INTRODUCTION
Schistosomiasis is endemic in 76 countries and territories (Engels et al. Reference Engels, Chitsulo, Montresor and Savioli2002), with an estimated 779 million people at risk of schistosomiasis and 207 million people infected (Steinmann et al. Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006). In China, Schistosoma japonicum infection remains an important public health problem, with an estimated 726 000 people infected in 2004 (Zhou et al. Reference Zhou, Guo, Wu, Jiang, Zheng, Dang, Wang, Xu, Zhu, Wu, Li, Xu, Chen, Wang, Zhu, Qiu, Dong, Zhao, Zhang, Zhao, Xia, Wang, Zhang, Lin, Chen and Hao2007a). At large scales, risk of Schistosoma infections is associated with climatic factors and proximity to permanent water bodies (Brooker, Reference Brooker2002; Yang et al. Reference Yang, Vounatsoub, Zhou, Tanner and Utzinger2005; Brooker et al. Reference Brooker, Clements and Bundy2006b; Peng et al. Reference Peng, Zhang, Zhuang, Zhou and Jiang2006).
Previous work has shown that schistosomiasis risk is focal at the county level in China. For example, studies have identified high numbers of acute schistosomiasis cases in Guichi, in an area covering approximately 2500 km2 (Zhang et al. Reference Zhang, Carpenter, Chen, Clark, Lynn, Peng, Zhou, Zhao and Jiang2008, Reference Zhang, Carpenter, Lynn, Chen, Bivand, Clark, Hui, Peng, Zhou, Zhao and Jiang2009a, Reference Zhang, Clark, Bivand, Chen, Carpenter, Peng, Zhou, Zhao and Jiangb). However, there is limited information on the determinants of small-scale spatial variation in schistosomiasis risk, and it is not known whether risk is heterogeneous within such foci. Apart from providing insight into the drivers of transmission, knowledge of small-scale variation is important because debate exists as to whether mass treatment with praziquantel or individual case detection is the optimal approach to schistosomiasis control (Williams et al. Reference Williams, Sleigh, Li, Feng, Davis, Chen, Ross, Bergquist and McManus2002). If schistosomiasis risk is relatively homogeneous within communities, this would support a mass treatment approach, whereas if substantial heterogeneity was found at this level, support would be added to the individual case detection approach. Identification of high-risk groups, such as occupational groups, could help to prioritize community pre-treatment screening programmes. The purpose of our study was to identify areas of high and low risk of schistosomiasis within a community in a highly endemic area of China, and the factors influencing small-scale spatial variation.
MATERIALS AND METHODS
Study area and population
This study was conducted in the Ximiao administrative village, Jiangxi province, located adjacent habitats of Oncomelania hupensis (the intermediate host snail of S. japonicum) in Poyang Lake, the biggest fresh water lake in China. The administrative village comprises 9 smaller ‘natural villages’ and is known to be a highly endemic area for S. japonicum. Residents in the village are infected with S. japonicum mainly through contacts with water in the lake or on the beach. The study area covers about 6 km2. Study participants included 920 (89·3%) of 1030 people in the resident population aged 5–80 years. The remaining 10·7% refused to participate in the study.
Written informed consent was obtained from all adult participants and from the parents or legal guardians of children. Ethical approval for the study was obtained from village (local government), county (anti-schistosomiasis) and provincial (schistosomiasis headquarters) authorities, and was endorsed by Fudan University.
Demographic and parasitological data
Demographic and parasitological data were obtained via a cross-sectional survey conducted in October 2007. The age, sex, education and occupation of survey participants were obtained from an existing database at the Xingzi Anti-schistosomiasis Station. Occupation was divided into 3 groups: farmers, fishermen and others (comprising students, teachers, businessmen and children in kindergarten, with small numbers in each of these subgroups). Participants were asked to produce a stool specimen and 50 g of each specimen was examined within 24 h of sample collection and 1–12 h after slide preparation (3 slides per sample) by quantitative Kato-Katz (KK) thick smear (Katz et al. Reference Katz, Chaves and Pellegrino1972). S. japonicum egg counts were expressed in eggs per gram of stool (EPG), averaged across the 3 slides. Individuals with a positive stool examination result were treated with a single oral dose of praziquantel (40 mg/kg).
