Introduction
Differences between populations in terms of sexual traits and/or mating preferences can result in speciation mediated by sexual selection (Butlin & Ritchie, Reference Butlin, Ritchie, Slater and Halliday1994; Ritchie, Reference Ritchie2007; Macedo & Machado, Reference Macedo and Machado2013). Sexual selection can favour characteristics that reinforce pre-zygotic reproductive isolation between subpopulations through the evolution of preferential mating associated with male secondary characters (Lande, Reference Lande1981, Reference Lande1982; Ritchie, Reference Ritchie2007). Among cryptic species, pre-zygotic isolation tends to evolve either as a direct factor (Wu et al., Reference Wu, Hollocher, Begun, Aquadro, Xu and Wu1995) or by reinforcing already existing post-zygotic isolation (Noor, Reference Noor1999). Sexual behaviour isolation is frequently a component of pre-zygotic isolation (Nanda & Singh, Reference Nanda and Singh2012), and male traits evaluated by the females during courtship are selected and enable reliable recognition of con-specifics, promoting isolation between related species and avoiding cross-mating (Boake et al., Reference Boake, Deangelis, Ebra and Andreadis1997).
The nominal species Anastrepha fraterculus represents a cryptic species complex (Stone, Reference Stone1942; Malavasi & Morgante, Reference Malavasi and Morgante1982; Steck, Reference Steck1991; Selivon & Perondini, Reference Selivon and Perondini1998; Hernández-Ortiz et al., Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2004; Selivon et al., Reference Selivon, Vretos, Fontes and Perondini2004; Selivon et al., Reference Selivon, Perondini and Morgante2005; Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Hernández-Ortiz et al., Reference Hernández-Ortiz, Bartolucci, Morales-valles, Frías and Selivon2012, Reference Hernández-Ortiz, Canal, Tigrero Salas, Ruíz-Hurtado and Dzul-Cauich2015; Canal et al., Reference Canal, Hernández-Ortiz, Tigrero-Salas and Selivon2015; Manni et al., Reference Manni, Lima, Guglielmino, Lanzavecchia, Juri, Vera, Cladera, Scolari, Gomulski, Bonizzoni, Gasperi, Silva and Malacrida2015) that shows pre- and post-zygotic reproductive incompatibilities among lineages (Selivon et al., Reference Selivon, Perondini and Morgante1999; Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Segura et al., Reference Segura, Vera, Rull, Wornoayporn, Islam and Robinson2011; Rull et al., Reference Rull, Abraham, Kovaleski, Segura, Mendoza, Liendo and Vera2013; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014; Vaníčková et al., Reference Vaníčková, Hernández-Ortiz, Joachim-Bravo, Dias, Roriz, Laumann, Mendonça, Paranhos and do Nascimento2015a). This species, known as the South American fruit fly, is a polyphagous species considered the most important native pest of native and commercial fruits in South America (Dutra et al., Reference Dutra, Fernandes, Nascimento, Quadros and Oliveira2007; Alberti et al., Reference Alberti, Confalonieri, Zandomeni and Vilardi2008). It is widely distributed from northern Mexico to the south of South America (Stone, Reference Stone1942; Aluja, Reference Aluja1994; Hernández-Ortiz et al., Reference Hernández-Ortiz, Canal, Tigrero Salas, Ruíz-Hurtado and Dzul-Cauich2015) and infests more than 116 hosts (Zucchi, Reference Zucchi2016). Currently, eight distinct morphotypes has been postulated to constitute the A. fraterculus cryptic species complex known as the Peruvian, Andean, Mexican, Venezuelan, Brazil-1, Brazil-2, Brazil-3 and Ecuadorian morphotypes (Hernandez-Ortiz et al., Reference Hernández-Ortiz, Bartolucci, Morales-valles, Frías and Selivon2012, Reference Hernández-Ortiz, Canal, Tigrero Salas, Ruíz-Hurtado and Dzul-Cauich2015).
The pre-zygotic incompatibilities found between the morphotypes described for this cryptic species complex (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Rull et al., Reference Rull, Abraham, Kovaleski, Segura, Mendoza, Liendo and Vera2013; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014; Roriz et al., Reference Roriz, Japyassú and Joachim-Bravo2017) suggest that reproductive barriers have evolved favouring isolation, comprising temporal and behavioural aspects associated with the mating system. The mating system in A. fraterculus is polygynous, in which the females discriminate among males congregated in displaying, lekking arenas (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom1999). Male courtship behaviour was described by Gomez Cendra et al. (Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011) as composed of 15 behavioural units arranged in three groups: pheromone emission, wing positioning and body movements. More recently, Passos-Roriz et al. (Reference Passos Roriz, Japyassú and Sordi Joachim-Bravo2018) reanalysed the courtship of A. fraterculus males and subdivided that behaviour into 26 behavioural units within eight groups. This repertoire of behaviours involves the emission of chemical, visual and acoustic signals.
