INTRODUCTION
Parasitic helminth worms have major economic impacts on the global livestock industry and chemical anthelmintics, in the absence of commercial vaccines, remain the cornerstone of their control (Keiser and Utzinger, Reference Keiser and Utzinger2010; Sutherland and Leathwick, Reference Sutherland and Leathwick2011). Unfortunately, there is increasing evidence that parasitic worms are becoming resistant to many anthelmintics and this represents a major threat to global food security. In attempts to meet the challenge of controlling parasite infection, whilst maintaining anthelmintic longevity, many practices that aim to reduce the selection pressure for anthelmintic resistance are being promoted. The aim of control strategies for ruminant gastro-intestinal nematodes is to reduce the risk of disease by controlling the level of infection in the host. Therefore, whilst grazing management practices may contribute to minimising infection levels, the use of broad-spectrum anthelmintics has been the primary method to control these parasites in livestock for over 50 years (Wolstenholme et al. Reference Wolstenholme, Fairweather, Prichard, Von Samson-Himmelstjerna and Sangster2004). Similarly, with fluke, where the aim is not only to reduce levels of infection, but to target the highly pathogenic juvenile stages, anthelmintics are the choice control method.
There are currently four classes of broad-spectrum anthelmintics for treatment of nematodes of ruminants; the benzimidazoles (BZs); the imidathiazoles/ tetrahydropyrimidines (levamisole, LEV), the macrocyclic lactones (MLs), including ivermectin (IVM) and moxidectin (MOX) and the amino-acetonitrile derivatives (AAD) (Wolstenholme et al. Reference Wolstenholme, Fairweather, Prichard, Von Samson-Himmelstjerna and Sangster2004; Kaminsky et al. Reference Kaminsky, Ducray, Jung, Clover, Rufener, Bouvier, Weber, Wenger, Wieland-Berghausen, Goebel, Gauvry, Pautrat, Skripsky, Froelich, Komoin-Oka, Westlund, Sluder and Maeser2008). For liver fluke a number of products are licensed with efficacy against adult fluke. However, the pro-drug triclabendazole (TCBZ) is a novel BZ that demonstrates excellent efficacy against the highly pathogenic juvenile fluke stages, as well as adults, and is, therefore, usually the drug of choice (Brennan et al. Reference Brennan, Fairweather, Trudgett, Hoey, Mccoy Mcconville, Meaney, Robinson, Mcferran, Ryan, Lanusse, Mottier, Alvarez, Solana, Virkel and Brophy2007). Thus, historically, the introduction of relatively inexpensive drugs onto the market has led to their widespread and frequent use, a further intensification of livestock production systems and, in turn, made possible the production of cheap and plentiful food for a growing world population (Wolstenholme et al. Reference Wolstenholme, Fairweather, Prichard, Von Samson-Himmelstjerna and Sangster2004). Not surprisingly, anti-parasitic agents hold the greatest percentage share of all products in the animal health market (McKellar and Jackson, Reference McKellar and Jackson2004).
In contrast, surprisingly, despite this global market and reality of widespread anthelmintic resistance within the ruminant and equine industries, not one fully resolved biochemical pathway(s) used by a parasitic helminth to bio-transform a commercial anthelmintic has been published. Potential detoxification routes within parasite tissues for anthelmintics were possibly resolved along with host pathways during the compound development phase, and it may not be commercially feasible to release datasets. However, the absence or unavailability of these datasets potentially curtail a molecular handle to measure drug resistance arising via the pharmacokinetic or non-drug target route. This information void also potentially hinders future strategies for parasitic helminth drug development by others. For example, targeted co-inhibition of a parasite detoxification protein could, in theory, increase the efficacy of an anti-parasite drug (‘resistance reversal’) and a working understanding of parasite detoxification pathways could allow for building selectivity into lead compound design.
The lack of progress towards understanding the mode of action, metabolism and detoxification of many anthelmintic classes is also likely fragmented as a legacy of the random drug discovery route. The research focus has been on the mechanisms of anthelmintic resistance at the presumed target protein/gene. To this end, significant advances have been made using this targeted one-gene approach, particularly with Benzimidazole resistance (Prichard et al. Reference Prichard, Von Samson-Himmelstjerna, Blackhall and Geary2007). The recent success in understanding the importance of detoxification mechanisms in pesticide resistance in field insects (Muller et al. 2008) and large-scale protein changes under drug treatment in the malarial protozoan parasite Plasmodium falciparum (Prieto et al. Reference Prieto, Koncarevic, Park, Yates and Becker2008) using genomic and functional genomics-led approaches supports a similar strategy for understanding anthelmintic metabolism in parasitic worms.