Households and snail habitat mapping
Homes of every person enrolled in the study area were visited and the locations of the households were determined using a hand-held global position system (GPS) GPSMAP 76 (Garmin Ltd, Olathe, KS, USA). Snail habitats (i.e. sites where snail colonies were found to be located) near the village were geo-located using the GPS. Distances from each household to the nearest snail habitat were computed using the Distance Matrix extension (www.jennessent.com/arcview/dist_matrix.htm) of the GIS ArcView 3.2 (ESRI, Redlands, CA, USA). In Poyang Lake area, the distance to the nearest snail habitat is the same as the distance to water contact sites since snail habitats are under water in summer. All infections occur within the lake basin in the bottomlands; there are no snails behind the dikes (Davis et al. Reference Davis, Wu, Williams, Liu, Lu, Chen, Zheng, McManus and Guo2006). The distances were divided into quartiles (⩽238 m, 238–419 m, 419–681 m and >681 m).
Spatial cluster detection
We implemented the spatial scan statistic using the software SatTScan (Kulldorff et al. Reference Kulldorff2008). This method is well described in the literature (Kulldorff, Reference Kulldorff1997) and tests whether events such as disease cases are distributed randomly over space and, if not, identifies the location of significant spatial clusters. The Bernoulli variant of the statistic was used because the outcome was a dichotomous variable (positive, with 1 or more S. japonicum eggs detected in the faeces, or negative). The test uses a moving circular window, the maximum size of which we set to include no more than 50% of the total population. The software was set to detect both high- and low-risk clusters. A likelihood ratio test was used to determine if there was a higher, or lower risk of schistosomiasis inside compared to outside each window and to define the ‘most likely’ cluster (i.e. the cluster that was least likely to have occurred by chance). A P-value was obtained by repeating the same analytic exercise on 9999 random replications of the data set, generated under the null hypothesis of no spatial clustering. Only statistically significant clusters (P⩽0·05) were reported. Clusters were mapped using ArcGIS 9.2 (ESRI, Redlands, CA, USA) in order to identify their physical location. Demographic, social and environmental predictor variables were then aggregated to the cluster areas in order to identify their possible relationship with schistosomiasis clustering.
Multivariate analysis
First, binary logistic regression and Bayesian logistic regression model (details are provided in the Appendix) were used to examine the relationship between individual infection status and gender, age, education, occupation and household distance to the nearest snail habitats. Second, multinomial logistic regression analyses were performed to identify risk factors for being located in high-risk cluster, low-risk cluster and non-cluster areas. Large-scale environmental variables such as normalized difference vegetation index (NDVI) and land surface temperature (LST) were not included in our study because they did not vary substantially within the small study area (Brooker, Reference Brooker2007; Raso et al. Reference Raso, Matthys, N'Goran, Tanner, Vounatsou and Utzinger2005). All variables were selected in above analyses according to their known association with schistosomiasis, and all were entered in the final models. All analyses were carried out using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) and WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge and Imperial College, London, UK).
RESULTS
Prevalence of schistosomiasis
Sixty of 920 persons (6·5%) were infected with S. japonicum. All cases were chronic. Based on chi-square tests, males had a significantly higher prevalence of schistosomiasis than females (χ2=4·62, P=0·03). There was no significant difference in risk of infection between age groups (P=0·44) and education levels (P=0·33). Most cases were fishermen (26/60, 43·3%) and occupation was significantly associated with risk of infection (χ2=21·15, P<0·001). Distance of household to the nearest snail habitat was marginally significant (χ2=7·61, P=0·055).
Spatial clusters of schistosomiasis
The spatial scan statistic detected 1 high-risk and 1 low-risk cluster (Fig. 1, Table 1). There was 1 case at 44 households, 2 cases at 6 households, and 4 cases at 1 household. The prevalence of infection in the high-risk cluster, low-risk cluster and non-cluster areas were 12·3%, 1·3% and 4·3%, respectively. The relative risks of infection in high and low-risk cluster areas were 3·94 and 0·16, respectively. The high-risk cluster had a radius of 550 m, and included 139/364 (38·2%) households and 42/60 (70·0%) cases. The low-risk cluster had a radius of 480 m, and included 93/364 (25·5%) households and 3/60 (5·0%) cases.

