Introduction
Biofuel production has increased worldwide during the last two decades (Balat and Balat Reference Balat and Balat2009), with United States and Brazil having the highest production volumes. Brazil has been successfully producing bio-ethanol from sugarcane (Saccharum officinarum L.) for more than three decades (Galdos et al. Reference Galdos, Cavalett, Seabra, Nogueira and Bonomi2013; Gupta and Verma Reference Gupta and Verma2015), and in the United States, ethanol has become an important liquid biofuel in the last 15 yr, mainly from starch fermentation in the case of corn (Zea mays L.) and more recently from lignocellulosic feedstocks (Gupta and Verma Reference Gupta and Verma2015; Sanchez and Cardona Reference Sanchez and Cardona2008). Despite benefits associated with potential reduction in greenhouse gas emissions, the increase in ethanol production generates important amounts of residuals that should be properly disposed of to minimize negative environmental impacts. For example, it is estimated that for every liter of bio-ethanol produced, at least 12 L of fermentation residuals (called vinasse or stillage) are generated and must be disposed of (Martinelli and Filoso Reference Martinelli and Filoso2008). Vinasse has chemical properties such as a low pH, corrosive potential, high chemical and biochemical oxygen demand, and high salt and nutrient levels (e.g., potassium, phosphorous, calcium) that limit the ways this fermentation residual can be disposed of or used for other purposes (Martinelli and Filoso Reference Martinelli and Filoso2008; Wilkie et al. Reference Wilkie, Riedesel and Owens2000).
Sugarcane mills producing bio-ethanol regularly dispose of vinasse by applying it dry or wet to fields as a soil amendment to recycle nutrients, increase organic matter, and favor soil aggregation and soil moisture retention (Jiang et al. 2012; Reyes-Cabrera et al. Reference Reyes-Cabrera, Erickson, Leon, Silveira, Rowland, Sollenberger and Morgan2017). Several studies have indicated that fresh liquid vinasse (i.e., without processing or long periods in oxidative tanks) can affect weed establishment and growth. For example, Christoffoleti and Bacchi (Reference Christoffoleti and Bacchi1985) determined that under field conditions at increasing rates of vinasse, Jamaican crabgrass (Digitaria horizontalis Willd.) and red tasselflower [Emilia sonchifolia (L.) D.C.] had their population densities increased by 3- to 4-fold at 91 d after treatment. Conversely, common purslane (Portulaca oleracea L.), arrowleaf sida (Sida rhombifolia L.), and purple nutsedge (Cyperus rotundus L.) had their populations reduced 50% to 60% compared with the control. Other studies conducted under controlled laboratory conditions confirmed that the response to vinasse is species dependent. However, reductions in seedling emergence have been reported in multiple cases (Azania et al. Reference Azania, Azania, Marques and Pavani2004; Soares Novo et al. Reference Soares Novo, Ramon, Do Lago and Marin2007; Soni et al. Reference Soni, Leon, Erickson, Ferrell, Silveira and Giarcanu2014), supporting the hypothesis that vinasse might contain compounds with herbicidal activity (Voll et al. Reference Voll, Adegas and Grazzeiro2010a) and could be a tool for weed management.
Soni et al. (Reference Soni, Leon, Erickson, Ferrell, Silveira and Giarcanu2014) found that the time of exposure to vinasse might be responsible for variation in the responses to this amendment. They determined that vinasse has a negative effect on weed germination and a positive effect on growth for established weeds. Using germination assays with soil, these researchers showed that vinasse dramatically reduced germination and seed viability, but large-seeded species such as sicklepod [Senna obtusifolia (L.) H. S. Irwin & Barneby] were more tolerant than small-seeded species such as Palmer amaranth (Amaranthus palmeri S. Watson). They also reported that vinasse increased the activity of soil microbiota associated with seeds, but seed viability loss and seedling mortality were due primarily to the direct effect of vinasse. This and other studies have reported reductions in germination and seedling emergence (Azania et al. Reference Azania, Marques, Pavani and Azania2003; Christoffoleti and Bacchi Reference Christoffoleti and Bacchi1985; Soni et al. Reference Soni, Leon, Erickson, Ferrell, Silveira and Giarcanu2014), but the methodologies used did not allow for determining whether the effect is on the seed embryo or seedling growth. This information is important to identify the best way to use vinasse’s herbicidal activity for weed management. For example, if the effect is primarily on nongerminated seeds, vinasse application to reduce seedbank viability can be done throughout the year. Conversely, if the activity is predominantly on seedlings once germination has started and the radicle has protruded through the seed coat, then vinasse application timing might be limited to the normal germination and seedling emergence periods of target species. Additionally, vinasse is a heterogeneous liquid by-product that has solid particles that can stay in suspension after agitation, but it is not known how much each phase contributes to the herbicidal effect this substance has on susceptible plants. Because vinasse is typically applied to the field as a liquid or dry amendment, determining whether herbicidal compounds are more likely to stay bound to solid particles or are easily available in water solutions would inform strategies to maximize its use for weed control.
