INTRODUCTION
Bergmann's Rule states that ‘within a broadly distributed taxon, species of larger size are found in colder environments, and species of smaller size are found in warmer regions, (Bergmann, Reference Bergmann1847). Although originally formulated in terms of species within a genus and derived principally for terrestrial endotherms, the rule has often been recast in terms of populations within a species. It has also been redacted in terms of latitude, and is most often applied to terrestrial vertebrates and fishes.
The rule has also found rich ground in the study of fossils. Roy (Reference Roy2008), for example, demonstrated body size evolution in two deep-sea ostracods in response to temperature. Poseidonamicus rudis Whatley et al., 1986 lived earlier under much warmer temperatures and was half the size of the later Poseidonamicus major Benson, 1972 living under cooler conditions. Similarly, Daley (Reference Daley1999) showed that variations in Upper Ordovician palaeonvironments controlled disparities in the maximum shell size of species of the bivalve genus Ambonychia Hall, 1847. The hypothesis has also been applied to extant marine animals by Timofeev (Reference Timofeev2001) who suggested that, in crustaceans, increasing latitude—that is, decreasing temperature—results in increased cell sizes and increased life spans, both of which lead to an increase in maximum body size with continued growth throughout life being characteristic of these arthropods. Kornicker (Reference Kornicker1967) showed that the myodocopid ostracod Eusarsiella zostericola Cohen & Kornicker, 1975 became larger down the North American Atlantic coastline from Massachusetts to Texas. Similarly, Hart & Hart (Reference Hart, Hart, Hart and Fuller1979) reported an increase in the size of ostracods with increases in latitude. Interestingly, the growth rate of the Tasmanian giant crab, Pseudocarcinus gigas (Lamarck, 1818), was shown to be faster and moult increment larger, but longitudinally, at the cooler, Tasmanian, end of the species’ range compared to the warmer, Western Australian (Levings, Reference Levings2008).
Timofeev (Reference Timofeev2001) hypothesized that an increased size of marine animals could be associated with increased oceanic depth, a phenomenon most obviously seen in mysids, euphausiids, decapods and isopods. Of the latter, the giant Bathynomus giganteus A Milne-Edwards, 1879 may reach up to 76 cm in overall length (Briones-Fourzán & Lozano-Alvarez, Reference Briones-Fourzán and Lozano-Alvarez1991). Similarly, female deep-ocean species of Architeuthis, can grow to an estimated maximum size of 13 m from the posterior fins to the tips of the two retractible tentacles (Roeleveld, Reference Roeleveld2002). In contrast, however, representatives of the deep sea Bivalvia are all small (Knudsen, Reference Knudsen1967; Reference Knudsen1970), the southern Australian nuculid Nucula pusilla Angas, 1877 being <3 mm in shell length, ranking it among the smallest known protobranchs and one of the smallest bivalves (Morton, Reference Morton2012a).
Decreases in maximum body size have also been correlated with changes in species richness both negatively and positively. For example, Roy & Martien (Reference Roy and Martien2001) showed for north-eastern Pacific bivalves that changes in mean body size associated with biogeographic boundaries were significantly larger than changes elsewhere along the latitudinal range. Latitudinal trends in mean body sizes and their variances, however, showed no correlation with trends in species richness in the same direction. Close to an order of magnitude difference in species numbers between tropical and polar latitudes did not appear to significantly affect either mean size or variance in size. These authors concluded that the spatial distributions of major environmental barriers along the north-eastern Pacific coastal margin play a major role in structuring the latitudinal distribution of body size, but not species richness in marine bivalves.
Notwithstanding, Morton (Reference Morton, Wilbur and Russell-Hunter1983) argued that the marine bivalves of Pacific islands, especially those associated with coral reefs, are relatively species-depauperate compared with continental ecosystems. This may result from high temperatures, low nutrient supplies and isolation. Using mean body size (shell length), Roy & Martien (Reference Roy and Martien2001) further identified a decreasing trend with latitude within the tropical Panamic Province and a similar latitudinal decrease in species richness in north-eastern Pacific bivalves. In a major review of the subject, however, Berke et al. (Reference Berke, Jablonski, Krug, Roy and Tomasovych2012) showed that although size-latitude trends are geographically (and taxonomically) common in bivalves, they vary widely in terms of sign and strength. The observed trends, moreover, varied considerably between hemispheres and among coastlines.
When considering the vast environmental variability potentially influencing and affecting species richness and maximum size, the conclusions of Berke et al. (Reference Berke, Jablonski, Krug, Roy and Tomasovych2012) seem eminently sensible. To give just a few examples, Bamber & Henderson (Reference Bamber and Henderson1985) showed that the sand smelt Atherina presbyter Cuvier, 1829 (Pisces: Atherinidae) demonstrated decreased size with increased environmental stress, and bred earlier and died younger, irrespective of geography. At a very specific level, moreover, Freeman et al. (Reference Freeman, Wright, Hewitt, Campbell and Szeto2013) demonstrated that the caenogastropod Haustrum vinosum (Lamarck, 1822) showed variations in growth rate and, thus, overall size, in the presence of the molluscivorous crab Carcinus maenas (Linnaeus, 1758) introduced into Australia. Such variations in size can have important effects upon, for example, reproduction. Nakaoka (Reference Nakaoka1994) showed that age, size at maturity, oocyte size, fecundity and reproductive effort varied between localities at different depths in the protobranch bivalve Yoldia notabilis Yokoyama, 1922. In the Azorean Archipelago, Cunha et al. (Reference Cunha, Amaral, Medeiros, Martins, Wallenstein, Couto, Neto and Rodrigues2008) showed that the shell morphometrics of the endemic Macaronesian limpet Patella candeii gomesii Drouet, 1858 varied in response to chronic heavy metal stress in proximity to shallow hydrothermal vent activity.
