Fallot-type absent pulmonary valve syndrome with patent ductus arteriosus and disconnected pulmonary arteries is an extremely rare condition.Reference Lakier, Stanger and Heymann1 Fallot-type absent pulmonary valve syndrome is usually associated with agenesis of ductus as patent ductus arteriosus during fetal period is incompatible with life. If patent ductus arteriosus is present, the branch pulmonary arteries get disconnected to allow survival.
A 9-month-old infant presented with breathlessness and was saturating 88–90%. Imaging studies revealed large subaortic malaligned ventricular septal defect with overriding of aorta, absent pulmonary valve, stenotic pulmonary valve annulus, hugely dilated main pulmonary artery, absent pulmonary artery confluence, main pulmonary artery continuing as right pulmonary artery, patent ductus arteriosus continuing as left pulmonary artery, moderate pulmonary stenosis, severe pulmonary regurgitation (Figs 1 and 2), and severe right bronchial obstruction (Fig 3). He underwent successful intracardiac repair ventricular septal defect closure, right pulmonary artery reduction plasty, implanting the left pulmonary artery on right pulmonary artery creating a confluence, and transannular patch augmentation of right ventricular outflow tract with bicuspid polytetrafluoroethylene valve implantation. Post-operative recovery was uneventful.
The unresolved question in developmental basis of Fallot-type absent pulmonary valve syndrome is the primary event causing the pathology, whether defective development of the pulmonary valveReference Berg, Thomsen and Geipel2 or pre-mature constriction of ductus leading to non-development of pulmonary valve.Reference Emmanoulides, Thanopoulos and Siassi3 Fallot-type absent pulmonary valve syndrome due to defective neural crest cells leads to diastolic collapse of main pulmonary artery and diastolic flow reversal in descending aorta to main pulmonary artery and right ventricle. The presence of patent ductus arteriosus leads to severe pulmonary regurgitation, right ventricular failure, and fetal demise. Survival is possible only if the ductus is either absent or restricted and not connected to main pulmonary artery. In our case, the presence of disconnected branch pulmonary arteries with left pulmonary artery not connected to main pulmonary artery and patent ductus arteriosus continuing as left pulmonary artery did not allow severe pulmonary regurgitation and right ventricular failure allowing the child to survive. Further, the left pulmonary artery arising from restrictive patent ductus arteriosus was not aneurysmally dilated, and hence no airway obstruction was present on the left side. The presence of normal lung on one side favoured a rapid post-operative recovery compared to Fallot-type absent pulmonary valve syndrome with absent ductus and well-formed aneurysmal pulmonary artery confluence.
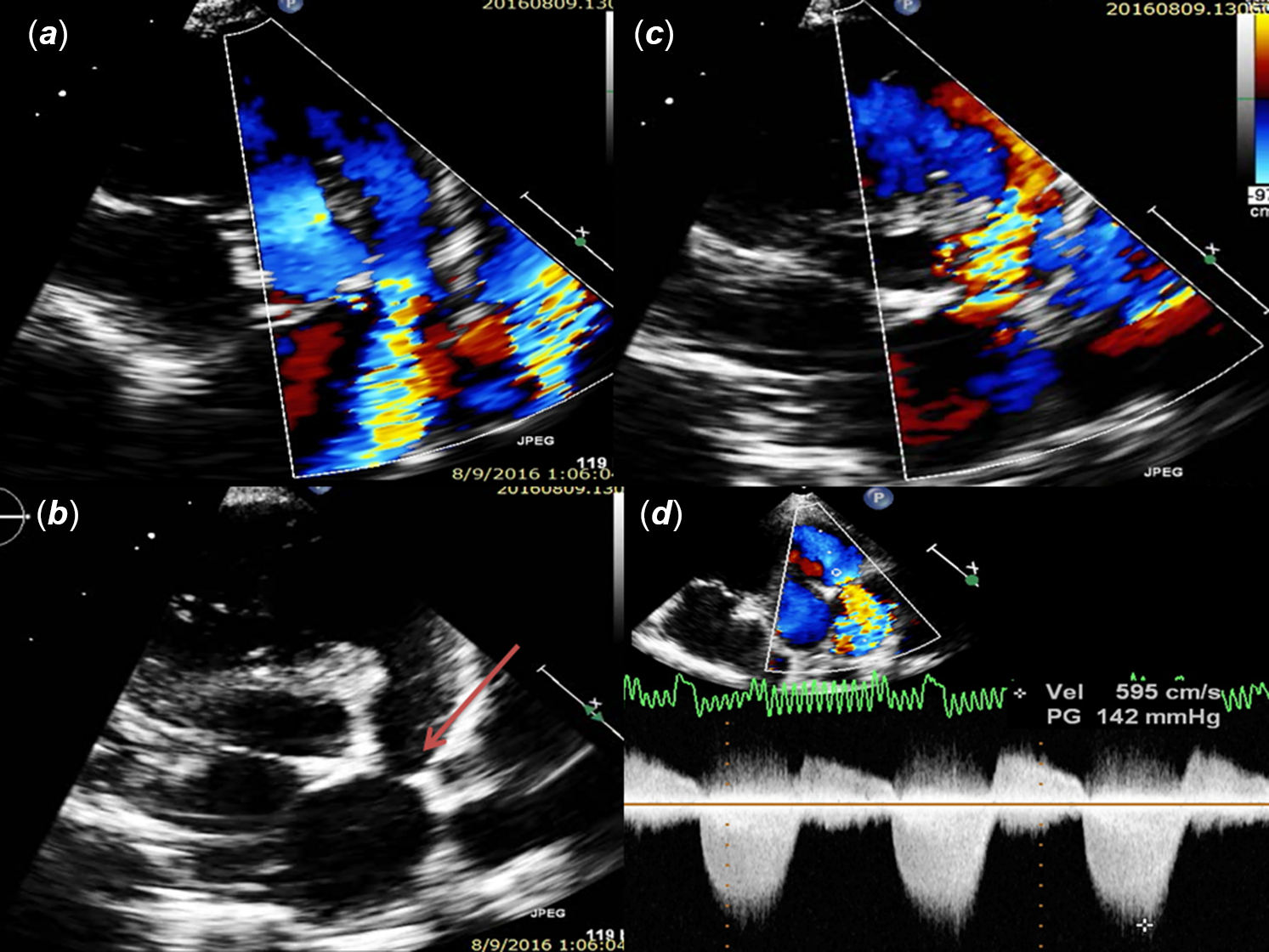
Figure 1. (a and b) Echocardiography parasternal short-axis view of right ventricular outflow tract showing severe annular stenosis due to echogenic ridge (Arrow) projecting in to the lumen; (c) moderate pulmonary regurgitation; (d) Doppler showing both pulmonary stenosis and regurgitation.