Chemical communication, mediated by the emission of volatile sexual pheromones or the presence of certain hydrocarbons in the cuticle, can contribute to the recognition and choice of reproductive partners in certain insect species (Antony & Jallon, Reference Antony and Jallon1982; Cobb & Jallon, Reference Cobb and Jallon1990; Giglio & Dyer, Reference Giglio and Dyer2013; Vaníčková et al., Reference Vaníčková, Virgilio, Tomčala, Břízová, Ekesi, Hoskovec, Kalinová, Do Nascimento and De Meyer2014). In the A. fraterculus complex, cuticular hydrocarbons differ between morphotypes as well as between males and females (Vaníčková et al., Reference Hernández-Ortiz, Gómez-Anaya, Sánchez, McPheron and Aluja2012, Reference Vaníčková, Břízová, Pompeiano, Ferreira, de Aquino, Tavares, Rodriguez, Mendonça, Canal and do Nascimento2015b, Reference Vaníčková, Břízová, Mendonça, Pompeiano and do Nascimentoc). Moreover, male-borne volatiles of distinct populations differ quantitatively even across populations belonging to the same morphotype (Břízová et al., Reference Břízová, Mendonça, Vanícková, Mendonça, Da Silva, Tomčala, Paranhos, Dias, Joachim-Bravo, Hoskovec, Kalinová and Do Nascimento2013). Juárez et al. (Reference Juárez, Devescovi, Břízová, Bachmann, Segura, Kalinová, Fernández, Ruiz, Yang, Teal, Cáceres, Vreysen, Hendrichs and Vera2015) reported that A. fraterculus females were attracted to male-borne volatiles of their respective populations; even though these authors argued that pair recognition probably occurred during courtship.
The evolution of differences in the time of sexual activity between populations may promote or accelerate reproductive isolation (Dobzhansky, Reference Dobzhansky1937). The time of sexual activity within the A. fraterculus complex has been studied for Brazil-1, Brazil-3, Mexican, Peruvian and Andean morphotypes (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Segura et al., Reference Segura, Vera, Rull, Wornoayporn, Islam and Robinson2011; Rull et al., Reference Rull, Abraham, Kovaleski, Segura, Mendoza, Liendo and Vera2013; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014; Dias et al., Reference Dias, Silva, Lima, Petitinga, Hernandez-Ortiz, Laumann, Paranhos, Uramoto, Zucchi and Joachim-Bravo2015; Roriz et al., Reference Roriz, Japyassú and Joachim-Bravo2017). Evidence shows that this trait varies between morphotypes and may contribute, in certain cases, to mating isolation.
The high complexity of A. fraterculus courtship (Aluja, Reference Aluja1994; Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom1999; Gomez Cendra et al., Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011) and the fact that some components of male courtship can be important for mate recognition (Ryan & Rand, Reference Ryan and Rand1993) support our hypothesis that there are differences in the courtship behaviours of the different morphotypes within this cryptic species complex. As such, in this work, we pursued to determine the temporal pattern of pheromone emission as well as to analyse and characterize male courtship of some morphotypes within the A. fraterculus cryptic species complex. These analyses should help elucidate the pre-zygotic mechanisms underlying the reproductive isolation found between different lineages of this cryptic species complex, emerging as a useful tool for lineage discrimination.
Materials and methods
Flies
The distinct populations of A. fraterculus were maintained at the Insect Pest Control Laboratory of the IAEA/FAO, Seibersdorf, Austria. Flies were reared on artificial diets according to the methods described by Vera et al. (Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006) and Rull et al. (Reference Rull, Abraham, Kovaleski, Segura, Islam, Wornoayporn, Dammalage, Tomas and Vera2012). The colonies were maintained at 25 ± 1°C, relative humidity 70 ± 10% and under a 12 h light:dark photoperiod (7:00 photophase; 19:00 scotophase). We used populations from San Miguel de Tucumán, Tucumán/Argentina (26°48′2″S, 65°13′3″W; F1 generation), Vacaria, Rio Grande do Sul/south of Brazil (28°27′32″S, 50°59′44″W; F32 generation), and Piracicaba, São Paulo/southeastern of Brazil (22°45′45″S, 47°50′33″W; F35 generation) as representatives of morphotype Brazil-1, a population from La Molina/Peru (12°4′55″S, 76°55′41″W; F30 generation) representing the Peruvian morphotype and a population from Ibagué, Tolima/Colombia (4°26′40″S, 75°14′32″W; F29 generation) representing the Andean morphotype. The taxonomic confirmations of the morphotypes were made by Dr Vicente Hernández-Ortiz of the Instituto de Ecología A.C. México.