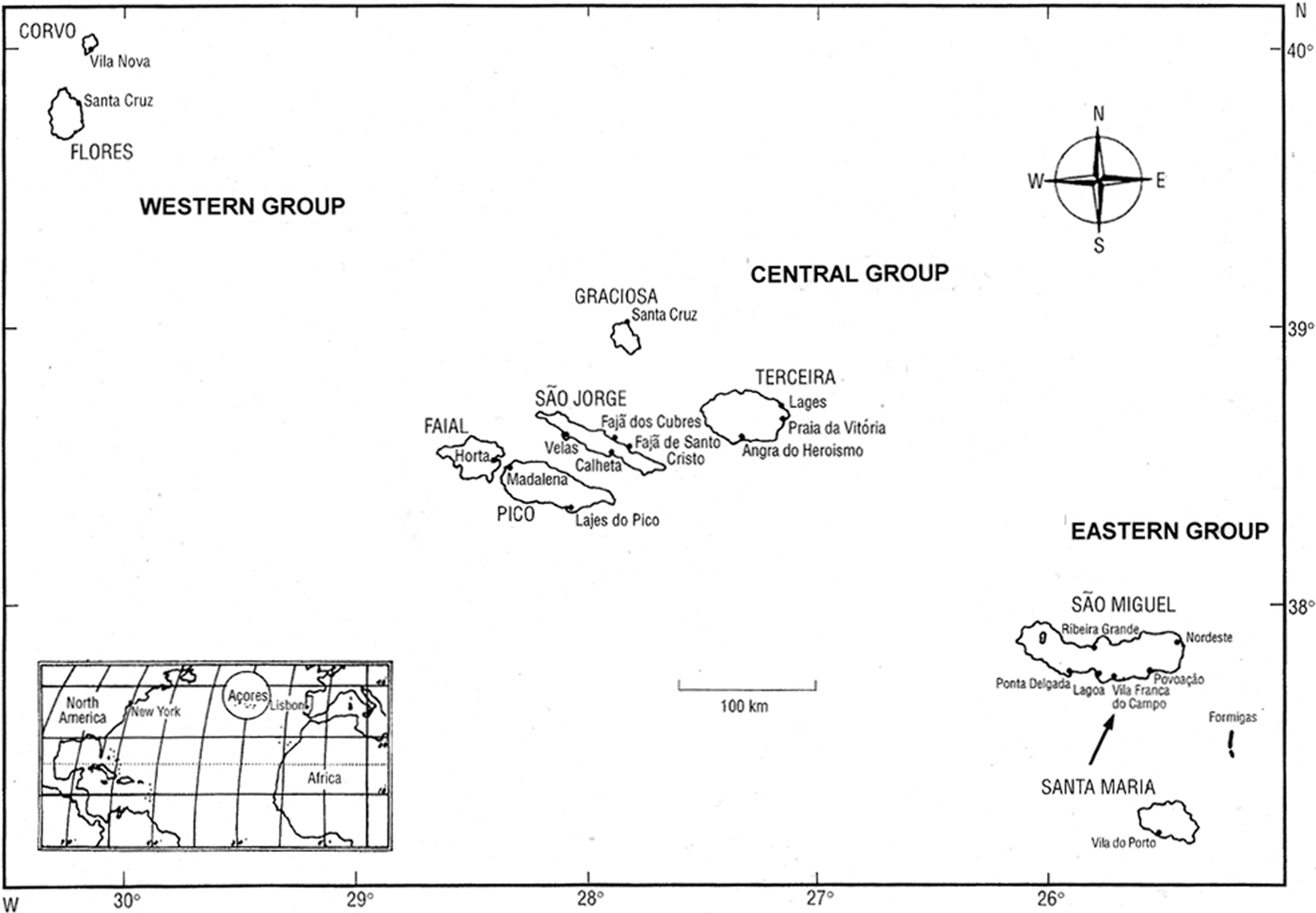
The Azorean Archipelago (36o55′–39o45′N 24o45′–31o17′W) is situated some 1300 km from the nearest continental land mass at Cabo de Roca in Portugal. Newfoundland is some 1700 km to the north-west. Because of its situation in the near centre of the North Atlantic, the nine islands of the archipelago have a Mediterranean climate and surface waters are warmed by the North Atlantic Drift—itself a branch of the Gulf Stream. Since the end of the last ice age some 8000 yr ago, the influence of Mediterranean water flowing out and westwards through the Straits of Gibraltar has re-asserted itself. Briggs (Reference Briggs1966), for example, argued that the present lack of Azorean marine endemism indicates that an older endemic fauna was eradicated (either wholly or in part) by a severe fall in sea surface temperatures during the Pleistocene and, thus that the present marine flora and fauna have arrived relatively recently. In contrast, Ávila et al. (Reference Ávila, Madeira, Mendes, Rebelo, Medeiros, Gomes, García-Talavera, Marques da Silva, Cachão, Hillaire-Marcel and Martins2008a) have argued, contrary to Briggs' theory, that the islands were largely unaffected by such a dramatic temperature fall and thus that the composition of the marine flora and fauna of the Azores today approximates what it has always been since the islands emerged from the sea. Hence, on Santa Maria, the oldest island and with a fossiliferous carbonate cap (Ávila et al., Reference Ávila, Madeira, Zazo, Kroh, Kirby, Marques da Silva, Cachão and Martins2009a), the last glaciation resulted in the local extirpation of warm water ‘guest’ species (Avila et al., 2008b). Since, however, the islands have emerged from the sea at times ranging from <1 million years ago (Pico) to >7 million years ago (Santa Maria) (Morton et al., Reference Morton, Britton and Martins1998), their colonizations have been different spatially as well as temporally. Since the islands' various ages encompass not one but more ice ages, moreover, it is clear that colonization has been sporadic but, nevertheless, represents continued larval recruitment principally from mainland habitats to the east and reflects faster inter-island colonization on more local physical and temporal scales.
