Introduction
Disruption of circadian rhythms due to rotating and night shift work or jet lag has been implicated in a variety of reproductive dysfunctions in women, including irregular, extended menstrual cycles, increased risk of pre-term birth, and overall reduced fecundity (Baker & Driver, Reference Baker and Driver2007; Lawson et al., Reference Lawson, Whelan, Lividoti, Spiegelman, Schernhammer and Rich-Edwards2011; Gamble et al., Reference Gamble, Resuehr and Johnson2013).
The circadian clock is cell autonomous and characterized by transcriptional–translational feedback loops (Reppert & Weaver, Reference Reppert and Weaver2002). Previous work has shown that BMAL1 (brain muscle arnt-like 1) protein (also known as MOP3 or ARNTL1), in a dimeric complex with the CLOCK protein, is an essential component that controls circadian driven gene expression (Lowrey & Takahashi, Reference Lowrey and Takahashi2011). Loss of Bmal1 expression disrupts behavioral rhythmicity and gene expression rhythms in the SCN and peripheral tissues (Bunger et al., Reference Bunger, Wilsbacher, Moran, Clendenin, Radcliffe, Hogenesch, Simon, Takahashi and Bradfield2000). In human, polymorphisms in BMAL1 and the CLOCK paralog NPAS2 also have been associated with altered pregnancy rates and risk of miscarriage (Kovanen et al., Reference Kovanen, Saarikoski, Aromaa, Lonnqvist and Partonen2010).
The effects of deletion of the core biological clock gene Bmal1 in female mice on early embryo development are still unclear. The original report of the Bmal1 null mice stated that they were viable, fertile, and with a normal distribution of genotype (Bunger et al., Reference Bunger, Wilsbacher, Moran, Clendenin, Radcliffe, Hogenesch, Simon, Takahashi and Bradfield2000). Later studies demonstrated that Bmal1 null mice are infertile, but reports on the early embryo development were not the same. Ratajczak et al. reported normal embryo development up to day 3.5 of gestation and suggested that the infertility of Bmal1 null mice was due primarily to poor implantation (Ratajczak et al., Reference Ratajczak, Boehle and Muglia2009). Boden et al. found Bmal1 knockout mice ovulate, but with poor embryo development after mating in both natural and hormone stimulated cycles, and it was not clear from their study if the failure to produce mature blastocysts was because of poor oocyte quality or because the oviduct failed to support the developing embryo (Boden et al., Reference Boden, Varcoe, Voultsios and Kennaway2010).
Data obtained in different organisms have established a tight connection between the biological clock and cellular redox signalling (Patel et al., Reference Patel, Velingkaar and Kondratov2014). Increased levels of reactive oxygen species (ROS) had been detected in some tissues of Bmal1 null mice, and this was considered to be correlated with reduced lifespan and various symptoms of premature aging observed in this model animal (Kondratov et al., Reference Kondratov, Kondratova, Gorbacheva, Vykhovanets and Antoch2006). In reproductive physiology, ROS is involved in normal ovulation, corpus luteum regression, while excess ROS is proven to be detrimental for reproductive function (Shkolnik et al., Reference Shkolnik, Tadmor, Ben-Dor, Nevo, Galiani and Dekel2011; Agarwal et al., Reference Agarwal, Aponte-Mellado, Premkumar, Shaman and Gupta2012; Al-Gubory et al., Reference Al-Gubory, Garrel, Faure and Sugino2012). We suppose the ROS levels in the reproductive organs of Bmal1−/− mice should also be changed and may be correlated with their reproductive function.
In this study, ovulation, in vivo and in vitro oocyte fertilization, embryo development, implantation and intracellular ROS levels in ovary and fallopian tubule were studied in female Bmal1+/+ and Bmal1−/− mice.
Materials and methods
Animals
Male and female heterozygous Bmal1 knockout mice on C57BL/6J background (5–6 weeks of age, 18–20 g) were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Homozygote animals were produced by breeding heterozygous pairs. The genotypes of the offspring were determined as previously described (Bunger et al., Reference Bunger, Wilsbacher, Moran, Clendenin, Radcliffe, Hogenesch, Simon, Takahashi and Bradfield2000). Wild-type male and female C57BL/6J mice (5–6 weeks of age, 18–20 g) were purchased from the Guangdong Province Laboratory Animal Center (Guangzhou, China). All mice were synchronized with a 12 h light/dark cycle, with the lights on from 6 a.m. (Zeitgeber time 0, ZT 0) to 6 p.m. (ZT 12) for 2 weeks, and with free access to regular chow food and water. The indoor temperature was maintained at a 20–25°C and relative humidity of 40–70%. All experimental procedures were approved by the Ethical Committee of The First Affiliated Hospital of Sun Yat-Sen University, and all efforts were made to minimize suffering.