The seed coat is an important selective barrier protecting and limiting the exposure of the embryo to multiple external compounds (Bewley and Black Reference Bewley and Black1994). Conversely, after radicle protrusion the seedling is directly exposed to the environment and can more easily absorb water and compounds in solution (Bewley and Black Reference Bewley and Black1994). Therefore, we hypothesized that vinasse herbicidal activity originates from the liquid phase and affects only seedling tissue after the radicle has protruded through the seed coat and that there is no effect on seed embryos of imbibed nongerminated seeds. To test these hypotheses and better inform the use of vinasse as a component of integrated weed management, we conducted a series of laboratory experiments to determine how exposure to vinasse during imbibition, germination, and seedling growth affected several weed and crop species.
Materials and Methods
Seed and Vinasse Sources
Laboratory experiments were conducted to assess the effect of vinasse on germination and radicle growth of A. palmeri, southern crabgrass [Digitaria ciliaris (Retz.) Koeler], S. obtusifolia, lettuce (Lactuca sativa L.), and wheat (Triticum aestivum L.). These species were selected to include a range of seed sizes, seed dormancy levels and mechanisms, and mono- and dicotyledonous groups. Amaranthus palmeri seed was obtained from Amlin Seed Service (Leland, MS), D. ciliaris seed from Estel Farm and Seeds (Thomas, OK), and lettuce and wheat seed from the West Florida Research and Education (Jay, FL) seed stock. Senna obtusifolia seed was collected from experimental fields with dense natural populations at the West Florida Research and Education Center. Senna obtusifolia seeds were scarified with sandpaper for 15 s to favor seed imbibition and germination. Preliminary germination and tetrazolium tests (Moore Reference Moore1985) confirmed that all seed lots had >85% viability and >65% germination potential.
Vinasse was produced by lignocellulosic fermentation of sugarcane bagasse to ethanol at the University of Florida Stan Mayfield Biorefinery Pilot Plant in Perry, FL. Residual ethanol in the crude vinasse was evaporated at 70 C to reduce the total volume 40%. This concentrated product was stored at 4 C and used as stock for the different experiments.
Vinasse was filtered with a No. 1 filter paper (Whatman, GE Healthcare Bio-Sciences, Pittsburgh, PA) followed by a 0.45-μm filter (Target2™ PVDF syringe filter, Thermo Scientific, Waltham, MA) to remove the solid phase. Then the remaining solution was centrifuged at 6,000 × g to eliminate any particles in suspension. The supernatant was transferred to a clean container. Additionally, for the salt and ionic phase experiment (see below), a fraction of the filtered liquid vinasse was run through an AG 1-X8 resin column (Bio-Rad, Hercules, CA) to reduce the concentration of salts and ionic compounds (Table 1).
Table 1 Chemical profile of stock solutions of total (solid + liquid), liquid, and liquid with reduced salt and ion concentrations (filtered) vinasse from lignocellulosic fermentation of sugarcane bagasse.

a Abbreviation: EC, electrical conductivity.
b Solid particles bigger than 0.45 µm were removed.
c Liquid vinasse was filtered with an AG 1-X8 resin column to reduce salt and ion concentrations.
Imbibition–Germination Experiment
An experiment was conducted to determine whether vinasse affects early-germination (i.e., imbibition) or late-germination (i.e., embryo growth and radicle protrusion) phases (Bewley and Black Reference Bewley and Black1994). Also, this experiment compared the effect of the liquid phase versus total vinasse (i.e., liquid plus solid phases). Seeds of the aforementioned weed and crop species were imbibed for 3 d at 25 C in petri dishes by placing them on top of a No. 1 filter paper saturated with 6 ml of a solution of total vinasse at 20% v/v (VT), a solution of liquid vinasse at 20% v/v (VL), or distilled-deionized water (WA). Preliminary studies determined that all seeds increased volume and fresh seed weight, confirming imbibition. After the imbibition phase, seeds were thoroughly rinsed with WA to remove vinasse residues. After being rinsed, seeds from each imbibition treatment (only nongerminated seeds) were separated in three groups and then transferred to petri dishes with a filter paper and 6 ml of either VT, VL, or WA for germination. Thus, there were seven imbibition–germination combinations: VT-VT, VL-VL, WA-VT, WA-VL, VT-WA, VL-WA, and WA-WA (VT-VL and VL-VT combinations were not included). Petri dishes were placed in a germinator chamber (CONVIRON A1000, Controlled Environments, Pembina, ND) with a 12-h light at 28 C and 12-h dark at 25 C regime to ensure favorable conditions for germination of the studied species (Chauhan and Johnson Reference Chauhan and Johnson2008; Creel et al. Reference Creel, Hoveland and Buchanan1968; Soni et al. Reference Soni, Leon, Erickson, Ferrell, Silveira and Giarcanu2014; Steckel et al. Reference Steckel, Sprague, Stoller and Wax2004). Seeds remained in the chamber for 10 d, and at the end of this period, germinated seeds were counted. Nongerminated seeds were transferred to a petri dish with a dry filter paper, dried at 25 C for 5 d, and subjected to a crush test to determine viability (Sawma and Mohler Reference Sawma and Mohler2002). A subsample of seeds classified as viable in the crush test was subjected to a tetrazolium test (Moore Reference Moore1985) to confirm viability. The experiment was arranged as a completely randomized design with three replications; each replication consisted of an experimental unit of 50 seeds petri dish−1 for a given species, and the experiment was conducted twice.