Although the marine flora and fauna of the Azores has been well-listed taxonomically (Borges et al., Reference Borges, Costa, Cunha, Gabriel, Gonçalves, Martins, Melo, Parente, Raposeiro, Rodrigues, Santos, Silva, Vieira and Vieira2010), there are fewer ecological studies and, in particular, there have been few detailed studies of the Azorean Bivalvia. Studies upon five intertidal and shallow subtidal bivalves in the Azores (Morton, Reference Morton and Martins1990a; Reference Morton and Martins1995; Reference Morton and Martins2009; in press), however, have collectively suggested that all the species described therein are smaller, much smaller, than continental conspecifics. This seems to be an example of Foster's Rule (Foster, Reference Foster1964), which proposes that some island creatures have evolved into larger versions of themselves while others have became smaller. Foster explained this by proposing that smaller taxa become larger in the absence of island predators and, more applicable to the case of the Azorean marine bivalves, larger taxa become smaller in the absence of food resources. Foster's Rule, therefore, complements that of Bergmann in that, with decreasing latitude (setting aside the confounding element of depth), ocean temperatures generally increase and productivity decreases. It should, therefore, be expected that not only maximum size, especially of suspension feeders, but also species richness and abundance, should decrease. Such a trend should, further, be compounded on such remote islands.
This study examines this hypothesis and describes the results obtained for a greater number of Azorean subtidal bivalves than hitherto identified by Morton (Reference Morton and Martins1990a; Reference Morton and Martins1995; Reference Morton and Martins2009; in press), examining the above suggestions of remote island dwarfism in more detail and discussing, if true, the reasons for such discrepancies. Specifically, we ask the questions: do (1) species richness, (2) relative abundance and (3) maximum size of Azorean bivalves differ from Northeast Atlantic and, more particularly, Mediterranean conspecifics?
MATERIALS AND METHODS
In 2006, a dredge survey of the benthic fauna of the southern seabed of São Miguel, Azores (Figure 1), was conducted for 60 stations at depths ranging from 5–350 m (see Martins et al., Reference Martins, Ávila, Borges, Madeira, Morton and Martins2009, fig. 1 for station identifications). All the molluscan taxa obtained from the survey were identified to species-level and preserved in 70% ethanol. A sub-sample obtained from three stations (5% of the total), that is, 6, 7 and 19, located at depths of 40–41 m, 167–189 m and 23 m, respectively, were analysed in detail to provide an assessment of the numbers of each identifiable bivalve species taxon present and their sizes (shell lengths). The three samples were chosen to represent different depths from the shore of the steeply sloping island because Martins et al. (Reference Martins, Patarra, Álvaro, Prestes and Neto2013) has shown that the distribution of benthic taxa offshore from São Miguel varies with depth and scale of the location, but not with the scale of the coast. Subsequently, the entire collection of bivalve specimens obtained from the 60 stations, encompassing the large scale of the coast, and previously rough-sorted, was examined to obtain a measure of the shell lengths of the largest individuals of each species obtained. The latter data have been compared with equivalent information obtained for conspecifics collected from elsewhere in the north-eastern Atlantic Ocean (Tebble, Reference Tebble1966) and the Mediterranean Sea (Poppe & Goto, Reference Poppe and Goto1993). Where possible also, literature-derived figures for maximum shell lengths of the species described are also identified and discussed.

Fig. 1. The Azorean Archipelago. Identified are the three constituent island groups. Inset, A map of the Central Atlantic showing (circled) the Azores. The arrow points to the study location off Vila Franca do Campo on São Miguel Island.
In addition, literature information on Azorean rocky intertidal bivalve species, that is, Lasaea adansoni (Montagu, 1803) and Trichomusculus semigranatus (Reeve, 1858), and the introduced species Venerupis decussata (Linnaeus, 1758) and Mytilus galloprovincialis Lamarck, 1819, have also been compared with information obtained for conspecifics from elsewhere.
RESULTS
Numbers of Azorean species
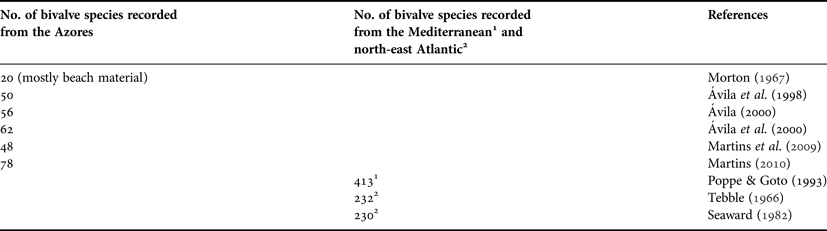
There is only one freshwater species of bivalve recorded from the Azores—the minute pisidiid Pisidium casertanum Suter, 1907. Table 1 identifies the numbers of species of marine bivalves recorded from the Azores. In 1965, Morton (Reference Morton1967) collected and identified 20 bivalve species from the island of São Jorge in the central group of islands (Figure 1). With no facilities for offshore collecting, these species largely comprised beach material, and such impoverishment results from the near absence of gently sloping sand beaches on São Jorge's precipitous coastline. This author was, however, the first to record the presence of the introduced Venerupis decussata (Linnaeus, 1758) from the lagoon at Fajã de Santo Cristo on the north shore of São Jorge. Thirty years later, Ávila et al. (Reference Ávila, Azevedo, Gonçalves, Fontes and Cardigos1998) recorded 50 species from Pico and Faial (Central Group), and Flores and Corvo (Western Group) but subsequently increased this to 56 overall (Ávila, Reference Ávila2000). Ávila et al. (Reference Ávila, Azevedo, Gonçalves, Fontes and Cardigos2000) recorded 62 species of bivalves from São Miguel (Eastern Group). Subsequently, Martins et al. (Reference Martins, Ávila, Borges, Madeira, Morton and Martins2009) recorded 48 species from the seabed south of Vila Franca do Campo, also on São Miguel, and the material from which forms the basis of this study. Most recently, Martins (Reference Martins, Borges, Costa, Cunha, Gabriel, Gonçalves, Martins, Melo, Parente, Raposeiro, Rodrigues, Santos, Silva, Vieira and Vieira2010) has combined all the available information and identified 78 putative species from Azorean waters. This study identifies 42 bivalve species from three (5%) of the 60 stations investigated by Martins (Reference Martins, Borges, Costa, Cunha, Gabriel, Gonçalves, Martins, Melo, Parente, Raposeiro, Rodrigues, Santos, Silva, Vieira and Vieira2010) from the seabed south of Vila Franca do Campo on São Miguel in the eastern group of the Azores (Table 2).