Natural ovulation
Estrous cycles were determined as described previously (Byers et al., Reference Byers, Wiles, Dunn and Taft2012). Bilateral oviducts of Bmal1+/+ and Bmal1−/− female mice were dissected in the early hours of the estrus morning (ZT 2–3) to examine the number of naturally ovulated oocytes.
Superovulation
Wild-type and Bmal1−/− female mice were superovulated by an intraperitoneal (i.p.) injection of 10 IU pregnant mare serum gonadotrophin (PMSG; Ningbo Second Hormone Factory, Ningbo, China) at 16:00 (ZT 10), followed 48 h later by an i.p. injection of 10 IU of human Chorionic Gonadotropin (hCG, Ningbo Second Hormone Factory, Ningbo, China). For the observation of mice oocytes, cumulus-oocyte complexes were collected in 3-(N-morpholino)propanesulfonic acid (MOPS) medium (VitroLife, Sweden) 8 h after hCG injection, and then denudated with hyaluronidase (VitroLife, Sweden).
In vivo fertilization and embryo development
In the in vivo group, after injection with hCG, these females were placed with male C57BL/6J mice overnight. The following morning (ZT 2), female mice with the presence of vaginal plugs were considered pregnant as day 0.5. These mice were then humanely killed by cervical dislocation 1 or 3 days later for the determination of fertilization rate or blastocyst number, by dissecting oviducts or flushing dissected uteri, respectively. Fertilization was assessed by the formation of 2-cell embryos.
In vitro fertilization and embryo development
For the in vitro fertilization group, 15 h after injection with hCG, female mice were humanely sacrificed by cervical dislocation. Oviduct dissection was carried out at 37°C, and cumulus–oocyte complexes (COC) were collected in G-MOPS (VitroLife, Sweden) supplemented with 5 mg/ml human serum albumin (HSA). COC were then wash and placed in G-IVF (VitroLife, Sweden) supplemented with 5 mg/ml HSA under paraffin oil (VitroLife, Sweden) and incubated in a modular incubator chamber at 37°C in 6% CO2, 5% O2 for 4 h with sperm, collected from the cauda epididymis of male C57BL/6J mice, that had been previously incubated for 1 h in G-IVF media (VitroLife, Sweden) for capacitation. Putative zygotes were placed in G1 media (10 zygotes/20 μl drop; VitroLife, Sweden) and checked for fertilization/cleavage the next morning. Embryos (2-cell) were transferred to a new drop of G1 media for the initial 48 h of culture and then in G2 (VitroLife, Sweden) before hatching.
Blastocyst vitrification and thawing
Blastocysts derived from female wild-type and Bmal1−/− mice after in vitro fertilization by sperm from male wild-type mice were vitrified using a vitrification kit (Kitazato Biopharma Co. Ltd, Shizuoka, Japan) in combination with open pulled straws for vitrification. The vitrification and thawing procedure was carried out according to the protocols.
Blastocyst transfer
After thawing, blastocysts were transferred to G2 medium and cultured for 1–2 h before they were transferred to day 2.5 pseudopregnancy female wild-type mice. Pseudopregnant female mice were mated to vasectomized males 3.5 days prior to embryo transfer. The morning after mating, females were checked for the presence of a vaginal plug, and this was considered day 0.5 of pseudopregnancy. Embryo transfer with the NSET device (ParaTechs, Lexington, KY, USA) was performed as described previously (Green et al., Reference Green, Bass and Spear2009). In brief, day 2.5 pseudopregnant mouse was placed on a wire-top cage and allowed to grip the bars. The small and large specula (ParaTechs) were placed sequentially into the vagina to open and expose the cervix. The NSET catheter was then inserted through the large speculum into the uterine horn. The device and specula were removed, and the mouse was returned to its home cage. Totally 12 blastocysts were transferred to uterine horn of pseudopregnant mouse. Pregnant females were killed 5 days later and implantation sites were evaluated.