Radicle Growth Experiment
To determine the relation between vinasse and radicle elongation reduction, seeds of A. palmeri, S. obtusifolia, D. ciliaris, and wheat recently germinated in petri dishes with a filter paper and water (radicle was 2- to 4-mm long) were placed in square dishes with a 1% agar-based media with different vinasse concentrations. Vinasse concentrations were 0%, 1%, 2.5%, 5%, 7.5%, 10%, 20%, and 40% v/v (dilutions from the stock solution). All media were diluted with water, mixed with agar, and autoclaved. After germinated seeds were placed in the media, dishes were sealed with parafilm. There were 5 germinated seeds dish−1 for a given species, and species were kept in separate dishes. Each germinated seed was considered an experimental unit, and the experiment included 10 experimental units species−1. The dishes were placed in a germination chamber as previously described and tipped vertically to allow unimpeded gravitropic growth. The experiment was a conducted as a completely randomized design with 10 replications, and it was conducted twice. After 7 d, radicle length was measured, and abnormal growth and injury symptoms were characterized (e.g., tissue swelling, necrosis, reduced root hair production).
Salt and Ionic Phase Experiment
Recently germinated seeds of A. palmeri, S. obtusifolia, D. ciliaris, and wheat were placed in a 1% agar-based media with 0%, 1%, 2.5%, 5%, 7.5%, and 10% v/v of VL and a solution with reduced salt and ion concentrations after running VL through an ionic exchange column (VC) as previously described. The experiment was a completely randomized design with 15 replications, and it was conducted twice. Measured variables were the same as the radicle growth experiment.
Data Collection and Analysis
Results were analyzed with ANOVA using PROC GLIMMIX of SAS (SAS Institute, Cary, NC). Main effects were species, treatment, experimental repetition, and their interaction. After finding no significant interactions between main effect and experimental repetition, this latter factor was considered random. The interaction between species and treatment was highly significant (P < 0.0001), so the data were analyzed separately by species. Treatment mean separation was done with Tukey’s honestly significant difference (α=0.05). PROC NLIN of SAS was used to describe dose responses of radicle growth to vinasse.
Results and Discussion
Imbibition–Germination Experiment
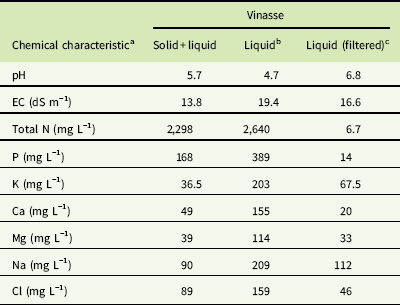
Lettuce seeds imbibed in vinasse and germinated in water exhibited no reduction in germination or increases in the proportion of nongerminated nonviable seeds compared with the control (i.e., WA-WA with >80% germination; Figure 1). Conversely, lettuce seeds that were imbibed in water and germinated in VT or VL exhibited less than 8% germination, and these germination levels were similar to those of seeds imbibed and germinated in VT and VL (Figure 1). Interestingly, the reduction in germination was directly proportional to the increase in nongerminated, nonviable seeds, which could include seeds that died before radicle protrusion and/or seeds that died soon after radicle protrusion.
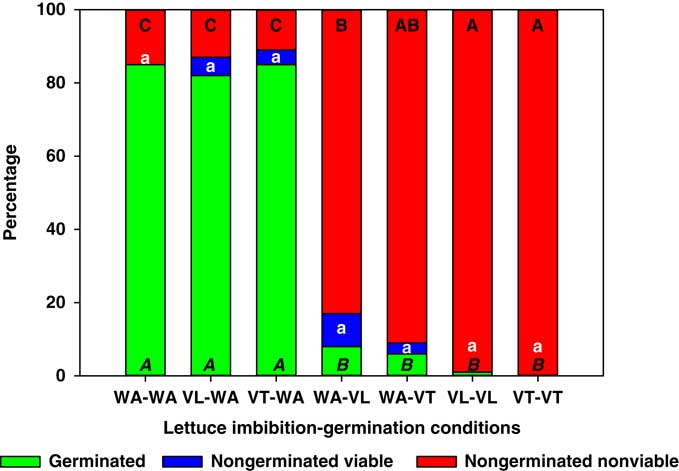
Figure 1 Germinated, nongerminated viable, and nongerminated nonviable fractions of lettuce seeds after imbibition for 3 d followed by germination for 10 d in water (WA), total vinasse (VT), or liquid vinasse (VL). The treatments are named with the imbibition and germination phases before and after the dash, respectively. Treatments with different letters within seed category were statistically different based on Tukey’s honestly significant difference (α=0.05). Italicized uppercase black, lowercase white, and regular uppercase black letters represent means separation for germinated, nongerminated viable, and nongerminated nonviable fractions, respectively.