Table 1. Numbers of species of bivalves recorded from Azorean waters over time as compared with numbers recorded from the Mediterranean and Northeast Atlantic.

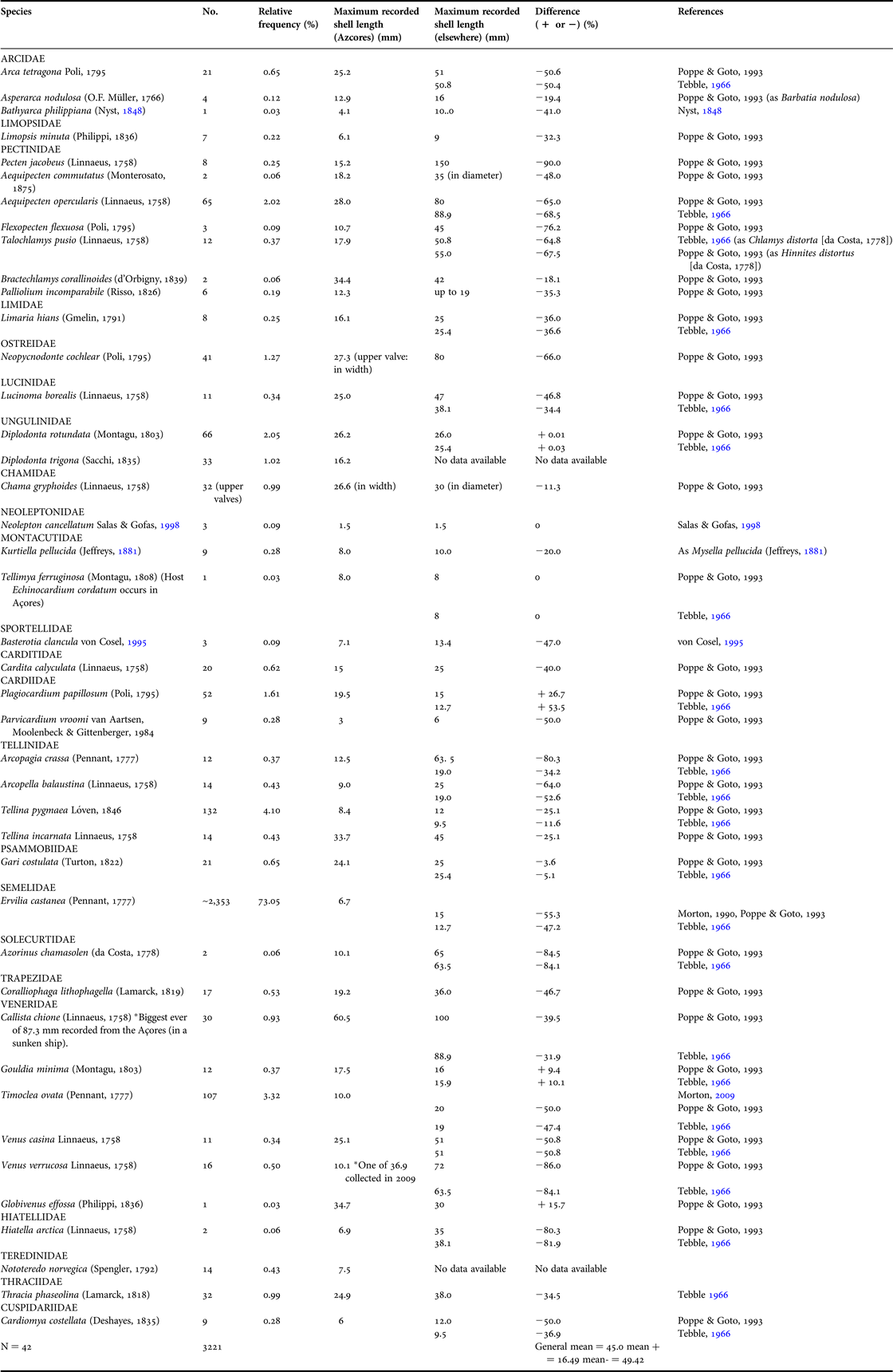
Table 2. A comparison of the numbers of individuals of bivalve species collected during dredging operations in the Azores in 2006 with the maximum shell length of each species collected. And a comparison and percentage difference with the maximum shell lengths of conspecifics recorded from the Mediterranean (Poppe & Goto, 1993) and Great Britain (Tebble, Reference Tebble1966).

Species composition and frequencies
As noted above, 42 bivalve species have been identified from the three sub-sampled stations on the seabed off the southern coast of São Miguel (Table 2). This compares reasonably well with the total of 48 species recorded by Martins et al. (Reference Martins, Ávila, Borges, Madeira, Morton and Martins2009) for the complete suite of 60 stations from the same locality. The identified species were dominated overwhelmingly by Ervilia castanea (73.3%) followed by Timoclea ovata (3.3%), both endobenthic suspension feeders (Morton, Reference Morton and Martins1990a, Reference Morton and Martins2009). Of the cemented epibenthic bivalves, important species were Neopycnodonte cochlear (1.27%) and Chama gryphoides (1%). A few byssally-attached species were recorded, for example, Arca tetragona (0.65%), Cardita calyculata (0.62%) and Hiatella arctica (0.06%). The dominant families represented in the samples were the epibenthic Pectinidae (seven species), especially Aeqipecten opercularis (2.02%), and the shallow-burrowing endobenthic Veneridae (six species), especially Callista chione (0.93%), and the deeper-burrowing Tellinidae and its tellinoid cousins (five species), especially Tellina pygmaea (4.11%).