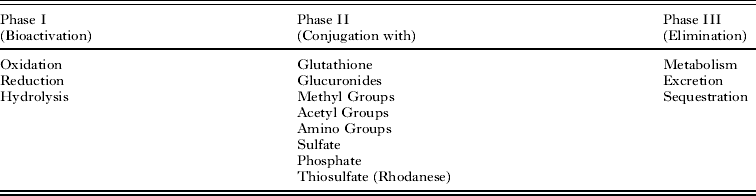
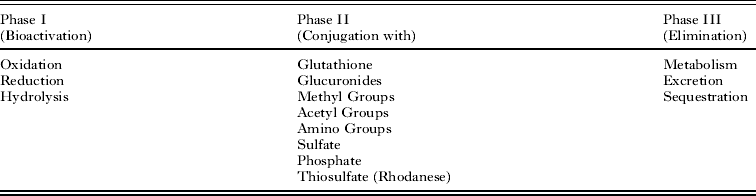
An anthelmintic (if metabolised and not secreted unchanged), in principle, can be neutralised or bio-transformed, in series or independently, by three protein level defence systems, termed Phase I to Phase III (Table 1; reviewed by Cvilink et al. Reference Cvilink, Skalova, Szotakova, Lamka, Kostiainen and Ketola2008). In brief, Phase I can add a reactive group, such as hydroxyl group, to the anthelmintic or uncover such a group on the toxic compound. In Phase II, a toxin is joined (conjugated) with a low weight endogenous component via insertion, such as the peptide glutathione, in order to increase solubility (polarity) and usually reduce reactivity. In Phase III, conjugated toxins can be further processed (metabolised) prior to being excreted by protein pumps or sequestered by abundant hydrophobic ligand binding proteins.
Table 1. The three potential phases of anthelmintic detoxification, adapted from Barrett (Reference Barrett1997).
However, the few studies that have been published at the metabolite and protein level tentatively suggest that parasitic helminths may have a reduced capacity to neutralise external toxins (xenobiotics) compared to their mammalian hosts (for reviews see Barrett et al. Reference Barrett, Saghir, Timanova, Clarke and Brophy1997; Cvilink et al. Reference Cvilink, Lamka and Skalova2009). Confirmation of this finding could prove a double edged sword for anthelmintic treatment. Firstly, reduced xenobiotic metabolism in parasitic helminths provides a limited template for the design of novel parasite-activated compounds (pro-drugs). In contrast, less pharmacokinetic capacity (non-drug target resistance routes) provides the parasitic helminth with fewer opportunities than their mammalian hosts to reduce the internal concentration of compound, and the parasite is less likely to innately possess or develop pharmacokinetic-based resistance mechanisms. Understanding the relative contribution of basic pharmacokinetic capacity compared to pharmacodynamic mechanisms (drug target-based routes) to anthelmintic resistance is an important step to resolve in support of new control strategies in field parasite population (Cvilink et al. Reference Cvilink, Lamka and Skalova2009).
To this end, a comparative mining of the genome of the Onchocercidae filarial nematode Brugia malayi suggests this particular tissue-dwelling nematode has relatively low pharmacokinetic capacity compared to the free–living nematode Caenorhabditis elegans. This finding suggests that natural selection favours broad detoxification defences in the free-living environment compared to a micro-host environment (Lindblom and Dodd, Reference Lindblom and Dodd2009), and not supporting the suitability of free-living helminths as anthelmintic response models. Induction of drug metabolising enzymes on drug exposure is the norm in organisms, but proteins clearly cannot be induced on exposure to anthelmintics if their genes are lost and not present in the genome. The downside for parasitic helminth control is that increased level of anthelmintic dosing will not lead to improved efficacy if target mutation or pharmacodynamics is found to be driving resistance development in parasitic helminth populations (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005).
To date, only a few experimental studies designed to track even partially the biotransformation of an activated anthelmintic within tissues of parasitic helminths have been published. These few investigations have only probed the in vitro ability of crude helminth extracts or isolated enzymes to potentially metabolise or interact with sometimes a parent anthelmintic compound. The problem of picking ‘most-likely.’ enzymes and ‘most-likely.’ anthelmintic form is that this compound might be bio-transformed by the enzyme, but it is not confirmed if this is an in vivo detoxification or if the selected study parasite enzyme is part of only a minor route for detoxification/biotransformation of only a minor pharmacologically active metabolite.
Thus, no integrated Phase I to Phase III case study on a confirmed active anthelmintic metabolite appears to have has been completed in a parasitic helminth. In addition, no studies have been published providing parasite protein biomarker panels that could be used to measure anthelmintic success or failure at the molecular dipstick level.
In principle, proteomics offers the opportunity with the support of genomics, reverse genetics (such as RNA interference [RNAi]) and pharmacokinetic studies to capture a global snap-shot of a parasite's response to anthelmintic challenge at the protein level, without pre-empting which enzyme activities or binding proteins are likely to be involved. This review re-visits anthelmintic metabolism in parasitic helminths using recently released genomics information and assesses the insight that experimental proteomics has made in the anthelmintic metabolism research area to date.