These results suggested that the vinasse herbicidal effect on lettuce acts at later stages of germination, after imbibition has been completed, and perhaps during radicle protrusion.
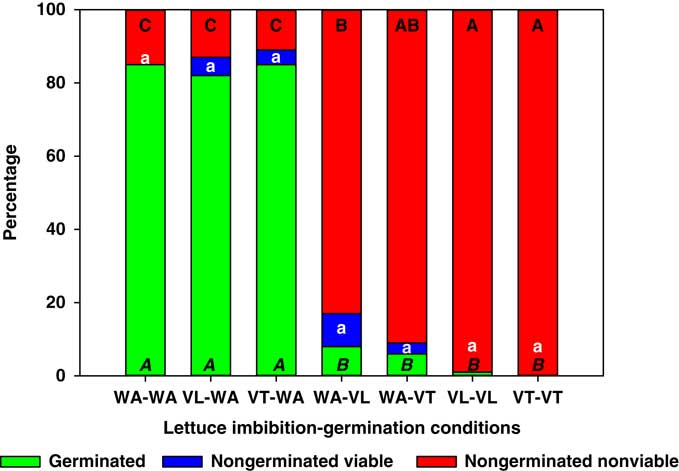
Unlike lettuce seeds, wheat seeds did not display reduced germination when imbibed or germinated with VT or VL, with the exception of the VT-VT treatment, which produced a 10% reduction in germination compared with WA-WA (Figure 2). In this case, the reduction in germination was also explained by an increase in the proportion of nongerminated nonviable seeds. Liebman and Sundberg (Reference Liebman and Sundberg2006) showed that species with larger seeds tend to be less susceptible to allelopathic compounds that affect seed germination, and wheat was among the most tolerant species studied by those researchers.
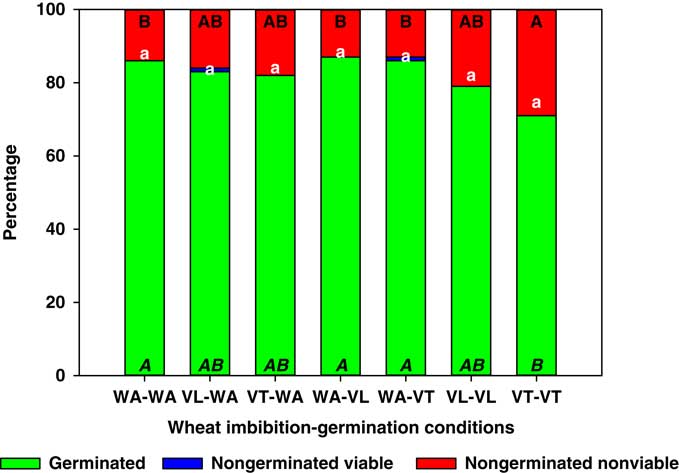
Figure 2 Germinated, nongerminated viable, and nongerminated nonviable fractions of wheat seeds after imbibition for 3 d followed by germination for 10 d in water (WA), total vinasse (VT), or liquid vinasse (VL). The treatments are named with the imbibition and germination phases before and after the dash, respectively. Treatments with different letters within seed category were statistically different based on Tukey’s honestly significant difference (α=0.05). Italicized uppercase black, lowercase white, and regular uppercase black letters represent means separation for germinated, nongerminated viable, and nongerminated nonviable fractions, respectively.
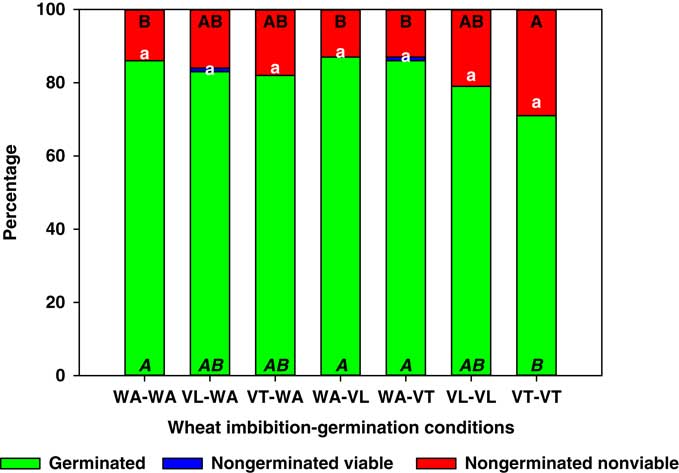
Weed seeds responded differently than crop seeds to vinasse exposure. For example, A. palmeri was considerably more susceptible to this fermentation residual than lettuce and wheat, and lower germination was not only observed for seeds that were continuously exposed to vinasse (VT-VT and VL-VL), but also in treatments with exposure only during the imbibition (VT-WA) or during the germination phase (WA-VT), when compared with WA-WA (Figure 3). Although most of the reduction in A. palmeri germination was explained by increases in nongerminated, nonviable seeds, several treatments differed in the proportion of nongerminated viable seeds. The most evident cases were VT-WA and VL-WA, which resulted in 51% and 31% nongerminated viable seeds, respectively, compared with 16% for the WA-WA control. Similarly, VL-WA and VT-WA caused a 6- and 7-fold increase in nongerminated viable seeds compared with WA-VL and WA-VT, respectively. These results indicate that imbibition on VT or VL increased A. palmeri seed dormancy.