Typically deeper-water species included Bathyarca philippiana, Asperarca nodulosa, Limopsis minuta and the predatory Cardiomya costellata, all occurring at very low frequencies. Similarly, two commensal species, that is, Tellimya ferruginosa, with Echinocardium cordatum Pennant, 1777 as a host, and Kurtiella pellucida (Jeffreys, Reference Jeffreys1881) with no, as yet, known host (Jeffreys, Reference Jeffreys1881)—all occurred at very low frequencies.
Suspension versus deposit feeders
Noticeably absent from the entire sample set were interstitial deposit feeders, including representatives of the Nuculidae and Nuculanidae. Others, such as Limopsis minuta (0.22%) and Lucinoma borealis (0.34%) were relatively uncommon. Lucinoma borealis has enlarged gills, which contain numerous prokaryotes in specialised cells (bacteriocytes) in the sub-filamentar region and which are responsible for the chemoautotrophic oxidation of sulphur (Dando et al., Reference Dando, Southwood and Southward1986). More collectively common were representatives of the Tellinidae and Psammobiidae, especially Tellina pygmaea (4.11%), which are typically considered to be surface deposit feeders (Yonge, Reference Yonge1949). Most of the other bivalves present in the sample, save for Cardiomya costellata, congenerics of which are predators typically of small epibenthic crustaceans (Reid & Crosby, Reference Reid and Crosby1980), were suspension feeders and can be regarded as non-specialist, possibly opportunistic, species.
Shell length comparisons
To date, only five bivalves that occur in the Azores have been investigated biologically and including data on maximum shell length sizes. Of these, only Trichomusculus semigranatus (reportedly = Gregariella subclavata Libassi, 1859; Poppe & Goto, Reference Poppe and Goto1993) inhabits the rocky intertidal (Morton, Reference Morton and Martins1995), while Ervilia castanea occurs in the shallow sub-tidal offshore from sandy beaches in the Azores (Morton, Reference Morton and Martins1990b). The other three species, Timoclea ovata (Morton, Reference Morton and Martins2009), Arcopagia crassa and Arcopella balaustina (Morton, in press), all occur in deeper waters, typically on the narrow shelf, that is, at ~ −100 m.
When the data on the maximum shell sizes of the Azorean offshore bivalves were compared with equivalent information on conspecifics from the north-east Atlantic (Tebble, Reference Tebble1966) and the Mediterranean (Poppe & Goto, Reference Poppe and Goto1993), a remarkable discrepancy was detected (Table 2). Firstly, comparing all the obtained species, the mean maximum shell lengths of Azorean individuals were 45.0% the size of continental conspecifics. Four (<10%) species were larger, that is, Diplodonta rotundata, the cockle Plagiocardium papillosum and the two venerids Gouldia minima and Globivenus effossa. On average, these species were 16.5% larger than mainland conspecifics, although much of this was accounted for by P. papillosum.
Collectively, the remaining 38 (90.4%) species were a mean of 49.4% smaller than mainland conspecifics. Such a figure is based on reference book data. Where individual reviews have been made, many species are shown to attain even larger sizes in the broader, non-Azorean, spectrum of their ranges. For example, Solem (Reference Solem1954), in a taxonomic review of the Trapezidae, points out that Coralliophaga lithophagella individuals (all from the Mediterranean) can reach a size (shell length) of 55 mm—50% more than the 36 mm suggested for this species by Poppe & Goto (Reference Poppe and Goto1993). Similarly, Basterotia clancula with a maximum shell length of 7.1 mm in the Azores is but 47% of the size of continental conspecifics (von Cosel, Reference Cosel von1995), but is not recorded from the Mediterranean.
Rocky intertidal species
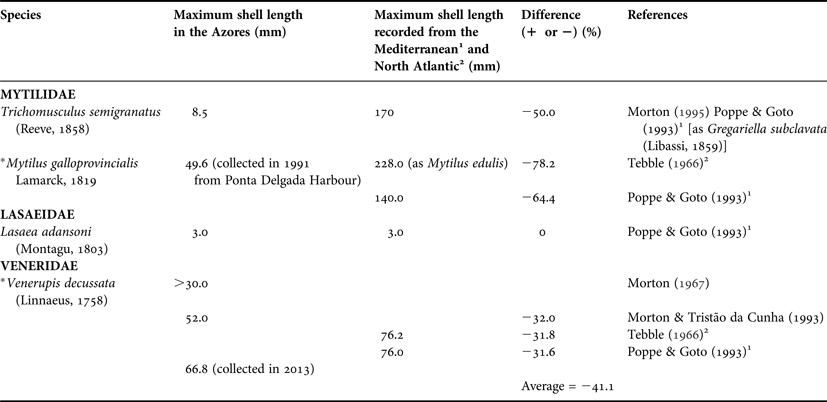
Lasaea adansonii is the same maximum shell length (~3 mm) in the Azores as conspecifics in the Mediterranean (Table 3). This species is possibly synonymous with Lasaea rubra (Montagu, 1803) (Poppe & Goto, Reference Poppe and Goto1993) and which, in the Northeast Atlantic, reaches a shell length of 3.2 mm (Tebble, Reference Tebble1966). Conversely, the Azorean rocky intertidal Trichomusculus semigranatus, with a maximum shell length of 8.5 mm is 50% smaller than conspecifics in the Mediterranean, which, as noted above, were identified as Gregariella subclavata by Poppe & Goto (Reference Poppe and Goto1993) (NB: Morton (Reference Morton, Morton and Tseng1982a) has made a detailed study of the solitary, coral-crevice dwelling, Indo-West Pacific Gregariella coralliophaga (Gmelin, 1791) and this species is not similar to the intertidal, algal turf inhabitant, Trichomusculus semigranatus, of the Azores. This taxonomic question will not, however, be discussed herein).