POTENTIAL PHASE I ANTHELMINTIC METABOLISM CAPACITY IN HELMINTHS
Traditionally, Phase I detoxification of chemical stressors in parasitic helminths was deemed via biochemically based studies to be associated more with reductive and hydrolytic-based metabolism and less by oxidative xenobiotic metabolising enzymes (XME). Genomics also predicts that hundreds of potential Phase I detoxification hydrolases and reductases can be present in helminth genomes (Dieterich et al. Reference Dieterich, Clifton, Schuster, Chinwalla, Delehaunty, Dinkelacker, Fulton, Fulton, Godfrey, Minx, Mitreva, Roeseler, Tian, Witte, Yang, Wilson and Sommer2008).
However, in vertebrates, and it appears most other invertebrates, the major Phase I route is oxidative via the cytochrome P450 Superfamily (CYPs). The inability of biochemical techniques to detect the usually major CYP activities in isolated microsomal preparations of adult helminths, in hindsight unfairly, raised the relative importance of reductive and hydrolytic transformations (Precious and Barrett, Reference Precious and Barrett1989). In insect pests, CYPs are established as a major route for insecticide resistance (Daborn et al. Reference Daborn, Yen, Bogwitz, Le Goff, Feil, Jeffers, Tijet, Perry, Heckel, Batterham, Feyereisen, Wilson and French-Constant2002). CYP activity has been demonstrated in range of parasitic protozoa (Barrett, Reference Barrett1998) and the structure of a CYP protein resolved binding to drugs from two trypanosomes (Chen et al. Reference Chen, Leung, Guilbert, Jacobson, Mckerrow and Podust2010).
Limited CYP-type biochemical level activity is detectable in nematode larval stages, but it is not yet confirmed whether this activity is simply metabolic house-keeping or detoxification (Kerboeuf et al. Reference Kerboeuf, Soubieux, Guilluy, Brazier and Riviere1995; Kotze et al. Reference Kotze, Dobson and Chandler2006). The few experimental studies completed in digeneans suggest that adult schistosomes, at least, have relatively high CYP-like activity. In Schistosoma mansoni, several antibodies to rat microsomal CYPs are shown to cross-react with schistosome extracts (Saeed et al. Reference Saeed, Mostafa, O'connor, Rafferty and Doenhoff2002). An inhibitor known to disrupt CYP function in other systems was found to enhance the susceptibility of a triclabendazole (TCBZ)-resistant F. hepatica isolate to TCBZ as judged by morphological tegumental surface scanning electron microscopy measurements (Devine et al. Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2010), although inhibition with the same CYP system inhibitor reduced TCBZ-sulphoxide formation equally in TCBZ-resistant and -sensitive isolates (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005).
Genomics also supports the possibility that biochemical assays might have been a ‘red herring.’ for CYP activity in adult parasitic helminths. Recent genomic and transcriptomics studies predict synthesis of many protein forms of the CYP superfamily within helminths. To date, the number of CYP genes ranges from 198 in the genome of the insect parasitic nematode Pristionchus pacificus (Dieterich et al. Reference Dieterich, Clifton, Schuster, Chinwalla, Delehaunty, Dinkelacker, Fulton, Fulton, Godfrey, Minx, Mitreva, Roeseler, Tian, Witte, Yang, Wilson and Sommer2008) to 27 CYP genes/partial genes in the plant parasitic nematode Meliodogyne incognita (Bird et al. Reference Bird, Williamson, Abad, Mccarter, Danchin, Castagnone-Sereno and Opperman2009).
CYP has now been extensively investigated in the free-living model nematode C. elegans, with 75 predicted CYP genes within 18 families, with CYPs found to be up-regulated on xenobiotic exposure (Kulas et al. Reference Kulas, Schmidt, Rothe, Schunck and Menzel2008) and anthelmintic exposure under genome wide transcript level analysis (Laing et al. Reference Laing, Ivens, Laing, Ravikumar, Butler, Woods and Gilleard2010). In addition, the gene encoding a receptor central to the regulation of CYP activity throughout the animal kingdom has been found in C. elegans (Lindblom and Dodd, Reference Lindblom and Dodd2009). Importantly, the key protein involved in CYP activity, NADP cytochrome P450 reductase has been identified in nematode genomes and nine CYP genes in C. elegans have altered phenotypes on large-scale RNAi gene knock-down experiments (Simmer et al. Reference Simmer, Moorman, Van Der Linden, Kuijk, Van Den Berghe, Kamath, Fraser, Ahringer and Plasterk2003).