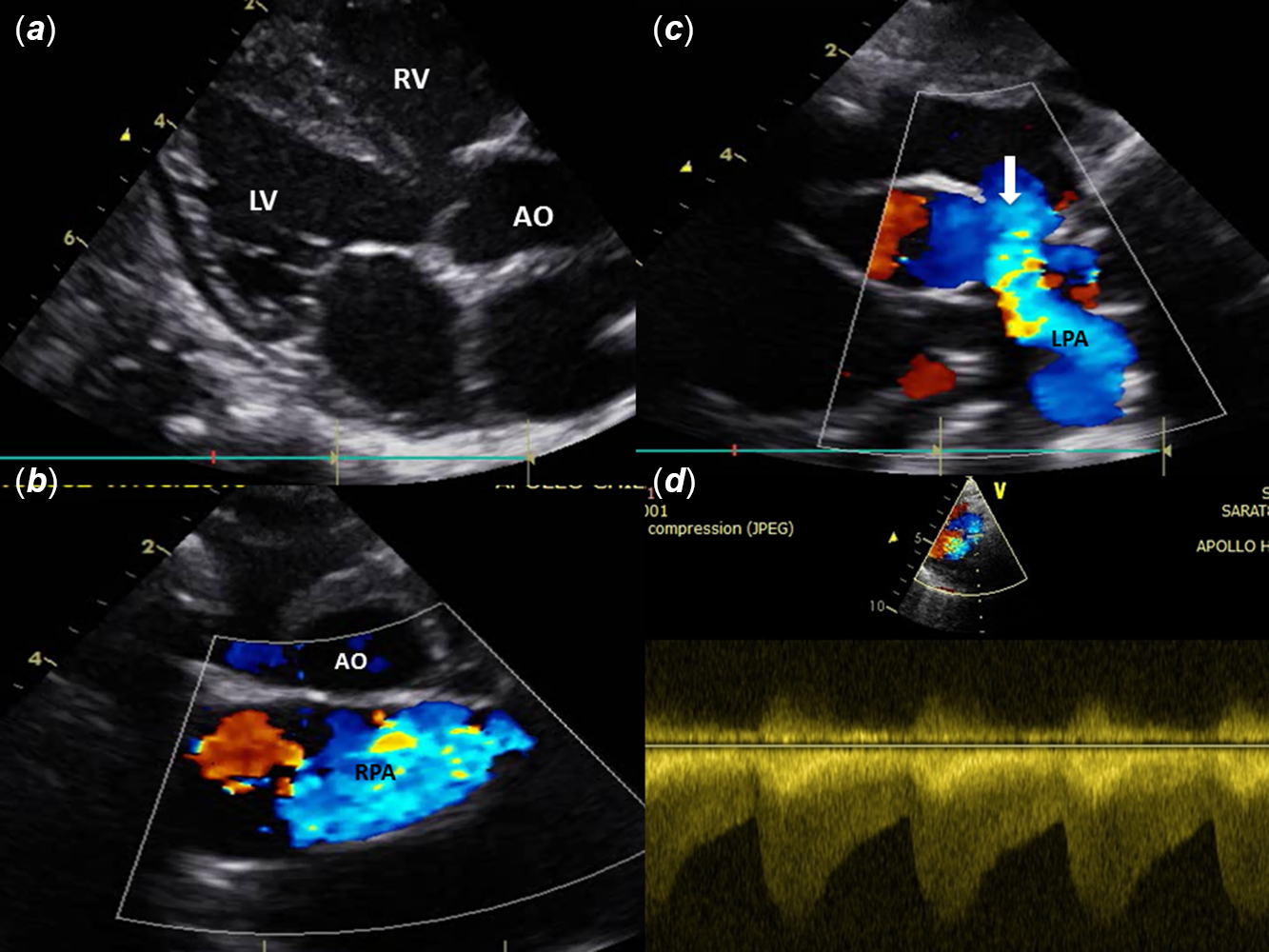
Figure 2. Echocardiography showing (a) large subaortic malaligned ventricular septal defect; (b) aneurysmally dilated right pulmonary artery; (c) patent ductus arteriosus continuing as left pulmonary artery; (d) Doppler showing continuous flow pattern in patent ductus arteriosus.

Figure 3. CT images showing (a and b) disconnected branch pulmonary arteries, patent ductus arteriosus continuing as left pulmonary artery; (c) main pulmonary artery continuing as right pulmonary artery); (d) right bronchial obstruction by aneurysmal right pulmonary artery.
Acknowledgement
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.