Table 3. A comparison of the maximum shell lengths recorded for rocky intertidal species from the Azores and conspecifics in the Mediterranean and the North Atlantic.

*, Introduced species.
Introduced species
The venerid Venerupis decussata was introduced into the waters of Fajã de Santo Cristo on São Jorge in the Azores in the un-recorded past (Morton, Reference Morton1967). This author recorded a maximum shell length, in 1965, of ~32 mm for this species in this locality although larger individuals were said to occur in deeper waters of the lagoon. Morton & Tristão da Cunha (Reference Morton and Tristão da Cunha1993) reported upon a maximum shell length for this species in Fajã de Santo Cristo of 52 mm. In 2013, however, a maximum shell length of 66.8 mm has been recorded (this study) (Table 3). This compares reasonably well with a reported maximum shell length of ca 76 mm in both the north-east Atlantic (Tebble, Reference Tebble1966) and the Mediterranean (Poppe & Goto, Reference Poppe and Goto1993).
Similar to Venerupis decussata, specimens of Callista chione (Linnaeus, 1758), one with a maximum shell length of up to 87.3 mm, have been recorded from Ponta Delgada Harbour, São Miguel, in the Azores, within a sunken ship (Table 2). Here, the species (this study) has attained shell lengths (87.3 mm) similar to those of North Atlantic (88.9 mm) and Mediterranean (100 mm) conspecifics (Tebble, Reference Tebble1966; Poppe & Goto, Reference Poppe and Goto1993). In this enclosed habitat in a more productive harbour, are more nutrients than elsewhere offshore attained to achieve this locally un-natural size?
The mytilid Mytilus galloprovincialis has been recorded occasionally from the Azores (Morton, Reference Morton1967). Individuals of this species were collected subsequently from the harbour of Ponta Delgada, again in São Miguel, in 1991 (this study). The largest such individual had a maximum shell length of 49.6 mm compared with attained shell lengths of 140 mm and 228 mm (as M. edulis) from the north-east Atlantic and Mediterranean, respectively (Tebble, Reference Tebble1966; Poppe & Goto, Reference Poppe and Goto1993).
DISCUSSION
This study reports upon 42 species of subtidal bivalves collected by dredging at three station sub-sets in Azorean waters to depths of <400 m. This is similar to the 48 species reported upon by Martins et al. (2009) for the larger set of 60 stations, suggesting that, generally speaking, most of the species occur over a wide depth range. Martins (Reference Martins, Borges, Costa, Cunha, Gabriel, Gonçalves, Martins, Melo, Parente, Raposeiro, Rodrigues, Santos, Silva, Vieira and Vieira2010), in a summary of all available information, recorded a total of 78 putative species from the Açores. This contrasts, for example, with the ~232 species recorded from around the coasts of Great Britain (Tebble, Reference Tebble1966). Seaward (Reference Seaward1982), similarly, identified 230 species from British waters. Further north, in the north-east Atlantic, species numbers decline and Sneli et al. (Reference Sneli, Schiøtte, Jensen, Wikander, Stokland and Sørensen2005) record only 126 species from the Faeroe Islands at ca 62oN. Regrettably, however, these authors did not provide sizes for any of the species they described from these remote northern islands.
In contrast, Poppe & Goto (Reference Poppe and Goto1993) record 413 species from European, especially, Mediterranean waters. Rodriguez & Sánchez (Reference Rodríguez and Sánchez1997) record 201 bivalve species from the Canary Islands, which, situated at ca 28o100′N 15o400′W and but 100 km from the coast of West Africa is, thus, influenced much more readily by larval input from mainland sources than the Azores. The Canaries also receive wind blown sand from the Sahara, and are thereby provided with a greater range of sediment characteristics favouring endobenthic bivalves.
As more studies are made, the numbers of bivalves recorded from the Azorean seabed has increased somewhat (Table 1), but this study of the reasonably common taxa suggest that species richness is no more than about 18% of that of the Mediterranean. In two reviews of the subject, Morton and Britton (Reference Morton and Britton2000a, Reference Morton and Brittonb) showed that the origins of the Azorean marine flora and fauna lie in the Mediterranean, although there is an occasional input of long-lived larvae from the Western Atlantic, for example the caenogastropod Charonia variegata (Lamarck, 1816) (Gofas & Beu, Reference Gofas and Beu2002), which is recorded locally only rarely as solitary individuals in comparison with the more widespread eastern species Charonia lampas (Linnaeus, 1758) (Morton, Reference Morton2012b). Such a view was supported by Ávila (Reference Ávila2000) and Ávila et al. (Reference Ávila, Marques da Silva, Schiebel, Cecca, Backeljau and Martins2009b). This is because, although surface waters are influenced by the more southerly flowing branch of the North Atlantic Drift, itself arising as the Gulf Stream, the Azorean islands are influenced at mid-water depths (1000–1500 m) by out-flowing Mediterranean water, which up-wells locally (Morton et al., Reference Morton, Britton and Martins1998). The America's are, additionally, further away from the Azores than continental southern Europe and North Africa, so that the chances of larval recruitment from the latter sources are greater than they are from the former (Morton & Britton, Reference Morton and Britton2000a, Reference Morton and Brittonb).
Morton (Reference Morton, Wilbur and Russell-Hunter1983) argued that the marine bivalve diversity of tropical islands, especially those with coral reefs, is relatively depauperate compared with continental ecosystems and this seems to apply also to the temperate, Mediterranean, climate of the Azores. Such a disparity may result from past extinctions (Ávila et al., Reference Ávila, Madeira, Marques da Silva, Cachão, Landau, Quartau and Martins2008b), isolation, low nutrient supplies and high temperatures. Conversely, the greater species richness of continental near-shore seas may also result from a greater diversity of habitats, for example estuaries created by river systems, singularly and largely lacking on stable islands and which, of themselves, enhance nutrient levels and, thus, productivity inshore.