CYP function is deemed essential for normal cellular metabolism in other organisms. In addition, it is predicted to be present in helminth genomes. Both points suggest CYP protein level absence will either reflect technical interference, the possibility that CYPs only have specific low level house-keeping metabolic functions (activities not associated with anthelmintic assault) or a unique biochemistry of adult helminths replacing CYP function. Others have suggested the absence is due to a limited access to oxygen required for CYP function (Barrett, Reference Barrett1998), although this seems unlikely as CYP function is universally required for essential house-keeping, and helminths have other functional oxidase enzymes. However, the possibility remains that alternative uncharacterized xenobiotic peroxidase(s) to CYP are expressed in adult parasitic helminths (Barrett, Reference Barrett1997). To this end, a number of candidate anthelmintic detoxification enzymes have been identified from studies on parasite reactive oxygen species (ROS) defence systems (reviewed by Cvilink et al. Reference Cvilink, Lamka and Skalova2009).
An inhibitor addition strategy was also used to show that another less well characterized Phase I system, flavin mono oxygenase system (FMO), is apparently important in TCBZ metabolism in microsomes of the digenean F. hepatica (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). The FMO gene family is smaller than the CYP superfamily and present in almost all phyla that have been examined (Cvilink et al. Reference Cvilink, Lamka and Skalova2009).
The anthelmintics albendazole (ABZ) and TCBZ are subject to Phase I sulphoxidation in a number of parasite helminths (Cvilink et al. Reference Cvilink, Lamka and Skalova2009), possibly by different enzymes in different cellular locations. Furthermore, in vivo, significant levels of Phase I oxidation of TCBZ-sulphoxide to the inactive TCBZ-sulphone is detectable in F. hepatica, with greater conversion in TCBZ-resistant F. hepatica isolate compared to TCBZ-sensitive isolate (Robinson et al. Reference Robinson, Lawson, Trudgett, Hoey and Fairweather2004). This is evidence of a new non-drug target resistance route in liver fluke (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005; Brennan et al. Reference Brennan, Fairweather, Trudgett, Hoey, Mccoy Mcconville, Meaney, Robinson, Mcferran, Ryan, Lanusse, Mottier, Alvarez, Solana, Virkel and Brophy2007). Phase I anthelmintic reduction has also been widely reported in nematodes (Cvilink et al. Reference Cvilink, Skalova, Szotakova, Lamka, Kostiainen and Ketola2008; Kohler and Bachmann, Reference Kohler and Bachmann1981) and cestodes (Barrett, Reference Barrett1997). Thus, overall, there is fragmented information at the protein level of Phase I detoxification capacity in parasitic helminths of medical and veterinary importance.
POTENTIAL PHASE II ANTHELMINTIC METABOLISM CAPACITY IN PARASITIC HELMINTHS
The majority of biochemically based Phase II studies have been limited to glutathione conjugation via glutathione transferases (GSTs), with only a few detailed investigations on other potential conjugation routes by glucose, sulphate, phosphate and N-acetylation (reviewed in Cvilink et al. Reference Cvilink, Lamka and Skalova2009). Genomic studies, such as in C. elegans and P. pacificus predict the presence of a range of proteins capable of Phase II detoxification such as UDP-glucuronosyltransferases and sulphotransferases (Dieterich et al. Reference Dieterich, Clifton, Schuster, Chinwalla, Delehaunty, Dinkelacker, Fulton, Fulton, Godfrey, Minx, Mitreva, Roeseler, Tian, Witte, Yang, Wilson and Sommer2008; Reichert and Menzel, Reference Reichert and Menzel2005).
GST is usually present in relatively high levels in the cytosol of many adult parasitic helminths. An interesting exception is lower GST levels in tissue-dwelling filarial nematodes (Brophy et al. Reference Brophy, Crowley and Barrett1990b) possibly correlating with suggested genome level absence of detoxification capacity. There are many GST forms within multi-GST families as would be expected of a xenobiotic defence protein superfamily, such as 54 GSTs in P. pacificus and 51 GSTs in C. elegans (Van Rossum et al. Reference Van Rossum, Brophy, Tait, Barrett and Jefferies2001). Many GSTs (as other detoxification proteins) also have house-keeping metabolic roles. For example, GSTs are possibly hydrophobic ligand transport proteins (Brophy and Barrett, Reference Brophy and Barrett1990), and their relatively high levels required for transport will be highlighted in proteomic studies, but this is not a detoxification role. Thus, global proteomics will often bring to prominence the abundant soluble GSTs that are relatively easy to assay and purify natively and express as recombinants. However, a highly effective detoxification enzyme might be present in levels undetectable by global proteomic studies, with its identification requiring sub-proteomic investigations, such as following an initial affinity chromatography step; in this manner a new GST form was found in F. hepatica (Chemale et al. Reference Chemale, Morphew, Moxon, Morassuti, Lacourse, Barrett, Johnston and Brophy2006).