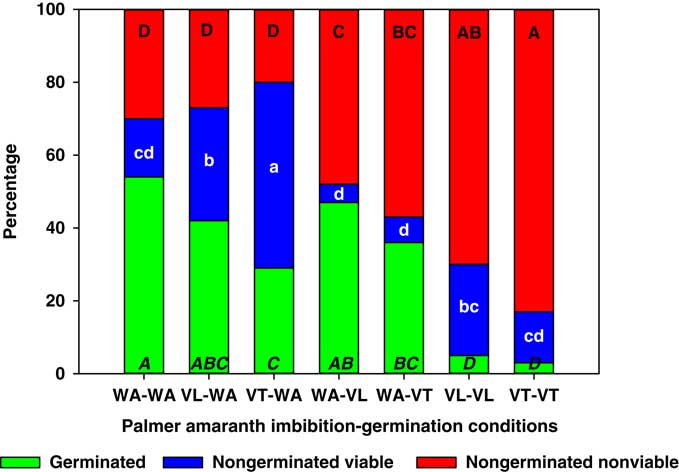
Figure 3 Germinated, nongerminated viable, and nongerminated nonviable fractions of Palmer amaranth (Amaranthus palmeri) seeds after imbibition for 3 d followed by germination for 10 d in water (WA), total vinasse (VT), or liquid vinasse (VL). The treatments are named with the imbibition and germination phases before and after the dash, respectively. Treatments with different letters within seed category were statistically different based on Tukey’s honestly significant difference (α=0.05). Italicized uppercase black, lowercase white, and regular uppercase black letters represent means separation for germinated, nongerminated viable, and nongerminated nonviable fractions, respectively.
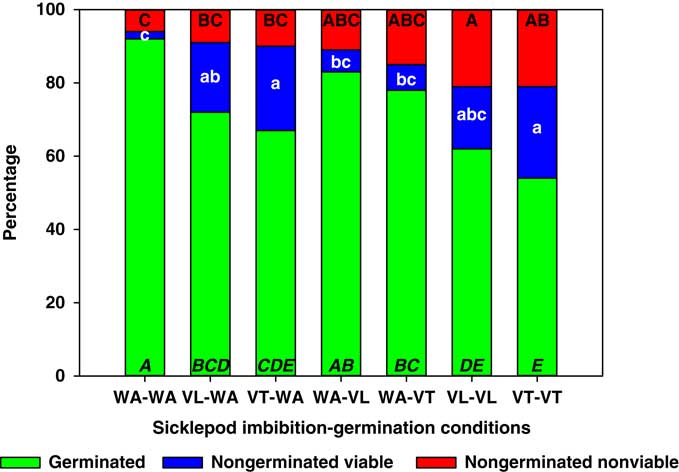
The germination of S. obtusifolia seeds in treatments WA-WA and WA-VL was similar (92% and 83%, respectively; Figure 4). The rest of the treatments resulted in reductions in germination, with VT-VT and VL-VL producing the lowest values (54% and 62%, respectively). These two treatments were also the only ones that increased the proportion of nongerminated nonviable seeds. Almost all treatments that imbibed S. obtusifolia seeds in vinasse, with the exception of VL-VL, increased the proportion of nongerminated viable seeds. In some cases, this increase was more than 15-fold (Figure 4). Senna obtusifolia seed dormancy is physical due to a hard seed coat (Baskin et al. Reference Baskin, Nan and Baskin1998; Creel et al. Reference Creel, Hoveland and Buchanan1968), but our scarification treatment consistently yielded at least 90% germination. Therefore, it is possible that imbibition in VT or VL might trigger some type of physiological dormancy (Baskin and Baskin Reference Baskin and Baskin2001; Baskin et al. Reference Baskin, Nan and Baskin1998), similar to the one observed in A. palmeri (Figure 3).
