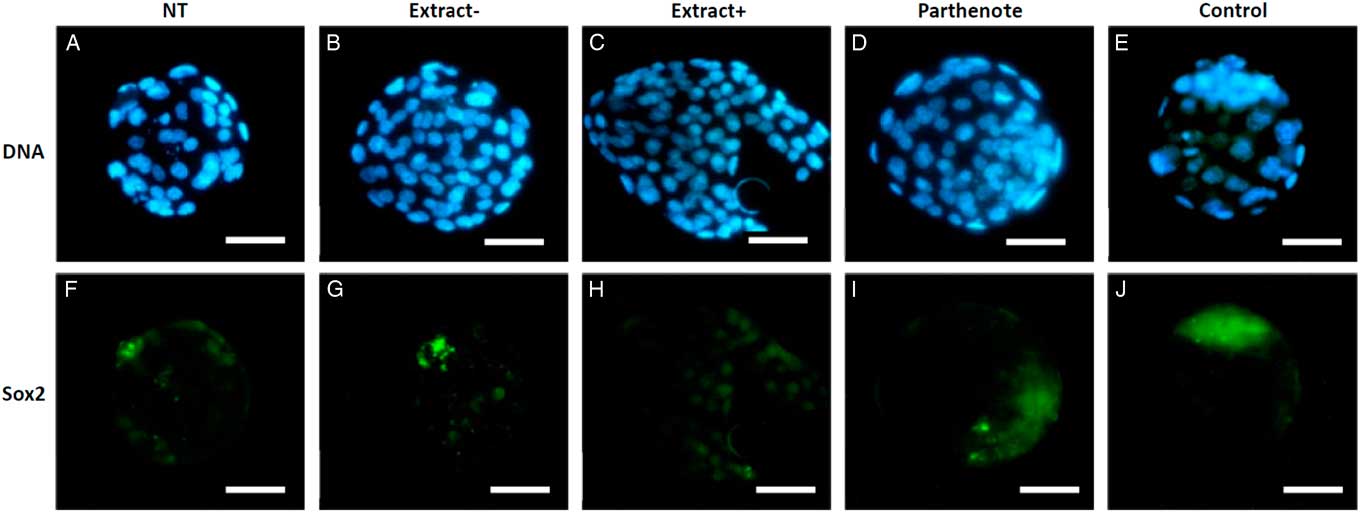
Figure 7 The expression of Sox2 in mouse blastocysts. Blastocysts derived from nuclear transfer (NT), NT with Xenopus egg extract (Extract+), NT with BRG1-depleted Xenopus egg extract (Extract−), parthenogenetically activated (Parthenote) and fertilized (Control) embryos were stained by immunocytochemistry to detect the expression of Sox2 (green, F–J). DNA was counterstained with DAPI (blue, A–E). Scale bars: 50 µm.