There is no mechanistic level evidence that GSTs actually conjugate anthelmintics, despite the number of GST-based reductionist biochemical investigations and identifications in proteomic studies. The only suggestion is that schistosome GST is involved in the biotransformation of the anthelmintic dichlorvos (O'Leary and Tracy, Reference O'leary and Tracy1991). However, the parent anthelmintic substrate is usually tested on GST, not a pro-drug activated form or a substrate modified by parasite Phase I metabolism. There is ample evidence, including proteomic leads, that isolated native or recombinant forms of helminth GSTs can bind to parent anthelmintic structures (Brophy et al. Reference Brophy, Crowley and Barrett1990a). To date, only one study tracked the binding of a pro-drug (triclabendazole sulphoxide) to F. hepatica proteins following comparative global proteomics (Chemale et al. Reference Chemale, Perally, Lacourse, Prescott, Jones, Ward, Meaney, Hoey, Brennan, Fairweather, Trudgett and Brophy2010). Loss-of-function phenotype RNAi protocols have been optimised in the free-living nematode C. elegans for the GST superfamily (LaCourse et al. Reference Lacourse, Perally, Hernandez-Viadel, Wright and Brophy2008) and offers opportunity to link GST to function under external anthelmintic stress in culture. However, in the C. elegans model at least there appears up-regulation of GST forms, at the protein level, to potentially compensate for down-regulation of other GST forms (LaCourse et al. Reference Lacourse, Perally, Hernandez-Viadel, Wright and Brophy2008). This study also showed that proteomics can track the success of RNAi knockdown experiments (termed Prot. RNAi).
In addition, further to the lack of demonstration of anthelmintic conjugation with glutathione, there is also limited evidence that parasitic helminths can Phase III metabolise glutathione conjugates, with only low activities of one enzyme detectable by enzymic assay in adult nematodes (cysteine conjugate β-lyase, Adcock et al. Reference Adcock, Brophy, Teesdale-Spittle and Buckberry1999). The focus on the importance of GST in the pre-genomics area has possibly deflected and discouraged other important Phase II investigations. For example, Phase II glucose conjugation of toxins mainly occurs in plants and not animals, but has been uncovered in parasitic nematodes (Cvilink et al. Reference Cvilink, Skalova, Szotakova, Lamka, Kostiainen and Ketola2008) and with novel glucoside metabolites of albendazole produced in the nematode model C. elegans (Laing et al. Reference Laing, Ivens, Laing, Ravikumar, Butler, Woods and Gilleard2010).
POTENTIAL PHASE III ANTHELMINTIC METABOLISM CAPACITY IN HELMINTHS
Anthelmintic conjugates from Phase I and Phase II, following further potential Phase III enzyme-directed modification would likely be removed from cells at membranes by ATP–binding cassette (ABC)- dependent transport family or sequestrated by an abundant binding protein. Additionally, a range of hydrolyase enzymes that could further process Phase II metabolites have been biochemically detected in parasitic helminths (Adcock et al. Reference Adcock, Brophy, Teesdale-Spittle and Buckberry1999; Cvilink et al. Reference Cvilink, Lamka and Skalova2009).
ABC transporters are found in both prokaryotes and eukaryotes and are associated with multidrug resistance. Genomic studies have predicted many ABC transporters in nematodes, with 129 genes in P. pacificus and 56 genes in C. elegans respectively (Dieterich et al. Reference Dieterich, Clifton, Schuster, Chinwalla, Delehaunty, Dinkelacker, Fulton, Fulton, Godfrey, Minx, Mitreva, Roeseler, Tian, Witte, Yang, Wilson and Sommer2008)). In parasitic helminths the experimental research effort has been directed towards the well-characterized P-glycoprotein member of the ABC transporters. For example, studies in F. hepatica show that TCBZ-resistant isolates accumulate less TCBZ and its major active metabolite than TCBZ-sensitive isolates, suggesting differences in rates of efflux pumping and presumed to be via P-glycoprotein. In addition, indirect evidence with inhibitors indicated that ABC transport pumps, including P-glycoprotein, in F. hepatica are associated with TCBZ resistance as reversal of ‘resistance’ occurred with inhibition. P-glycoprotein ABC pumps are also associated with anthelmintic resistance in nematodes (reviewed by Alvarez et al. Reference Alvarez, Merino, Molina, Pulido, Mckellar and Prieto2006).
Sequestration is deemed an alternative of excretion and, to date, only descriptive studies of this route have been undertaken in helminths. Binding of toxins by abundant helminth ligand-binding proteins has been described for both GST (discussed above) and lipid binding proteins (LBPs). LBPs (Barrett et al. 1997) have clearly been demonstrated to bind anthelmintics using purified or recombinant protein forms and both GST and LBP can be present at concentrations of up to 50 μ m. The binding of anthelmintic may either reduce the effective drug concentration, rendering the buffered compound more likely to be metabolised by a complex or, conversely, may simply disrupt GST/LBP house-keeping functions. One novel example of sequestration may be the binding of toxic ingested haem from the host to GST proteins from blood-feeding gastro-intestinal nematodes (Van Rossum et al. Reference Van Rossum, Brophy, Tait, Barrett and Jefferies2004). In addition, metallothioneins bind toxic metals and have been extensively investigated and identified in the C. elegans model (Hoeckner et al. Reference Hoeckner, Dallinger and Stuerzenbaum2011), but interaction with anthelmintics have not yet been investigated.