Fig. 1. Map of the household distribution of schistosomiasis cases and the nearby snail habitats at Ximiao village, Poyang Lake region, China and the locations of high and low clusters of cases as identified by the spatial scan statistic.
Table 1. Characteristics of spatial clusters of Schistosoma japonicum infection detected using the spatial scan statistic, Poyang Lake region, China, 2007

* High risk-cluster with relative risk >1; no cluster-aggregation of non-clustered population. Low risk-cluster with relative risk <1.
† RR-Relative risk.
§ LLR-Log likelihood ratio.
Risk factors
In the binary logistic regression analysis (Table 2), males were at greater risk than females (OR 1·75, 95% CI 1·0, 3·1) and fishermen were at greater risk than farmers and other occupational groups (OR 4·6, 95% CI 2·1, 10·1 for fishermen versus farmers and OR 9·9, 95% CI 1·5, 64·1 for fishermen versus other occupational groups). In the Bayesian logistic regression model (Table 3), statistically significant correlations suggested that infection prevalence was higher in males and fishermen, but no association was found between prevalence and age, education and distance to snail habitats. In multinomial logistic regression analyses (Table 4), all variables except for gender and age were significantly associated with being in a high-risk, low-risk or no cluster area (P<0·001). People who had a higher education level, fishermen and people who lived in closer proximity to snail habitats were more likely to be located in a high-risk cluster.
Table 2. Prevalence (%) and unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for schistosomiasis stratified by personal characteristics, Poyang Lake region, China, 2007

* Derived from the univariate analysis without controlling for potential confounders.
† Adjusted for other variables as potential confounders.
§ Significant variables (P<0·05).
The reference group for sex was female, for age was 60–80 years, for education was >6 years, for occupation was others, and for distance was >681 m.
Table 3. Bayesian logistic regression model of prevalence of infection with Schistosoma japonicum among individuals aged 5–80 at 364 households, Poyang Lake region, China, 2007Footnote *
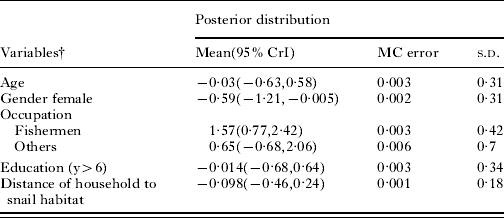
* CrI: Bayesian credible interval.
MC: Monte Carlo.
s.d.: Standard Deviation.
† The reference group for sex was male, for education was ⩽6 years, for occupation was farmer.
Table 4. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for relative risk (RR) of high cluster (RR=3·94) and low cluster (RR=0·16) against non-cluster from a multinomial regression model

* Derived from the univariate analysis without controlling for potential confounders.
† Adjusted for other variables as potential confounders.
§ Significant variables (P<0·05).
¶ The reference group for sex was female, for age was 60–80 years, for education was >6 years, for occupation was others, and for distance was >681 m.
DISCUSSION
The epidemiology of schistosomiasis japonicum over small areas remains poorly understood, and this is particularly true in China, where both the prevalence and mean intensity of S. japonicum infection in endemic areas has decreased dramatically in recent decades to historically low levels (Jiang et al. Reference Jiang, Wang, Guo, Chen, Zhou and Engels2002; Zhou et al. Reference Zhou, Wang, Chen, Wu, Jiang, Chen, Zheng and Utzinger2005). This study has demonstrated substantial variation in the prevalence of schistosomiasis at a small spatial scale (about 6 km2) in the Poyang lake region from the results of the spatial scan statistic. Education, occupation and distance to snail habitats were significant factors for infection with S. japonicum for the probability of living in a high-risk area. However, at individual levels, only demographic factors (gender, occupation) were significant predictors of infection. Occupation, specifically being a fisherman, was strongly associated both with the individual's risk of schistosomiasis and the probability of living in a high-risk area. The results are consistent with previous findings (Li et al. Reference Li, Ross, Yu, Li, Williams and McManus1997; Huang and Manderson, Reference Huang and Manderson2005; Raso et al. Reference Raso, Li, Zhao, Balen, Williams and McManus2009; Yang et al. Reference Yang, Zhao, Li, Krewski and Shi2009). In the study area most fishermen are involved daily in activities that put them in contact with infected water, whereas the farmers work mostly in the fields where there are no snail habitats or infected water, and so they come into contact less regularly with the schistosome transmission areas.