Raines & Huber (Reference Raines and Huber2012) investigated the bivalves of Easter Island and Salas y Gómez in the Pacific Ocean showing that the number of species was much higher than recorded in all earlier reports. The actual number of 71 species more than quadrupled the first systematic review of Rehder (Reference Rehder1980), who recorded but 15 bivalves from the two islands, as did Kay (Reference Kay, Maragos, Peterson and Eldredge1995). A similar case was identified by Huber (Reference Huber2010) for the Marquesas Islands with around 80 species, as compared with much lower earlier records. Nevertheless, the overall number of species recorded from Easter Island and Salas y Gómez waters, as compared to the richest areas for bivalves globally, that is, the Philippines and Indonesia where in excess of 1500 bivalve species are recorded from (Huber, Reference Huber2010), are simply miniature in comparison. This reflects the sparse habitat diversity typical of remote and insular environments, as first suggested by Morton (Reference Morton, Wilbur and Russell-Hunter1983). Interestingly, however, the bivalve species richness of the remote Pacific islands of Easter Island and Salas y Gómez is of the same order of magnitude as the numbers of species recorded from the Azores, that is ca 78 putative species (Martins, Reference Martins, Borges, Costa, Cunha, Gabriel, Gonçalves, Martins, Melo, Parente, Raposeiro, Rodrigues, Santos, Silva, Vieira and Vieira2010).
Most of the species present in Easter Island and Salas y Gómez waters, as noted too for the Marquesas (Huber, Reference Huber2010), occur in large numbers (Raines & Huber, Reference Raines and Huber2012). This is in contrast to the species-richest areas, where the high diversity is contrasted with limited numbers. This is not the case for the Azores, however, where only a few species are recorded in large, sometimes overwhelming, numbers—notably Ervilia castanea (Morton, 1990 and Table 2) and, in some locations, the rocky intertidal Lasaea adansonii (Ávila et al., Reference Ávila, Santos, Penteado, Rodrigues, Quintino and Machado2005). The overwhelming majority of the other species are sparsely distributed.
Most interestingly, however, the sizes of most bivalve species within Easter Island and Salas y Gómez waters were shown to be much smaller than in either continental or larger island situations. Except for Arcopsis sculptilis Reeve, 1844 and Spondylus exiguus Lamprell & Healy, 2001, which reach maximum sizes at Easter Island, most of the wider-ranging species were <50% of their known maximum possible shell length sizes. This situation also seems to apply to the Azores, where 38 of the 42 subtidal species herein reported upon were smaller than continental conspecifics by the same ca 50%. Conversely, also as seen in Easter Island and Salas y Gómez waters, four species were larger in the Azores. In these islands too, moreover, one species, Callista chione, attains approximately the same size as elsewhere—not, however, on the natural seabed but, inexplicably, within the confines of a sunken vessel within Ponta Delgada Harbour on São Miguel (Table 2). Such a pattern is also reflected in the Azorean rocky intertidal where Trichomusculus semigranatus is half the maximum shell length (Morton, Reference Morton and Martins1995; Table 3) of Mediterranean conspecifics (as Gregariella subclavata [Poppe & Goto, 1993]). Interestingly, Lasaea adansonii is of the same size in the Azores as elsewhere and, here, has a genetic character similar to mainland, Iberian, conspecifics (Ó Foighil & Josefowicz, Reference Ó Foighil and Josefowicz1999). According to these authors, this may result from the species being either rafted to the islands or, possibly, introduced, as evidenced by one island clade, from São Miguel, having a genetic distinctiveness suggestive of a human-mediated transport within the recent historical past.
Morton et al. (Reference Morton, Britton and Martins1998; figures 1–16) provided an illustration of summer sea surface temperatures in the North Atlantic Ocean along the 2°C isotherm. Although summer temperatures between the north-eastern Atlantic limits of the ranges of the species herein discussed differ by as much as 12°C, such a disparity is considerably less obvious when comparing seawater temperatures in the Mediterranean, where the great majority of Azorean species herein discussed also occur, with those of the Azores. This is because and as noted earlier, at mid-water depths (1000–1500 m), the Azorean islands are influenced by out-flowing Mediterranean water, which up-well locally (Morton et al., Reference Morton, Britton and Martins1998). Hence, although variations in temperature related to latitude and depth may influence growth and regulate ultimate size, there would seem to be another, more important, factor involved in the generalized dwarfism demonstrated by the suite of Azorean bivalves herein discussed. That is, colder water conspecifics may be able to invest more of the available resources into shell growth as those needed for reproduction are lessened because the temperatures that stimulate it are achieved only briefly. This could explain Bergmann's Rule in the sea. Conversely, warmer water conspecifics experience temperatures that lengthen the reproductive cycle thereby leaving fewer resources available for growth. This disparity is, however, compounded by another factor that affects, especially, individuals occupying the waters of remote oceanic islands.
We refer to ocean productivity. Situated in the near centre of the North Atlantic, the Azorean Archipelago is characterized by low ocean productivity and oligotrophic waters (Huskin et al., Reference Huskin, Anadon, Medinal, Head and Harris2001). These authors showed that zooplankton grazing, which controls phytoplankton populations and mediates vertical carbon flux through faecal pellet sinking, is lower in ocean centres. This is clearly of importance for any interstitial deposit-feeding bivalves, such as nearly all protobranchs, that is, nuculids and nuculanids (Allen, Reference Allen1954, Reference Allen1978), which are characteristic of colder, deeper, waters, but are un-recorded from the Azores. Deeper water arcoids, such as species of Bathyarca (Morton, Reference Morton1982b) and limopsids (Oliver & Allen, Reference Oliver and Allen1980; Morton, Reference Morton2013), are similarly rare in the Azorean seabed and represented by only one species each.