PROTEOMICS AS A TOOL TO UNCOVER ANTHELMINTIC METABOLISM PATHWAYS
Next-generation sequencing of helminth transciptomes and genomes can reveal potential pathways for anthelmintic metabolism. However, gene annotation based on previous biochemical studies in other systems will not reveal novel parasitic helminth detoxification processes. Metabolome and proteomic level studies are required to help confirm the detoxification status of a protein and associated pathways. A new metabolite and protein level drive is required to realise the potential of the post-genomic era and support the development of new interactive databases such as HelmCoP, Helminth Control and Prevention (Abubucker et al. Reference Abubucker, Martin, Taylor and Mitreva2011). Thus, information from genomes should not simply be used to clone and express ‘likely enzymes’ for assessing anthelmintic metabolism and return to reductionist biology approaches.
As highlighted above, proteomics, unlike classical reductionist biochemistry, has the potential to reveal systematically the complete (soluble and membrane) dynamic protein response (relative expression, modifications and interactions) in a parasitic helminth to an environmental challenge (Barrett et al. Reference Barrett, Jefferies and Brophy2000). To date, most proteomic investigations in parasitic helminths have been descriptive in technically straightforward soluble fractions without environment challenge to address a physiological question. These pioneering proteomic studies showed that helminth proteins could be identified (Jefferies et al. Reference Jefferies, Campbell, Van Rossum, Barrett and Brophy2001), without the luxury of a freely available annotated complete protein database, as is available for the model nematode C. elegans for example (Jones et al. Reference Jones, Staffa, Perally, Lacourse, Brophy and Hamilton2010). Despite this success, there remains a dearth of transcript and gene prediction data for helminths making many potential proteomics experiments extremely challenging, but possible with available databases (Millares et al. Reference Millares, Lacourse, Perally, Ward, Prescott, Hodgkinson, Brophy and Rees2012).
Descriptive proteomics studies have supported understanding of candidate anthelmintic metabolism proteins, such as the soluble GST superfamily. The GST complement of first the model nematode C. elegans (Van Rossum et al. Reference Van Rossum, Brophy, Tait, Barrett and Jefferies2001) and, more recently, in F. hepatica (Chemale et al. Reference Chemale, Morphew, Moxon, Morassuti, Lacourse, Barrett, Johnston and Brophy2006) was resolved via a sub-proteomics approach. In the 2006 study, the authors incorporated two affinity chromatography steps, 2DE proteomics and peptide sequencing to resolve new GST family members. The Fasciola-based study, in parallel with probing an EST dataset, identified two new forms of GST (Chemale et al. Reference Chemale, Morphew, Moxon, Morassuti, Lacourse, Barrett, Johnston and Brophy2006). The disadvantage of affinity chromatography-based sub-proteomic approaches is that although proteins can be concentrated by selection away from other cellular proteins, all the proteins of interest need to bind to an affinity matrix in order to reveal the full expressed complement which is not always accomplished. However, sub-proteomics studies such as those based on membrane fractions, could probe for the expression of Phase I (CYPs) or Phase III (P-glycoprotein ATP pump) proteins but are yet to be completed due to the technical complexities associated with analysing membrane fractions.
With the success of RNAi, initially in nematodes and more recently in flatworms, the ability to delineate anthelmintic metabolising or interacting proteins or protein superfamilies can be achieved by combining RNAi with proteomics (LaCourse et al. Reference Lacourse, Perally, Hernandez-Viadel, Wright and Brophy2008). The combination of both techniques should allow targeted experiments to unravel the complexities of anthelmintic metabolism.
To date, the findings from many descriptive proteomics studies have not been fully realised despite identifying a large variety of proposed xenobiotic detoxification proteins in parasitic helminths (Table 2). Gel-based proteomics is not focused on normalising data and more abundant soluble/xenobiotic detoxification proteins are more readily identified, such as the GSTs, hydrophobic ligand-binding proteins and peroxidises. Interestingly, proteins with detoxification roles are detected in helminth excretory and secretory products (Table 2), suggesting near surface detoxification in a xenobiotic rich host environment. However, descriptive proteomics studies could be enhanced quantitatively to further benefit the understanding of anthelmintic metabolism. Coupling the global separation of helminth proteins to partial least squares–discriminant analysis (PLS-DA) offers the possibility to group proteins and compare innate metabolic pathways in isolates. This approach has been used to protein fingerprint F. hepatica isolates in order to develop training sets to support the identification of anthelmintic resistant isolates from those susceptible to treatment (MacKintosh, 2011) and has the potential to identify new bio-markers.