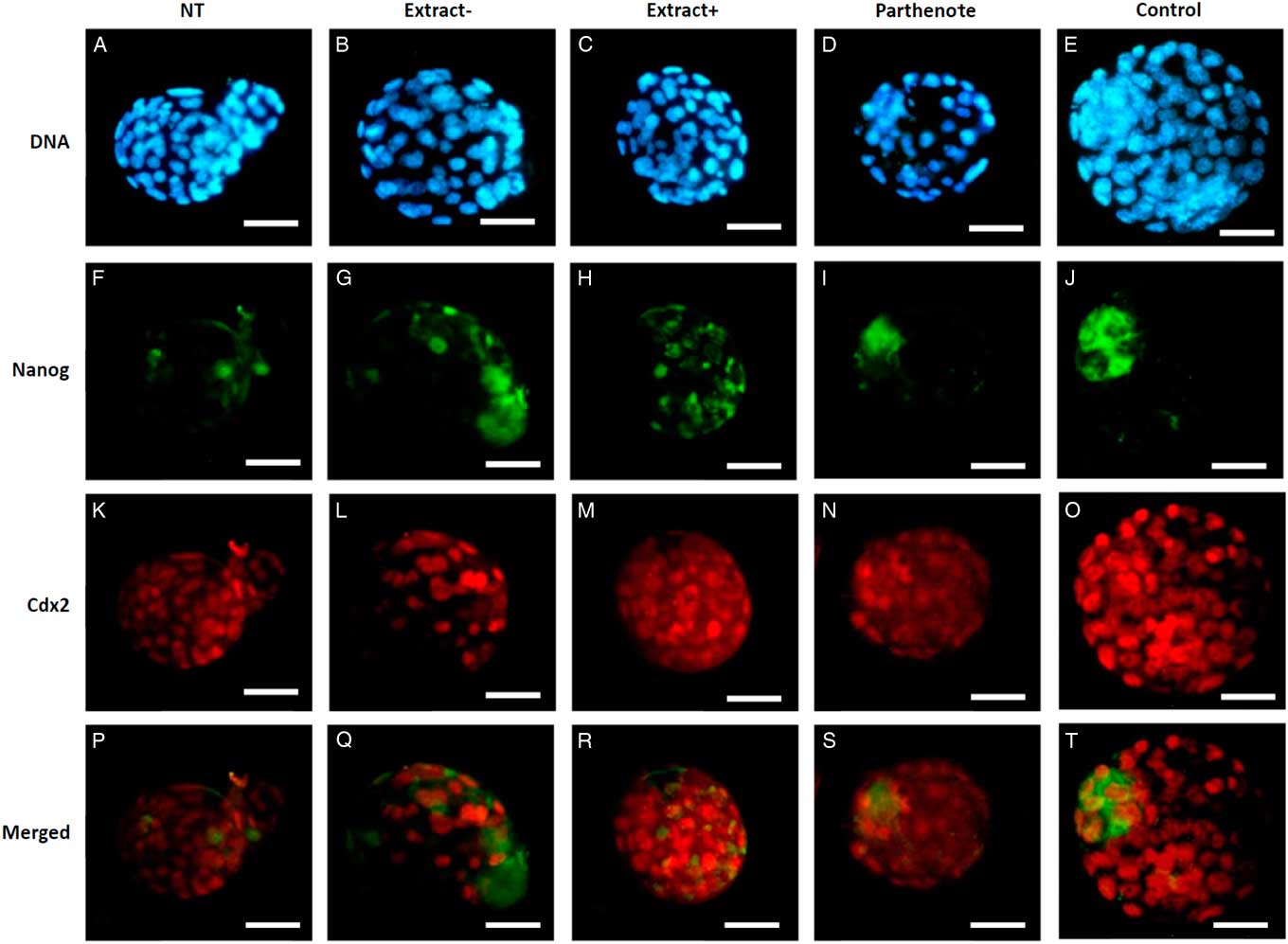
Figure 8 The expression of Nanog and Cdx2 in cloned mouse blastocysts. Blastocysts derived from nuclear transfer (NT), NT with Xenopus egg extract (Extract+), NT with BRG1-depleted Xenopus egg extract (Extract−), parthenogenetically activated (Parthenote) and fertilized (Control) embryos were stained by immunocytochemistry to detect the expression of Nanog (green, F–J) and Cdx2 (red, K–O). DNA was counterstained with DAPI (blue, A–E). (P–T) Merged images. Scale bars: 50 µm.
Discussion
Differentiated cells can be reprogrammed to an undifferentiated state using oocyte cytoplasm after SCNT, implying that unknown factors capable of reprogramming exist in eggs. Recent reports have indicated that Xenopus egg extracts have the ability to reprogramme mammalian somatic cells in vitro (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004; Alberio et al., Reference Alberio, Johnson, Stick and Campbell2005; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007). In this study, the function of Xenopus egg extracts in somatic cell reprogramming treatment was tested. In addition to the morphological change, i.e. NIH/3T3 cells started to form colony-like shapes 4 days after extract treatment, weak expression of OCT4 was found in the extract-treated Oct4−EGFP NIH/3T3 cells (data not shown). This result is in agreement with a previous study in which morphological change corresponded to the appearance of spliced oct4 transcripts (Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007). AP staining, as a pluripotent marker, was also observed in ETCs (Fig. 1). Therefore, these results demonstrated that ETCs were undergoing reprogramming.