Living nearby to snail habitats was significantly associated with the probability of living in a high-risk area. A common assumption is that with increased distance from infected water, water contact becomes less frequent and therefore the infection risk decreases (Raso et al. Reference Raso, Li, Zhao, Balen, Williams and McManus2009). It is surprising that there was no association between distance to snail habitats or infected water and the individual's risk of schistosomiasis. The results confirmed previous findings (Gazzinelli et al. Reference Gazzinelli, Hightower, LoVerde, Haddad, Pereira, Bethony, Correa-Oliveira and Kloos2006; Raso et al. Reference Raso, Li, Zhao, Balen, Williams and McManus2009) and suggest that demographic factors (gender, occupation) rather than distance to infected water are driving human transmission at small spatial scales.
The spatial variations in schistosomiasis risk at small and large scales were determined by previous studies (Kloos et al. Reference Kloos, Fulford, Butterworth, Sturrock, Ouma, Kariuki, Thiongo, Dalton and Klumpp1997, Reference Kloos, Gazzinelli and Van Zuyle1998; Brooker et al. Reference Brooker, Alexander, Geiger, Moyeed, Stander, Fleming, Hotez, Correa-Oliveira and Bethony2006a; Clennon et al. Reference Clennon, Mungai, Muchiri, King and Kitron2006; Gazzinelli et al. Reference Gazzinelli, Hightower, LoVerde, Haddad, Pereira, Bethony, Correa-Oliveira and Kloos2006; Steinmann et al. Reference Steinmann, Zhou, Matthys, Li, Li, Chen, Yang, Fan, Jia, Vounatsou and Utzinger2007; Wu et al. Reference Wu, Wang, Utzinger, Yang, Kristensen, Berquist, Zhao, Dang and Zhou2007; Pullan et al. Reference Pullan, Bethony, Geiger, Cundill, Correa-Oliveira, Quinnell and Brooker2008; Raso et al. Reference Raso, Li, Zhao, Balen, Williams and McManus2009). Other Chinese studies (Steinmann et al. Reference Steinmann, Zhou, Matthys, Li, Li, Chen, Yang, Fan, Jia, Vounatsou and Utzinger2007; Wu et al. Reference Wu, Wang, Utzinger, Yang, Kristensen, Berquist, Zhao, Dang and Zhou2007; Raso et al. Reference Raso, Li, Zhao, Balen, Williams and McManus2009) investigated schistosomiasis risk at the village level, but ours is the first to investigate risk at the household level.
The results suggest that, due to considerable heterogeneity between occupational and social groups, and between different areas at this small spatial scale, targeted chemotherapy using pre-treatment screening (particularly in high-risk occupational groups) might be more efficient than mass drug administration. In the study area, we should focus on the high-risk cluster which had a radius of 550 m, and included 139 households and 42 cases. Since the praziquantel-based control of schistosomiasis japonica in China alone is unlikely to eradicate the parasite (Zhou et al. Reference Zhou, Zhao and Jiang2007c), other control and prevention programmes should be considered. It is necessary to integrate praziquantel-based control of schistosomiasis with health education aimed at altering behaviour among high-risk groups. Targeted chemotherapy using pre-treatment screening, combined with the comprehensive control strategy aimed at reducing the roles of humans and bovines (cattle and buffalo) as sources of infection for snails (Wang et al. Reference Wang, Chen, Guo, Zeng, Hong, Xiong, Wu, Wang, Wang, Xia, Hao, Chin and Zhou2009) may be a good strategy to control transmission of S. japonicum in the study area. Further studies are required to determine the relative cost-effectiveness of the different approaches.