Determination of intracellular ROS
Intracellular ROS levels were determined using the fluorescent marker 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; S0033; Beyotime, China), according to the manufacturer's instructions. Briefly, mice in specific estrus stage were killed before bilateral oviducts and ovaries were quickly and gently cut using fine forceps under a dissecting microscope, homogenized in phosphate-buffered saline (PBS) on ice and filtered. Cells were collected and incubated with DCFH-DA at a final concentration of 10 μM for 20 min at 37°C in the dark. After washing with serum free cell culture medium twice, cells were resuspended in PBS in the presence of 2 mM freshly prepared H2O2 or not. Samples with or without H2O2 stimulation were analyzed by fluorescence activated cell sorting (FACS) Canto instrument flow cytometer system (Becton Dickson, Inc.), with an excitation at 488 nm and emission at 529 nm. Ten thousand events were analyzed in each sample. The fluorescence data were further analyzed with WinMDI 2.9 software (Scripps Research Institute, Jupiter, FL, USA). Intracellular ROS levels were expressed as the average dichlorodihydrofluorescein (DCF) fluorescence intensity of the cells.
Statistical analysis
Statistical analyses were performed using the t-test as appropriate. All P-values quoted are two-sided, and values <0.05 indicate statistical significance. Analyses were performed using the SPSS statistical package.
Results
Female Bmal1−/− mice are infertile, even though we detected vaginal plugs after mating to wild-type males.
The naturally ovulated oocyte number of Bmal1+/+ and Bmal1−/− mice was 7.8 ± 0.8 and 5.2 ± 0.8 (n = 6), respectively. Compared with Bmal1+/+ mice, the obtained oocyte number of Bmal1−/− mice decreased (P < 0.001). No major difference was found in the obtained oocyte number between Bmal1−/− and Bmal1+/+ mice after superovulation (26.9 ± 3 versus 30.1 ± 5.2, n = 6, P = 0.283) (Fig. 1 A). However, the total abnormal oocyte (degeneration, fragmentation, etc.) ratio increased in Bmal1−/− mice (20.4 ± 3.5% versus 11.7 ± 2.0%, n = 6, P = 0.001).

Figure 1 Oocyte number, fertilization rate and blastocyst number in female Bmal1 +/+ and Bmal1−/− mice under in vivo and in vitro conditions after superovulation. (A) Obtained oocyte number in female Bmal1 +/+ and Bmal1−/− mice after superovulation. (B) Fertilization rate in female Bmal1 +/+ and Bmal1−/− mice under in vivo and in vitro conditions after superovulation. (C) Obtained blastocyst number in female Bmal1 +/+ and Bmal1−/− mice under in vivo and in vitro conditions after superovulation. All values represent the mean ± standard error of the mean (SEM) (n = 6). *P < 0.05.
In Bmal1+/+ wild-type mice, no significant difference was found in the fertilization rate (70.3 ± 5.7% versus 66.1 ± 5.9%, n = 6, P > 0.05) (Fig. 1 B), blastocyst number (15.2 ± 1.9 versus 13.9 ± 2.1, n = 6, P > 0.05) (Fig. 1 C) between in vivo and in vitro conditions.
However, significantly lower levels of fertilization rate (33.2 ± 3.3% versus 70.3 ± 5.7%, n = 6, P < 0.001) (Fig. 1 B) and obtained blastocyst number (3.0 ± 0.8 versus 15.2 ± 1.9, n = 6, P < 0.001) (Fig. 1 C) were detected in Bmal1−/− mice compared with that of Bmal1+/+ mice after superovulation and mated with wild-type male mice in vivo.
Similarly, significantly lower levels of fertilization rate (57.4 ± 2.0% versus 66.1 ± 5.9%, n = 6, P < 0.001) (Fig. 1 B) and blastocyst number (5.0 ± 2.2 versus 13.9 ± 2.1, n = 6, P < 0.05) (Fig. 1 C) were observed in Bmal1−/− mice after superovulation and in vitro fertilized with sperm from wild-type male mice.
Interestingly, in vitro fertilization rate of oocytes derived from Bmal1−/− increased significantly compared with in vivo study (57.4% ± 2.0 versus 33.2 ± 3.3%, n = 6, P < 0.01) (Fig. 1 B). There was also a trend for blastocyst number to increase in vitro, but no significant difference was found (5.0 ± 2.2 versus 3.0 ± 0.8, n = 6, P >0.05) (Fig. 1 C).
Obtained blastocysts derived from female Bmal1 +/+ and Bmal1−/− mice under in vitro environment were transferred to pseudopregnant wild-type mice, the implantation sites 5 days later was 8.0 ± 0.8 and 5.3 ± 1.0, respectively (n = 4, P = 0.005), as shown in Fig. 2.