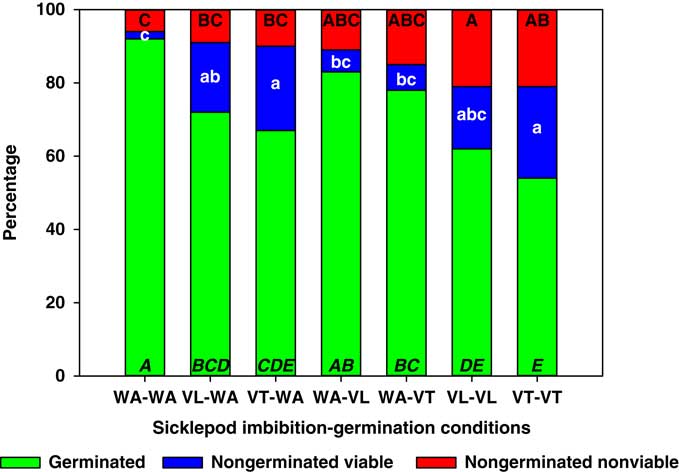
Figure 4 Germinated, nongerminated viable, and nongerminated nonviable fractions of sicklepod (Senna obtusifolia) seeds after imbibition for 3 d followed by germination for 10 d in water (WA), total vinasse (VT), or liquid vinasse (VL). The treatments are named with the imbibition and germination phases before and after the dash, respectively. Treatments with different letters within seed category were statistically different based on Tukey’s honestly significant difference (α=0.05). Italicized uppercase black, lowercase white, and regular uppercase black letters represent means separation for germinated, nongerminated viable, and nongerminated nonviable fractions, respectively.
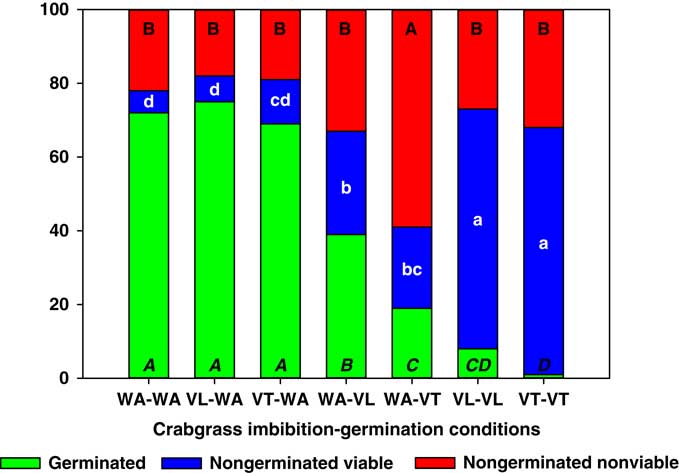
Digitaria ciliaris seeds only had reduced germination when germinated in VT or VL (Figure 5). However, this reduction was not always proportional to increases in nongerminated nonviable seeds. In fact, VT-VT and VL-VL treatments produced the same proportion of nongerminated nonviable seeds as the WA-WA control, but these two vinasse treatments resulted in a dramatic 10-fold increase in the proportion of nongerminated viable seeds compared with WA-WA. A smaller increase in nongerminated viable seeds was also observed with the WA-VT and WA-VL treatments, but unlike A. palmeri and S. obtusifolia, imbibition in VT or VL and germination in water did not increase the proportion of nongerminated viable seeds. Therefore, the inhibitory effect of vinasse on D. ciliaris germination might occur after imbibition and once seed metabolic activity has increased during the late-germination phase (Bewley and Black Reference Bewley and Black1994).
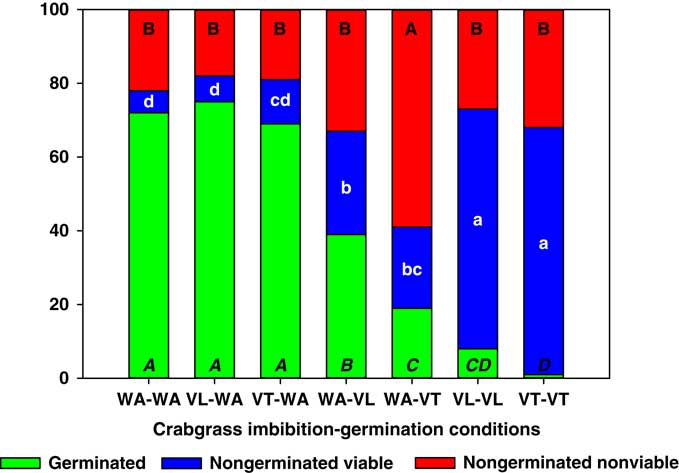
Figure 5 Germinated, nongerminated viable, and nongerminated nonviable fractions of crabgrass seeds after imbibition for 3 d followed by germination for 10 d in water (WA), total vinasse (VT), or liquid vinasse (VL). The treatments are named with the imbibition and germination phases before and after the dash, respectively. Treatments with different letters within seed category were statistically different based on Tukey’s honestly significant difference (α=0.05). Italicized uppercase black, lowercase white, and regular uppercase black letters represent means separation for germinated, nongerminated viable, and nongerminated nonviable fractions, respectively.