Transcription factors, such as Oct4, Sox2, and Nanog, have emerged to play a key role in determining the fate of ES cells. When Oct4, Sox2, and Nanog are expressed, self-renewal genes ‘turn ON’ and differentiation genes ‘turn OFF’ (Chickarmane et al., Reference Chickarmane, Troein, Nuber, Sauro and Peterson2006). Activation of endogenous oct4 or nanog genes has become a more stringent selection strategy for isolating pluripotent cells in which positive colonies resembled ES cells and the reprogramming process was mostly complete (Wernig et al., Reference Wernig, Meissner, Foreman, Brambrink, Ku, Hochedlinger, Bernstein and Jaenisch2007; Okita et al., Reference Okita, Ichisaka and Yamanaka2007; Duinsbergen et al., Reference Duinsbergen, Eriksson, ’t Hoen, Frisén and Mikkers2008; Kim et al., Reference Kim, Zaehres, Wu, Gentile, Ko, Sebastiano, Araúzo-Bravo, Ruau, Han, Zenke and Schöler2008). Our results demonstrated that the reprogramming molecules in Xenopus egg extract could induce pluripotent oct4 gene promoter reactivation, and pluripotent marker genes (e.g. oct4, nanog and fgf4) were expressed in ETCs (Figs 2 and 3). Therefore, the reprogramming function of Xenopus egg extract was demonstrated.
Most losses of cloned embryos occur during post-implantation development, and might be attributed to incomplete reprogramming of donor somatic cells. Epigenetic modifications are responsible for nuclear reprogramming in cloned embryos (Reik et al., Reference Reik, Dean and Walter2001, Reference Reik, Santos and Dean2003). Nevertheless, the complete outcome of animal cloning could be influenced by any step of the SCNT procedure (Campbell et al., Reference Campbell, Fisher, Chen, Choi, Kelly, Lee and Xhu2007), including selection of both recipient and donor cells, manipulation of NT, and methods of activation. Therefore, the following experiment was conducted to test the effect of Xenopus egg extracts on the development of cloned mouse embryos. The percentages of cloned embryos were significantly (P<0.01) higher in the Extract group than the NT group during the preimplantation stage from 2-cell to blastocyst stages (Table 1). These results demonstrated that cloned mouse embryos showed much improved development following co-injection with Xenopus egg extract.
Another NT study found that the treatment of donor cells with Xenopus oocyte extract increased cloned embryo development in pigs (Bui et al., Reference Bui, Kwon, Kang, Oh, Park, Park, Paik, Van Thuan and Kim2012). Similar to our results, a higher percentage of embryos developed into blastocysts in the Xenopus egg extract-treated group than in the control group. The authors also found that DNA methylation in the donor cells decreased after extract treatment for 2.5 h (Yang et al., Reference Yang, Mao, Walters, Zhao, Teson, Lee and Prather2012). The present study suggests that factors contained in mature Xenopus egg extract enhanced the development of mouse SCNT embryos, however further investigation is required to identify these factors. A previous study screened for factors involved in reprogramming in amphibian egg extracts and identified the chromatin remodelling ATPase BRG1 by antibody depletion or as dominant-negative BRG1. The experiment showed that nuclear reprogramming of human somatic cells by in vitro permeabilization with Xenopus egg extract treatment required BRG1 (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004). For this reason, we tried to analyze if BRG1 is a key factor in Xenopus egg extracts for SCNT.