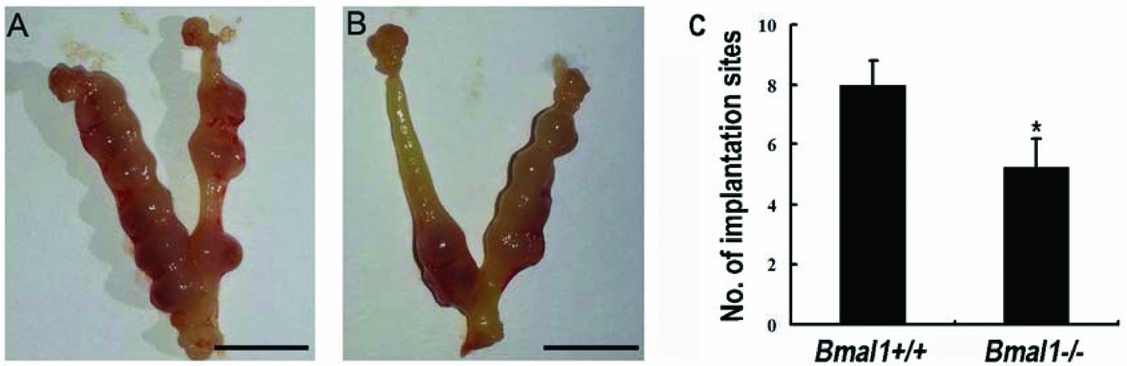
Figure 2 Number of implantation sites in pseudopregnant Bmal1 +/+ mice 5 days after transfer of blastocysts derived from female Bmal1 +/+ or Bmal1−/− mice. (A) The transferred blastocysts were derived from female Bmal1 +/+ mice. (B) The transferred blastocysts were derived from female Bmal1−/− mice. (C) Blastocysts derived from female Bmal1−/− formed fewer implantation sites compared with that derived from female Bmal1 +/+ mice (*P < 0.05). Scale bar = 1 cm. Values represent the mean ± standard error of the mean (SEM) (n = 6).
At ZT 10 and 14 on the day of proestrous, the ROS level in the ovary cells of Bmal1−/− mice significantly increased compared with that of Bmal1+/+ mice (n = 4, both P < 0.01) (Fig. 3); Similarly, the ROS level of oviduct cells in Bmal1−/− mice significantly increased compared with that in Bmal1+/+ mice at ZT4 on the day of metestrus, (n = 4, P < 0.001) (Fig. 4).

Figure 3 ROS levels in the ovary cells of Bmal1 +/+ and Bmal1−/− mice at ZT10 and ZT 14 on the day of proestrous. Representative histogram of flow cytometric analysis of intracellular ROS staining with DCFH-DA at ZT 10 and 14 in the ovary cells of Bmal1 +/+ (A, B) and Bmal1−/− (C, D) mice, respectively. Comparative ROS levels (expressed as fluorescence intensity) in the ovary cells of Bmal1 +/+ and Bmal1−/− mice at ZT 10 (E) and 14 (F) on proestrous day. Values represent the mean ± standard error of the mean (SEM) (n = 4, in each time point and mice type). *P < 0.05, compared with respective Bmal1 +/+ mice.

Figure 4 ROS levels in the oviduct cells of Bmal1 +/+ and Bmal1−/− mice at ZT4 on the day of metestrus. Representative histogram of flow cytometric analysis of intracellular ROS staining with DCFH-DA at ZT 4 in the oviduct cells of Bmal1 +/+ (A) and Bmal1−/− (B) mice. Comparative ROS levels (expressed as fluorescence intensity) in the oviduct cells of Bmal1 +/+ and Bmal1−/− mice at ZT4 on metestrus day (C). Values represent the mean ± standard error of the mean (SEM) (n = 4, in each mice type). *P < 0.05 compared with respective Bmal1 +/+ mice.