In this imbibition–germination experiment, there are at least two caveats worth noting. First, we acknowledge that germination is a continuum (Bewley and Black Reference Bewley and Black1994), and our classification system using only two categories—early (i.e., imbibition) and late phases (i.e., embryo growth and radicle protrusion included)—is arbitrary. Therefore, it is possible that there was overlap, especially with respect to respiration and embryo growth between these two categories (Bewley and Black Reference Bewley and Black1994). However, our methodology allowed us to determine with certainty whether exposure to vinasse only during imbibition was enough to affect the seeds, or whether longer exposure or exposure at late-germination phases might be necessary to elicit injury on the seed and seedling. More detailed studies will be needed to determine whether the effect of vinasse at late-germination phases occurs before radicle protrusion, or whether opening of the seed coat by the radicle, which allows direct exposure to vinasse, is the critical factor for embryo injury. Second, our ability to identify changes in seed dormancy relied on the assumption that the crush test provided an accurate representation of seed viability. However, it is possible that after vinasse exposure, some nongerminated seeds were classified as viable because they maintained enough structural integrity to pass the crush test, but in reality, their embryos were not viable (i.e., they were false positives). In our opinion, this is unlikely, because dissection of random subsamples of nongerminated viable seeds exhibited embryo integrity and viability based on tetrazolium tests. Also, although we did not do this consistently for all experimental units, we confirmed that seeds that had been classified as nongerminated viable did germinate a few weeks later when water was supplied. In future studies, it will be important to incorporate an additional germination phase for nongerminated viable seeds to properly describe their dormancy and viability.
Radicle Growth
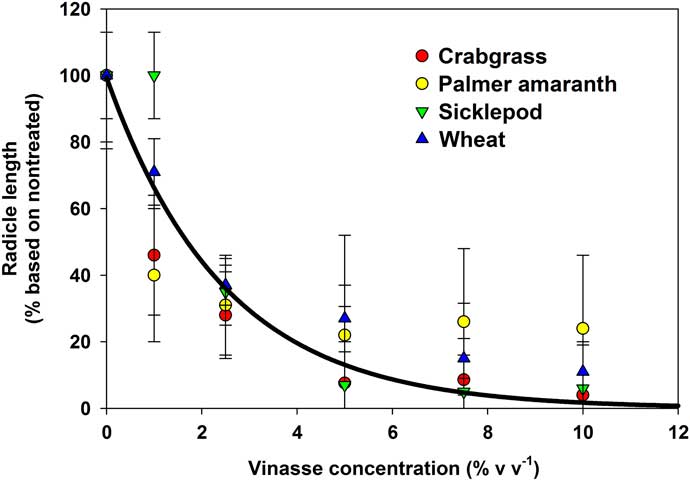
The imbibition–germination experiment indicated that most of vinasse herbicidal activity occurs after imbibition, likely once radicle protrusion has started. Therefore, to confirm that vinasse can inhibit radicle growth in seedlings, a dose–response experiment was conducted by growing recently germinated seeds in different VL concentrations. Using two-parameter exponential decay models, it was confirmed that as vinasse concentration increased, radicle growth decreased, regardless of the species (r2=0.52; P < 0.001; Figure 6). It is worth noting that even S. obtusifolia, which was less susceptible to vinasse in the imbibition–germination experiment, showed reductions in radicle growth at low concentrations and high levels of necrosis at high concentrations (Figure 7).
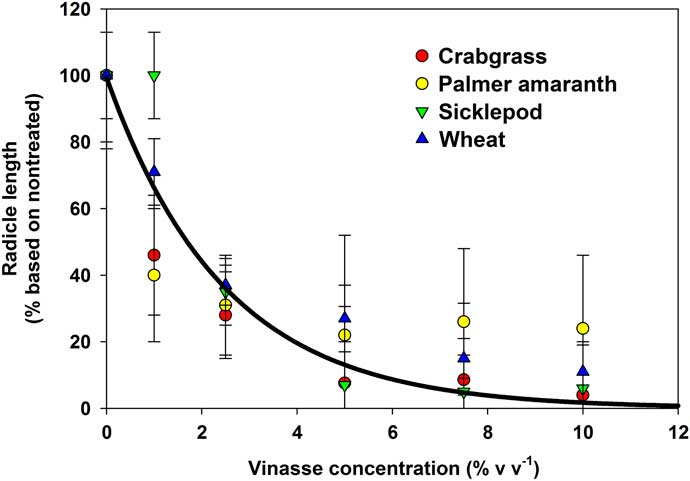
Figure 6 Radicle length of seedlings of four species relative to nontreated controls after 7 d of exposure to increasing concentrations of liquid vinasse. Error bars represent 95% confidence intervals with n=20 for each species. No differences in response to vinasse were observed among species (P > 0.21). The solid line represents the best-fit overall model [radicle length (%)=99.4635 * exp(–0.4058 * vinasse concentration)], r2=0.52, P < 0.0001.

Figure 7 Sicklepod (Senna obtusifolia) radicle growth inhibition and injury in response to increasing concentrations of liquid vinasse.