BRG1 (Brahma-related gene-1) is the central catalytic subunit of various chromatin-modifying enzymatic complexes. BRG1 can alter the chromatin structure of target promoters using the energy derived from ATP hydrolysis, and is a major co-regulator of transcription (Trotter and Archer, Reference Trotter and Archer2008; Singhal et al., Reference Singhal, Graumann, Wu, Araúzo-Bravo, Han, Greber, Gentile, Mann and Schöler2010; Takebayashi et al., Reference Takebayashi, Lei, Ryba, Sasaki, Dileep, Battaglia, Gao, Fang, Fan, Esteban, Tang, Crabtree, Wang and Gilbert2013). In early embryo development, BRG1 regulates zygotic genome activation (ZGA) (Bultman et al., Reference Bultman, Gebuhr, Pan, Svoboda, Schultz and Magnuson2006) and is required for preimplantation embryo development (Kidder et al., Reference Kidder, Palmer and Knott2009; Carey et al., Reference Carey, Cao, Choi, Ganguly, Wilson, Paul and Knott2015). Zygotic genome activation is induced by nuclear reprogramming after fertilization. This event involves many regulator systems, e.g. chromatin remodelling, histone modification, and DNA demethylation (Tadros and Lipshitz, Reference Tadros and Lipshitz2009). In a study by Bultman and colleagues (Bultman et al., Reference Bultman, Gebuhr, Pan, Svoboda, Schultz and Magnuson2006), depletion of maternal BRG1 reduced dimethyl-H3K4 concentrations, but this reduction did not affect global amounts of histone acetylation. Knockdown of BRG1 using RNA interference in blastocysts caused abnormal expression of pluripotency genes (e.g. oct4 and nanog) (Kidder et al., Reference Kidder, Palmer and Knott2009). Another study showed that BRG1 is necessary for Nanog silencing in the trophoblast lineage. BRG1 depletion in preimplantation embryos revealed that BRG1 cooperates with histone deacetylase 1 (HDAC1) to regulate Nanog expression (Carey et al., Reference Carey, Cao, Choi, Ganguly, Wilson, Paul and Knott2015).
BRG1 is not only required for zygote to embryo development but is also an essential protein for ES cells. SWI/SNF-BRG1 regulates pluripotency-related genes and self-renewal in ES cells (Bultman et al., Reference Bultman, Gebuhr, Yee, La Mantia, Nicholson, Gilliam, Randazzo, Metzger, Chambon, Crabtree and Magnuson2000; Kidder et al., Reference Kidder, Palmer and Knott2009). Therefore, BRG1 has different roles in different circumstances to regulate key pluripotency genes, lineage-specific genes and epigenetic modifier genes. This study found that the BRG1 protein in Xenopus egg extracts is not necessary for SCNT. BRG1-depleted Xenopus egg extract (Extract−) or Xenopus egg extracts (Extract+) was co-injected into enucleated oocytes with cumulus cells. Interesting results were seen including significantly higher development rates during early cleavage in the in vitro culture period for the NT group (Extract−), compared with the NT (Extract+) and control NT groups. Development of morulae and blastocyst was not significantly different among the three groups, but was increased in the Extract− group compared with the other groups (Table 2). In addition, expression of Oct4 in the ICM in blastocysts was more confined in the NT (Extract−) than in the control NT group and was slightly improved compared with the NT (Extract+) group (Fig. 6). Both experiments indicated that the NT group (Extract−) gave the better results, which differed from findings from other studies. Even though BRG1 was depleted from the Xenopus egg extract, the recipient oocyte still contains maternal BRG1. Therefore additional molecules of Xenopus egg extract could improve the reprogramming process in SCNT, but increased BRG1 was not necessary.