Discussion
Previous reports on the early embryo development of Bmal1 null mice were not consistent. In this study, oocyte morphology, in vivo and in vitro oocyte fertilization, early embryo development and their implantation potential of female Bmal1−/− mice were investigated. Our study, consistent with recent studies (Ratajczak et al., Reference Ratajczak, Boehle and Muglia2009; Boden et al., Reference Boden, Varcoe, Voultsios and Kennaway2010), showed that female Bmal1−/− mice were infertile. Chu et al. found that Bmal1−/− females tends to yield fewer oocytes than wild-type, but the difference did not reach significance; Furthermore, they observed that cumulus–oocyte complexes in distended ampullae of female Bmal1−/− mice were morphologically indistinguishable from wild-type controls (Chu et al., Reference Chu, Zhu, Blum, Mai, Leliavski, Fahrenkrug, Oster, Boehm and Storch2013). Our result also showed that female Bmal1−/− mice were able to ovulate spontaneously, but relatively fewer oocytes were retrieved, indicating the process of follicle development and/or ovulation was at least partly affected. Due to the fewer number of naturally ovulated oocytes, experiments on in vivo and in vitro oocyte fertilization and embryo development were carried out after superovulation. No significant difference was observed in the obtained oocyte number, this is consistent with Borden's result (Boden et al., Reference Boden, Varcoe, Voultsios and Kennaway2010). However, after removing cumulus cells, we first found that the ratio of abnormal oocytes, including degeneration, big polar body and fragmentation increased significantly in female Bmal1−/− mice, indicating adverse environment existed before ovulation.
Ratajczak et al. found normal embryo development up to day 3.5 of gestation (Ratajczak et al., Reference Ratajczak, Boehle and Muglia2009). Later, Boden firstly reported lower insemination rate and embryo development in Bmal1−/− mice in vivo. Under both in vivo and in vitro conditions, we found that oocyte fertilization rate and early embryo development decreased in female Bmal1−/− mice. It was not possible to remove abnormal oocytes before fertilization, so it is reasonable that the decreased oocyte fertilization rate in female Bmal1−/− mice was at least partly due to the increased abnormal oocytes rate. To our surprise, we found that the in vitro fertilization rate of oocytes derived from Bmal1−/− increased significantly compared with in vivo study. Moreover, the blastocyst number also had a trend to increase under in vitro condition. In our experiment, oocytes were collected for in vitro fertilization 15 h after hCG injection, and fertilization was assessed by the formation of 2-cell embryos in the next moring (ZT 2) for both in vivo and in vitro fertilization. In vivo, the oviduct is the site of fertilization and early embryo development. This result indicated that detrimental factors existed in oviduct of Bmal1−/− female, except for the decreased oocyte quality.
In recent years, the existence of circadian rhythm in the ovary has been demonstrated extensively (Sellix & Menaker, Reference Sellix and Menaker2010). It is also known that the oviduct rhythmically expresses the core clock genes, several transcription factors and enzyme regulators important for the protection of the embryo (Kennaway et al., Reference Kennaway, Varcoe and Mau2003). Tight connections between biological clock and cellular redox signaling have been established in different organisms. As we speculated, the ROS level in the ovary cells on the day of proestrus at ZT 10 and 14, and in the oviduct cells on the day of metaestrus at ZT 4 were significantly higher in Bmal1−/− mice compared with that of Bmal1+/+ mice, respectively. In wild-type mice, luteinizing hormone (LH) surge generally occurs just prior to the active period on proestrus day (approximately ZT 11) (Chu et al., Reference Chu, Zhu, Blum, Mai, Leliavski, Fahrenkrug, Oster, Boehm and Storch2013). Our results indicated that around the time of ovulation and fertilization, oocytes/early embryos are exposed to the environment of excess ROS in Bmal1−/− females. Previous studies demonstrated that oxidative stress impairs oocyte quality, fertilization and embryo development (Matsuzuka et al., Reference Matsuzuka, Ozawa, Nakamura, Ushitani, Hirabayashi and Kanai2005; Tamura et al., Reference Tamura, Takasaki, Miwa, Taniguchi, Maekawa, Asada, Taketani, Matsuoka, Yamagata, Shimamura, Morioka, Ishikawa, Reiter and Sugino2008). So the decreased oocyte quality, fertilization and embryo development in vivo in Bmal1−/− females may be caused by excess ROS in these organs.