Table 2. Mining of experimental parasitic helminth proteomic data from publications in refereed journals for evidence of potential anthelmintic metabolising, sequestration and efflux pump proteins

Investigative comparative proteomics has already provided some new insights to support understanding of anthelmintic metabolism. There are many potential routes for a parasite to reduce the concentration of a toxic anthelmintic in their cells: reduce uptake, increase excretion, change or lose a target, not activating a pro-drug and via toxin detoxification pathways. As a case study, a proteomics experiment with defined isolates of F. hepatica exposed to TCBZ provided evidence following recombinant expression that a fatty acid binding protein (FABP) was a new type of Phase III anthelmintic sequestration protein associated with resistant isolates while providing further evidence of the role of Mu class GST in binding of anthelmintics (Chemale et al. Reference Chemale, Perally, Lacourse, Prescott, Jones, Ward, Meaney, Hoey, Brennan, Fairweather, Trudgett and Brophy2010). This single proteomics experiment highlighted the dynamics of protein response to anthelmintic exposure and supports previous reductionist biochemistry in independent laboratories that when the data are combined suggested broad TCBZ-induced changes in liver fluke. RNAi assays will confirm the role of FABP in liver fluke anthelmintic response and nucleic acid sequencing studies will reveal if any markers of TCBZ resistance are found in the FABP gene.
CHALLENGES TO PROTEOMICS TECHNOLOGIES TO SUPPORT UNDERSTANDING OF ANTHELMINTIC METABOLISM
To date, anthelmintic response and metabolism studies have also been neglected by proteomics approaches, with only one study of soluble responses in liver fluke published (Chemale et al. Reference Chemale, Perally, Lacourse, Prescott, Jones, Ward, Meaney, Hoey, Brennan, Fairweather, Trudgett and Brophy2010).
The success of proteomics is entwined with the delivery of genomes or transcriptome databases that will provide the searchable databases to link function with sequence. In contrast, the success of helminth genomes requires an experimental lead to predict functional annotation. It is predicted that there will be over thirty helminth genomes available at least in draft form within the next five years (Abubucker et al. Reference Abubucker, Martin, Taylor and Mitreva2011).
Helminth proteomic studies have confirmed the presence of post-translational modifications (PTM) (Jefferies et al. Reference Jefferies, Campbell, Van Rossum, Barrett and Brophy2001; Chemale et al. Reference Chemale, Van Rossum, Jefferies, Barrett, Brophy, Ferreira and Zaha2003) but, to date, there have been no proteomic studies probing the extent or the functional significance of PTM on helminth proteins. With over four hundred potential modifications, and an average of ten PTMs per protein, it is clear that for a full mechanistic understanding of helminth biochemistry, including anthelmintic metabolism, a significant time investment is required in this area of functional proteomics.
Most cellular processes are completed by proteins that temporarily assemble into multimeric complexes. Thus protein-protein interactions are important in protein function. For example, the blocking of such protein-protein interactions by the anthelmintic group benzimidazoles (Bzs) leads to the disruption of tubulin polymerisation (Prichard et al. Reference Prichard, Von Samson-Himmelstjerna, Blackhall and Geary2007). At present, experiments on helminth protein complexes associated with anthelmintic metabolism have been investigated using ‘pull down’ proteomics approaches. For example, using an anthelmintic as a ‘bait’ has shown some success studying interacting partners of albendazole (Chambers et al. Reference Chambers, Ryan, Hoey, Trudgett, Mcferran, Fairweather and Timson2010). In addition, using known anthelmintic interacting/ metabolising proteins, such as GST (Greetham et al. Reference Greetham, Morgan, Campbell, Van Rossum, Barrett and Brophy2004) and beta-tubulin (Fig. 1) as ‘baits’, pools of interacting proteins can be identified. Thus, protein networks associated with anthelmintic metabolism/interaction can be established and the effect of anthelmintic pressure on these interactions examined (Fig. 2).

Fig. 1. Understanding anthelmintic action using ‘pull down’ proteomic methods. A) Schematic representation of ‘pull down’ methods and their application to studying anthelmintic action where the ‘bait’ (1) can either be a protein of interest or an anthelmintic. The bait, attached to a solid substrate, can be loaded into a column (2) before being allowed to interact with a protein lysate (3); or the bait and lysate can be pre-incubated before loading into a column. At this stage, if using a protein as bait, an anthelmintic (X) can be introduced to disrupt potential interactions. The interactions are washed substantially to remove non-specific binding partners (4) and then eluted and analysed directly via 1D or 2D SDS-PAGE followed by mass spectrometry or directly by mass spectrometry using gel free methods (5). This method has been demonstrated in B) and C) to investigate protein interactions of C. elegans (wild type N2) beta-tubulin (Ben-1) using Dynabeads® TALON® Co2+ affinity purification as the solid support incubated with wild type N2 protein lysate. B) Interactions of wild type N2 Ben-1 Beta-tubulin with N2 lysate purified with Dynabeads® TALON®. C) Interactions of wild type N2 lysate and Dynabeads® TALON® only. FT – flow through. 20 mm I-III – Immidazole washes 1–3 containing 20 mm immidazole. 150 mm and 500 mm correspond to 150 mm and 500 mm immidazole washes. TALON beads represents the Dynabeads® TALON® boiled in SDS buffer. Both B) and C) 12% SDS PAGE Coomassie blue stained. Arrowed are C. elegans Ben-1(upper arrow) and Sorbitol dehydrogenase (lower arrow) a known interacting partner of the tubulin complex (Morphew, unpublished).