Previous research also reported the replacement of somatic cell proteins, such as lamin A/C (LMNA) and heterochromatin protein 1a (HP1a), by specific Xenopus oocyte/egg components, e.g. histone B4 and lamin III (Alberio et al., Reference Alberio, Johnson, Stick and Campbell2005; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007, Reference Miyamoto, Yamashita, Tsukiyama, Kitamura, Minami, Yamada and Imai2008; Bian et al., Reference Bian, Alberio, Allegrucci, Campbell and Johnson2009). NT is also associated with a series of chromatin component exchanges between the transplanted nucleus and the surrounding environment of the recipient egg or oocyte (Jullien et al., Reference Jullien, Pasque, Halley-Stott, Miyamoto and Gurdon2011). For example, the nuclei of mouse NIH/3T3 cells expressing somatic linker histone H1C were transplanted into Xenopus oocytes, and began to express oocyte linker histone B4 and reprogramme transplanted nuclei through exchange with chromatin components (Jullien et al., Reference Jullien, Pasque, Halley-Stott, Miyamoto and Gurdon2011). Most importantly, the uptake of linker histone B4 is required for chromatin remodelling and efficient pluripotency gene activation, such as the oct4 gene (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004; Miyamoto et al., Reference Miyamoto, Furusawa, Ohnuki, Goel, Tokunaga, Minami, Yamada, Ohsumi and Imai2007, Reference Miyamoto, Yamashita, Tsukiyama, Kitamura, Minami, Yamada and Imai2008; Jullien et al., Reference Jullien, Pasque, Halley-Stott, Miyamoto and Gurdon2011). Our results also demonstrated that the Xenopus oocyte/egg components improved the expression of the oct4 pluripotency gene in the ICM after NT treatment (Fig. 6).
Different pluripotency genes could be regulated by different systems, such as Nanog, and Nanog transcription is regulated by BRG1 during blastocyst formation (Carey et al., Reference Carey, Cao, Choi, Ganguly, Wilson, Paul and Knott2015). Nanog is key for the first cell lineage in preimplantation embryo development. In BRG1-depleted blastocysts, Nanog protein was abnormally expressed in both the ICM and TE lineages. BRG1 has a role in modulating the trophoblast lineage by chromatin remodelling activity as part of the BRG1/BAF 155 complex in conjunction with histone deacetylation (HDAC1) to silence Nanog expression (Carey et al., Reference Carey, Cao, Choi, Ganguly, Wilson, Paul and Knott2015). Compared with our results, both the Extract+ and Extract− groups enhanced Nanog expression in the blastocyst after NT treatment (Fig. 8F−H). However, Nanog expression was more contracted in the ICM in the Extract− group than in the Extract+ group (Fig. 8G , H). The results showed that, apart from BRG1, some other components in the Xenopus egg extracts enhanced nuclear reprogramming and Nanog expression. From our results and Carey’s study (Carey et al., Reference Carey, Cao, Choi, Ganguly, Wilson, Paul and Knott2015), it is clear that more research is needed to understand Nanog regulation in preimplantation embryos.
In summary, the results reported here demonstrate that Xenopus oocyte/egg extracts can improve the reprogramming process of SCNT. In particular, this process can help the mouse cloned embryo to develop to the 2-cell stage and allow more embryos to develop to the blastocyst stage. Additionally, components in the extract enhance the expression of pluripotency genes, such as Oct4 and Nanog. Although BRG1 is an important oocyte/egg-derived component that influences nuclear reprogramming, significantly higher developmental rates of cloned embryos during early cleavage were found in the BRG1-depleted Xenopus egg extract (Extract−) group. Therefore, many other unknown components in Xenopus oocyte/egg extracts regulate reprogramming, and further study is required.
Acknowledgements
The authors would like to the Ministry of Science and Technology (MOST), as well as the Ministry of Education (MOE) for financial support. We would like to thank Dr Chien-Hong Chen for guidance in micromanipulation techniques, and Prof. Chih-Feng Chen for assistance in statistical analysis.
Conflicts of interest
The authors claim that there are no conflicts of interest.
Financial support
In addition to the financial support by grants NSC 95–2313-B-005–021 and NSC 95–2313-B-005–035-MY2 from MOST, this study was supported in part by the iEGG and Animal Biotechnology Center from Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (MOE-107-S-0023-H).
Ethical standards
The use of animals and procedures for animal handling and treatments were approved by the Institutional Animal Use and Care Committee (IACUC) at the National Chung Hsing University.