In our embryo transfer experiment, blastocysts derived from Bmal1+/+ and Bmal1−/− female mice were transferred to wild-type pseudopregnant mice, whose ovary and uterus were normal. Furthermore, the blastocysts derived from female Bmal1−/− mice were all heterozygous and it has been indicated the developmental potential of heterozygous Bma11 embryos were not influenced (Boden et al., Reference Boden, Varcoe, Voultsios and Kennaway2010). Thus, the decreased implantation potential of blastocysts derived from female Bmal1−/− mice observed in our experiment indicated their quality was negatively affected. Ratajczak et al. suggested that implantation failure due to impaired steroidogenesis cause infertility of Bmal1−/− females. After receiving daily injections of progesterone, 38% (five of 13) Bmal1−/− females displayed implantation sites at day 10.5 (Ratajczak et al., Reference Ratajczak, Boehle and Muglia2009). Using a cell-specific knockout mice model, in which Bmal1 was deleted in the steroidogenic cells, Liu et al. observed embryonic implantation failure. Through supplementation of progesterone or transplantation of wild-type ovaries to these conditional knockout female mice, they showed that clock gene Bmal1 in ovary steroidogenic cells is crucial for embryonic implantation (Liu et al., Reference Liu, Johnson, Shen, Wallisser, Krentz, Moran, Sullivan, Glover, Parlow, Drinkwater, Schuler and Bradfield2014). Although blastocyst quality was not determined in these studies, after progesterone supplementation, lower implantation rate observed in global Bmal1−/− female mice (38%) compared with that of the conditional knockout mice (62.5%), in which oviduct biological rhythm was not disrupted, also suggested blastocyst quality was impaired in female Bmal1−/− mice. Taking together, we consider the decreased blastocyst quality also contributes to the implantation failure in Bmal1−/− female mice.
Bedaiwy et al. found high day 1 ROS levels in culture media were associated with lower pregnancy rates in both in vitro fertilization and intracytoplasmic sperm injection cycles in human (Bedaiwy et al., Reference Bedaiwy, Falcone, Mohamed, Aleem, Sharma, Worley, Thornton and Agarwal2004). In murine, melatonin treatment reduced ROS production and increased the efficiency of blastocyst implantation (Wang et al., Reference Wang, Tian, Zhang, Tan, Reiter and Liu2013). The increased intracellular ROS levels detected in our experiment in oviduct of female Bmal1−/− mice may explain the lower implantation rate of blastocyst derived from these mice.
Generally, in vitro fertilization is also affected by excessive ROS in embryo culture media. However in our experiment, in wild-type female mice, no significant difference was found in the fertilization rate and blastocyst number between in vivo and in vitro conditions, although blastocyst morphology seemed better under in vivo conditions. It is possible that, in Bmal1−/− females, the relatively higher fertilization rate and blastocyst number in vitro was due to the effect of potent antioxidant contained in G-series culture mediums we used in our experiment; while in vivo the oocytes/early embryos were exposed to excess ROS in the oviduct.
Widely held view is that the proestrus LH surge is an obligatory prerequisite for ovulation. The phenomenon that natural ovulation was unaffected in Bmal1 null mice has been considered surprising, since the loss of central rhythmicity should have compromised the LH surge mechanisms (Boden et al., Reference Boden, Varcoe, Voultsios and Kennaway2010). After detecting blood samples taken at consecutive time points on proestrus and estrus day, Chu et al. surprisingly found that Bmal1−/− females lack the proestrus LH surge, with only a trend towards slightly higher LH abundance at one hour before lights off (ZT 11) (Chu et al., Reference Chu, Zhu, Blum, Mai, Leliavski, Fahrenkrug, Oster, Boehm and Storch2013). These authors challenged the traditional view and considered that LH surge is not required for spontaneous ovulation. H2O2 could fully mimic the effect of LH, bringing about an extensive mucification/expansion of the follicle-enclosed cumulus–oocyte complexes, which is essential for normal ovulation (Shkolnik et al., Reference Shkolnik, Tadmor, Ben-Dor, Nevo, Galiani and Dekel2011). Our result that excess ROS existed in the ovary of Bmal1−/− females around the time of ovulation may explain why natural ovulation was not affect in this animal model.
To sum up, we have determined that disruption of circadian rhythms in ovary decreases oocyte quality and subsequent fertilization; loss of circadian rhythms in oviduct also decreases fertilization rate and embryo developmental potential. However, we could not exclude the influence from central and other periphery system, which were also affected by the disruption of biological clock. In conclusion, deletion of the core biological clock gene Bmal1 significantly decreases oocyte quality, fertilization, embryo development and implantation potential in female mice, and these may be caused by excess ROS levels generated in ovary and oviduct. However, due to the relatively small sample size, more research will be needed to confirm this.
Acknowledgements
This study was supported by the following grants: National Basic Research Program of China (973 Program, grant no. 2012CB947604); National Natural Science Foundation of China (31071272); National Health and Family Planning Commission of China (201402004).