Vinasse injury on seed germination and radicle growth might be explained by the high concentration of salts and ions that this fermentation residual has, which could create a high osmotic potential and salinity levels that would dehydrate the root tip. Azania et al. (Reference Azania, Marques, Pavani and Azania2003) evaluated the effect of vinasse osmotic potential on the germination of sida and spreading liverseed grass [Urochloa decumbens (Stapf.) R. Webster] from 12% to 100% v/v, but they did not find any effects on germination and viability that could be attributed to this factor. However, the seeds were mainly dormant, and germination percentages were low, so radicle exposure to vinasse was limited. When we compared the radicle elongation for VL with normal and reduced concentrations of salts and ionic compounds (Table 1), we did not find any differences between these two vinasse types (P > 0.12). Most of the tested vinasse concentrations (≤40% v/v) had electrical conductivity (EC) values ranging from 0.5 to 5 dS m−1, which are usually not limiting for root growth in many species (Hanson Reference Hanson1990). Therefore, our results suggest that vinasse’s herbicidal activity is not a passive dehydration process of the radicle, and there might be biologically active compounds, likely in the liquid organic phase, that are responsible for these herbicidal properties. Voll et al. (Reference Voll, Gazzeiro and Adegas2010b, Reference Voll, Krzyzanowski, Gazziero and Adegas2010c) documented inhibitory effects of aconitic acid on weed seed germination and proposed that the presence of this compound in vinasse might be related to what they considered vinasse’s allelopathic effects (Voll et al. Reference Voll, Adegas and Grazzeiro2010a). However, currently there is no direct evidence that aconitic acid is solely or directly responsible for vinasse negative effect on germination and seedling growth.
Our original hypothesis that vinasse herbicidal activity is in the liquid phase was partially confirmed. Although in most cases, the maximum reduction in germination and increases in mortality were observed with VT, eliminating the solid phase from vinasse (VL) only slightly modified the results when compared with VT. Therefore, it might be feasible to isolate from the vinasse liquid phase those chemicals responsible for herbicidal activity and to purify and concentrate them. In this way, one could separate the soil amendment component of vinasse, which requires transportation of large volumes, and the herbicidal component, which might represent applications of smaller volumes of concentrated purified solutions.
These results suggested that vinasse should be used primarily to reduce weed seedling emergence, and to achieve this goal, applications should be done when conditions are favorable for germination of the target species. Interestingly, we were able to detect increases in seed dormancy in several species when seeds were imbibed in vinasse solutions. To our knowledge, there are no reports of dormancy induction triggered by vinasse. Abscisic acid (ABA) is the compound more commonly associated with induction of primary and secondary dormancy (Bewley Reference Bewley1997; Leymarie et al. Reference Leymarie, Robayo-Romero, Gendreau, Benech-Arnold and Corbineau2008), and it has been reported that stress and ABA responses overlap and both are involved in dormancy induction (Cadman et al. Reference Cadman, Toorop, Hilhorst and Finch-Savage2006). Therefore, it is possible that the stress caused by vinasse induced dormancy via ABA. Although we do not know the physiological or biochemical processes that may be involved in dormancy induction, or whether it involves the same chemical(s) involved in seedling growth inhibition, this characteristic would also be advantageous for weed management. For example, in cases of weed species with high and intermediate susceptibility, such as A. palmeri and crabgrass, respectively, vinasse not only will reduce the number of established plants by killing individuals going through the seed germination–seedling emergence transition but also by inducing dormancy in nongerminated seeds at the time of application. Although the duration of this induced dormancy is currently unknown, even if it is transient, this delay would give time for crop canopy closure to shade late-emerging weeds and for biotic factors such as microbial activity and seed predators to reduce the viable seedbank (Hartzler et al. Reference Hartzler, Battles and Nordby2004; Kremer Reference Kremer1993; Westerman et al. Reference Westerman, Hofman, Vet and van der Werf2003).
The results of the present study confirmed that vinasse has herbicidal properties that could be used for weed management purposes. Because the herbicidal effects of vinasse are likely to be transient due to rapid soil microbial degradation (Martinelli and Filoso Reference Martinelli and Filoso2008; Wilkie et al. Reference Wilkie, Riedesel and Owens2000; Yang et al. Reference Yang, Liu, Wu, Tan and Li2013), applications of vinasse as a soil amendment might not coincide with weed seedling emergence. Therefore, developing strategies to separate the application as amendment and as herbicide could help maximize both functions. Although it is possible that crop seedlings and roots might be affected by a concentrated liquid vinasse or an isolated herbicidal compound, crop seeds are considerably larger than weed seeds, so it is likely that differential sensitivity will allow the use of selective rates that provide weed suppression and/or control while causing minimal or no injury to the crop (Lopez et al. Reference Lopez, Cabrera and Murillo1992; Murillo et al. Reference Murillo, Cabrera and Lopez1993).
Based on the present and previous studies, vinasse has the potential of being a valuable tool for weed management, especially in bioenergy cropping systems. However, it might be necessary to manage the variability associated with differences in feedstock, fermentation processes, and soil types, which might affect both the concentration of herbicidal compounds and their stability. However, considering the large volumes of vinasse that are produced annually during bio-ethanol production, it is worth exploring in more detail the use of this by-product for weed management either by using concentrated solutions or by identifying the chemicals directly responsible for the herbicidal activity, and engineering purification methods to develop new natural herbicides (Dayan et al. Reference Dayan, Cantrell and Duke2009; Duke et al. Reference Duke, Dayan, Romagni and Rimando2000).
Acknowledgements
We want to thank Neeta Soni for technical advice on vinasse processing and rate selection and Michael Dozier for technical support.