One limitation of the present analysis is the method used for diagnosis of schistosomiasis. Some studies have shown that repeated egg counts of one individual by Kato-Katz thick smear can vary considerably, and that many infected individuals remain undetected if only a single examination is performed, particularly in low-transmission areas (Utzinger et al. Reference Utzinger, Booth, N'Goran, Muller, Tanner and Lengeler2001; Berhe et al. Reference Berhe, Medhin, Erko, Smith, Gedamu, Bereded, Moore, Habte, Redda, Gebre-Michael and Gundersen2004; Zhou et al. Reference Zhou, Yang, Wang, Zhao, Wei, Peng and Jiang2007b). However, the Kato-Katz technique is a better method for population screening of S. japonicum in moderate and high endemic areas than the hatching test and indirect haemagglutination assay (IHA) (Yu et al. Reference Yu, de Vlas, Jiang and Gryseels2007). Also of concern is the potential bias caused by those who refused to participate. Unfortunately, information on these individuals was not collected so it was not possible to estimate the magnitude or direction of any bias. With regard to the spatial scan statistic, a disadvantage is the subjective nature of the selection of the maximum cluster size (Lawson, Reference Lawson2006). However, the spatial scan statistic is statistically robust (using Monte Carlo simulation for significance testing) and has wide application in infectious disease and parasitic infection studies (Cousens et al. Reference Cousens, Smith, Ward, Everington, Knight, Zeidler, Stewart, Smith-Bathgate, Macleod, Mackenzie and Will2001; Chaput et al. Reference Chaput, Meek and Heimer2002; Enemark et al. Reference Enemark, Ahrens, Juel, Petersen, Petersen, Andersen, Lind and Thamsborg2002; Mostashari et al. Reference Mostashari, Kulldorff, Hartman, Miller and Kulasekera2003; Brooker et al. Reference Brooker, Clarke, Njagi, Polack, Mugo, Estambale, Muchiri, Magnussen and Cox2004; Jennings et al. Reference Jennings, Curriero, Celentano and Ellen2005; Langkjer et al. Reference Langkjer, Vigre, Enemark and Maddox-Hyttell2007).
In summary, this study identified high-risk areas of schistosomiasis and associated factors that partly explain the observed spatial heterogeneity. The results support a targeted approach to schistosomiasis control based on pre-treatment screening. The spatial scan statistic and geographical information systems (GIS) combined with Bayesian geostatistical models and multinomial logistic regression analyses can provide powerful tools for understanding the epidemiology of diseases and for improving disease prevention and control, especially in vector-borne or environment-related diseases. Future studies should be aimed at better understanding the dynamics and spatial heterogeneity of schistosomiasis at local scales and we propose to extend our study to other ecological settings, such as mountainous regions.
ACKNOWLEDGMENTS
This study would have been impossible without the assistance and cooperation with the Xingzi Anti-schistosomiasis Station. We are indebted to the local communities and administrative authorities for their collaboration. We are also indebted to two anonymous reviewers for helpful comments and suggestions. This research was partially supported by the National Natural Science Foundation of China (grant number 30590374), by the National High Technology Research and Development Program of China (grant number 2006AA02Z402), by the National Major Project on Science and Technology (grant number 2008ZX10004-011), and by Shanghai Leading Academic Discipline Project (grant number B118).
APPENDIX
Model building and assessment
The model was of the form
where Y i was the positive infection status of individuals, pi was the risk for positive infection status in individuals.
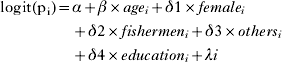
where α was the intercept, β was the coefficient for age, δ1 was the coefficient for female gender, δ2 was the coefficient for occupation as fishermen, δ3 was the coefficient for occupation as others, δ4 was the coefficient for education (year >6).
where ε was the coefficient for distances of households to the nearest snail habitat, θi was defined by the isotropic, exponentially decaying correlation function
where d ij are the distances between pairs of points i and j, and φ is the rate of decline of spatial correlation per unit of distance. Non-informative priors were specified for the intercept (uniform prior with bounds −∞ and +∞) and the coefficients (normal prior with mean=0 and precision, the inverse of variance=1×10−4). The prior distribution of φ was also uniform with upper and lower bounds set at 0·5 and 200. The precision of θi was given a non-informative prior gamma.
Model fitting used Markov Chain Monte Carlo simulation techniques. A burn-in of 1000 iterations was allowed, followed by 10 000 iterations where values for the intercept and coefficients were stored. Diagnostic tests for convergence of the stored variables were undertaken, including visual examination of history and density plots of the model parameters, and by computing Monte Carlo errors (MCE; if MCE was less than 0·05, it was decided that sufficient iterations had been conducted): convergence was successfully achieved after 10 000 iterations. The chains were also examined for autocorrelation by visual examination of the inbuilt autocorrelation function of WinBUGS.