Although the waters around the Azores are generally characterized by low levels of productivity, the presence of hydrodynamic features such as fronts and topographic features such as seamounts may help sustain enhanced, but localised, levels of plankton biomass and production. This, in turn, may help to sustain the surface deposit-feeding bivalves and suspension feeders that locally characterize the Azorean sea bed, especially if, as suggested herein, most appear to be non-specialists that, as a consequence, can more generally occupy a wider range of depths and sediment types.
Most of the bivalves discussed herein are suspension feeders and, as described, this, in turn, is associated with low population sizes, except for Ervilia castanea, which here overwhelmingly occupies high-energy inshore habitats and their associated higher productivities. Nevertheless, this species too attains only half the shell length achieved by continental conspecifics. The introduced Venerupis decussata, found solely within the lagoonal environment of Fajã de Santo Cristo on São Jorge, is similarly somewhat smaller than its mainland conspecifics (Table 3), although it is abundant enough to warrant artisanal exploitation. Interestingly, however, as also identified in Table 3, maximum shell length in the only fajã it inhabits on São Jorge seems to be increasing, that is, from ~32 mm in 1965, when it was first studied (Morton, Reference Morton1967), 52 mm in 1992 (Morton & Tristão da Cunha, Reference Morton and Tristão da Cunha1993) to 66.8 mm in 2013 (this study). Whether this is a real trend or not, however, is unknown but Fisher et al. (Reference Fisher, Frank and Leggett2010) have suggested that size truncation is commonplace in marine species that are exploited commercially. The largest individuals of V. decussata collected in 1965 (Morton, Reference Morton1967) were ~32 mm in shell length with an average of ~25 mm. At the latter size, conspecifics in the Eastern Adriatic Sea are only two years old (Jurić et al., Reference Jurić, Bušelić, Ezgeta-Balić, Vrgoč and Peharda2012) suggesting that size truncation may be occurring with this species in the fajã. If, however, the suggested temporal trend in increasing shell length is true, it is not known why it is occurring. Perhaps the productivity of the fajã is increasing for example? The lagoonal habitat, reduced salinities and productivity of the unique habitat V. decussata solely occupies in the Azores does, however, explain its continued survival here.
In contrast, Mytilus galloprovincialis is only rarely recorded from Azorean habitats, as herein described (Table 3) but, despite what must be a continual stream of introductions either on the hulls of sailing vessels or in the ballast waters of commercial vessels, has never been able to survive for long enough to create reproductively viable populations. And here too it has never reached the shell lengths the species attains in similar mainland habitats. This study therefore argues for the role of nutrients, or rather their deficiency, in regulating Azorean bivalve species richness, population sizes and individual maximum size. In waters of locally higher productivities, however, population densities (for example Ervilia castanea) increase, but not maximum shell length.
Vermeij (Reference Vermeij1990) hypothesized for a number of bivalve taxa that a generalized decline in nutrients available for suspension-feeders from the western rim of the tropical Pacific to the oceanic islands of Micronesia and Polynesia could account for their low diversity (as discussed above) and, more importantly, their small adult body size (also as discussed above). Such a hypothesis would thus also seem to be applicable to the Azores and requires further testing—as does the question: why are they so small? Is it because each species grows normally, but dies young? Or perhaps, here, more energy is expended on reproduction than shell growth? Or, do they grow slowly and thus have more growth rings in their shells than equal-sized conspecifics from elsewhere because of low ocean productivity? And, finally, is it really the case that in bivalves, maximum shell size is (everywhere) correlated with growth rate and maturation age as suggested by Ridgway et al. (Reference Ridgway, Richardson and Austad2011)?
CONCLUSIONS
1. Morton (Reference Morton, Wilbur and Russell-Hunter1983) argued that the marine bivalves of Pacific islands, especially those with coral reefs, are species-depauperate compared with continental ecosystems and this seems to also apply to the Azores with a temperate, Mediterranean, climate.
2. The bivalve species richness of the remote Pacific Ocean islands of Easter Island and Salas y Gómez is of the same order of magnitude (60–70) as the numbers of species recorded from the Atlantic Ocean Azores.
3. In the Azores, only a few species are recorded in large numbers—notably Ervilia castanea and, in some locations, the rocky intertidal Lasaea adansonii. The overwhelming majority of other species are sparsely distributed.
4. The sizes of most bivalve species within Easter Island and Salas y Gómez are much smaller (<50%) than in continental situations. This situation also seems to apply to the Azores, where 38 of the 42 subtidal species herein reported upon were smaller than continental conspecifics by the same ~50%.
5. Such a pattern is also reflected in the rocky Azorean intertidal where Trichomusculus semigranatus is half the maximum shell length of conspecifics elsewhere.
6. Although summer temperatures between the north-eastern Atlantic limits of the ranges of the species herein discussed differ by as much as 12°C, such a disparity is considerably less obvious when comparing temperatures in the Mediterranean, where virtually all the Azorean taxa discussed herein also occur, with those of the Azores.
7. Situated in the near centre of the North Atlantic, the Azorean Archipelago is characterized by low ocean productivity and oligotrophic waters. We believe that this might account for not only low species richness but also small population and individual sizes, and explain particularly the near absence of protobranch and limopsid interstitial deposit feeders.
8. This study therefore argues for the role of nutrients, or rather their deficiency, in regulating Azorean bivalve species richness, population sizes and individual maximum size. In inshore waters of locally higher productivities, however, population densities increase but not shell length sizes.
9. This conclusion seems to support the rule of Foster (Reference Foster1964), complemented by that of Bergmann (Reference Bergmann1847), who proposed that larger taxa become smaller in the absence of food resources and, thus, in part, a consequence of oceanic isolation.