Fig. 2. Anthelmintic disruption of protein-protein interactions. A) Schematic representation of ‘pull down’ proteomics to study anthelmintic action using a known interacting protein as ‘bait’ (1). The protein bait, attached to a solid substrate, can be loaded into a column (2) before being allowed to interact with a protein lysate (3) with and without the presence of anthelmintic (X). Protein interactions formed with and without anthelmintic (4) can be analysed as previously detailed (Fig. 1). This is demonstrated in B) and C) showing protein-protein interactions of C. elegans (wild type N2) beta-tubulin (Ben-1) interrupted by benzimidazoles. Ben-1 bound to Dynabeads® TALON® Co2+ were incubated with wild type N2 protein lysate in the presence of benzimidazole (B) and absence of benzimidazole (C) to look for disruption of protein interactions. Both B) and C) 12% SDS PAGE Coomassie blue stained. Arrowed are C. elegans Ben-1(upper arrow) in both B) and C) and Sorbitol dehydrogenase (lower arrow) in C) only (Morphew unpublished).
TAP (tandem affinity purification) methodology provides for isolation of proteins as native complexes from cells for subsequent proteomics analysis, but in helminths this powerful tool has to date only been used in the model nematode C. elegans (Lee and Lee, Reference Lee and Lee2004). As heterologous protein expression of parasitic nematode anthemintic target proteins has previously been successful in the C. elegans host (Kwa et al. Reference Kwa, Veenstra, Vandijk and Roos1995) then the potential of TAP technology for understanding anthelmintic metabolism, at least in nematodes, is a real possibility.
The main challenge for helminth proteomics remains the detection and measurement of integral membrane-bound proteins, such as those important in Phase I and Phase III anthelmintic metabolism. To date, there has been progress understanding the protein complement in the tegument of digeneans (Mulvenna et al. Reference Mulvenna, Sripa, Brindley, Gorman, Jones, Colgrave, Jones, Nawaratna, Laha, Suttiprapa, Smout and Loukas2010; Castro-Borges et al. Reference Castro-Borges, Simpson, Dowle, Curwen, Thomas-Oates, Beynon and Wilson2011; Wilson et al. Reference Hewitson, Harcus, Murray, Van Agtmaal, Filbey, Grainger, Bridgett, Blaxter, Ashton, Ashford, Curwen, Wilson, Dowle and Maizels2011). Yet, these flatworm studies have produced only a few recognised Phase I and III proteins potentially involved in anthelmintic metabolism (Table 2). The experimental difficulties with protein solubility and low abundance making integral membrane protein identification a challenge for both gel based and gel free proteomics.
CONCLUSIONS
Anthelmintics are essential for parasitic helminth control but there is fragmented information on drug metabolism in parasites derived from reductionist biology, and possibly red herrings (limited CYP and relative importance of GST). To date, most proteomic studies on parasitic helminths have been on descriptive on technically easy soluble fractions and not focused on addressing a biological question, such as response and protein induction to a specific anthelmintic assault and unravelling any relationship between anthelmintic metabolism to anthelmintic resistance. The new genome information will provide templates to link sequence via function via more functional proteomic investigations. Overcoming the difficult technical challenge of optimisation of membrane proteomics in helminths is essential to effectively study Phase I CYPs and Phase III ABC transporter pumps.
With the current information available on parasite protein complements fragmented and major technical challenges resolving membrane proteins, then the future of protein-level research directed towards anthelmintic metabolism is challenging. However, the availability of new genomes/ transcriptomes from parasitic helminths should inspire more parasitic worm laboratories to address the challenges of membrane proteomics analyses, using the information available from specialist membrane proteomic developers. The new sequences provide templates of the capacity of xenobiotic metabolism in parasitic helminths and proteomics will confirm the routes of individual anthelmintic response and inform, stimulate and support those interested in developing new anthelmintics for neglected diseases. At present, the limited information does suggests addressing challenges will be rewarded as there are differences in detail between the biotransformation routes between parasites and hosts.
ACKNOWLEDGEMENTS
This paper is dedicated to the memory of Professor John Barrett.
PMB and RMM thank the BBSRC and DFID for financial support (grant BBH0092561) and the English Beef and Lamb Executive (EBLEX), Hybu Cig Cymru/Meat Promotion Wales (HCC) and Quality Meat Scotland (QMS) for funding NMK.