After adult emergence, the flies were separated by sex and isolated in cylindrical acrylic cages, with free access to water and a diet composed of wheat germ, hydrolysed yeast and sugar (1:1:3) until sexual maturity (males: 10–25 days; females: 15–25 days). The experiments were undertaken in the Insect Pest Control Laboratory (IAEA, FAO, Seibersdorf, Austria) within arenas under these same colony maintenance conditions.
Temporal patterns of pheromone emission
To analyse the time of pheromone emission, at least 24 h before the test, ten marked (with Acrilex non-toxic paint) males of the same population were placed in cylindrical acrylic cages (29 × 20 cm). Ten females held in acrylic boxes (9 × 7 × 9 cm), with air flow in the top, were placed in the centre of each cylindrical male cage to stimulate male pheromone emission. Flies were kept for at least 12 h in darkness before the experiment to ensure the experiment began at the onset of the photophase. Pheromone emission frequency was recorded individually every 30 min, from 08:30 to 17:30 h. Males were considered to be emitting pheromone when they everted the anal epithelium at the distal portion of the abdomen and/or the prominent lateral glands (Lima et al., Reference Lima, House and do Nascimento2001). Ten replicates were made for each population.
Male courtship sequences
To characterize male courtship sequence, two males and two females (simulating a small lek) of the same population were released into a small glass cage (9 × 7 × 9 cm) containing a lemon leaf (Citrus sp.) as a substrate for their sexual interactions (Briceño et al., Reference Briceño, Eberhad, Vilardi, Cayol and Shelly2007 with modifications). Their behaviours were recorded for up to 30 min using a digital video camera (Geovision-GV-BX 220D-3, 2 M, variable focus lens, at 30 fps) positioned frontally to the glass cage under artificial light conditions. The videos were saved in an AVI format (GeoVision Multicam Surveillance version 8.5.4.0). Recordings were performed during the period of sexual pheromone emission. Only one male mated was analysed in each session and ten successful courtship replicates (i.e., that resulted in mating) were performed for each population.
Statistical analyses
To analyse temporal correlations in pheromone emission among populations, we generated an autologistic Generalized Linear Model (GLM) (Besag, Reference Besag1972). This methodology compared the populations using time as a co-variable. We assumed a Poisson distribution for the number of males that emitted pheromones. The Log was utilized as a linking function of the GLM. An automodel was constructed using the co-variance of five consecutive data collection times, using the Vacaria population as the reference within the model.
Male courtship ethograms followed Passos Roriz et al. (Reference Passos Roriz, Japyassú and Sordi Joachim-Bravo2018) and Gomez Cendra et al. (Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011) and are described in Appendix B. Only the behaviour of males that mated successfully was analysed. The frequency of the behavioural units was compared among the different populations of A. fraterculus through a GLM assuming that the data followed a Poisson distribution, using Log as the linking function, and model hypothesis test for multiple comparisons.
The behavioural sequences were analysed using EthoSeq software (Japyassú et al., Reference Japyassú, Alberts, Izar and Sato2006). The program produces a first-order transition matrix from the raw individual sequence data, and uses graph theory to produce hierarchical representations with directed trees (DiTree). The DiTree maximizes the sums of the transition probabilities between all of the behavioural units, generating the most parsimonious hierarchical graph, or trees (Japyassú et al., Reference Japyassú, Alberts, Izar and Sato2006). To analyse the courtship in detail, we selected the tree whose root indicated copulation success (ending with mating) to produce courtship routines (most probable, linear sequences of behaviour). The most frequent routines were analysed through a canonical discriminant analysis to evaluate their contribution to the discrimination between populations. The behavioural routines were included in the analyses using the stepwise mode, with the Wilks’ λ method to enter and remove the discriminant functions (F = 0.05 and F = 0.10, respectively).
The statistical analyses were performed using free R studio software, except the discriminant analyses which were executed on STATISTICA 7.1 software (Stat soft. 1984–2005).
Results
Temporal patterns of pheromone emission
The GLM analysed was significant, demonstrating differences in the pattern of pheromone emission across morphotypes (table 1). The initiation, ending and peak calling time varied among the morphotypes (fig. 1). Males from Vacaria, Tucumán and Piracicaba (morphotypes Brazil-1) start calling behaviour at the start of photophase and continued emitting pheromones for at the least 5 h. There were also small within morphotype differences: Vacaria, Tucumán and Piracicaba males all displayed peak calling early in the day, but Vacaria males called at a relatively high level until noon, whereas calling by Tucumán, and Piracicaba decreased steadily through the morning. Males from Peru initiated pheromone emission behaviour 4 h after the onset of the lights and called at high levels over the rest of the day. This population showed the greatest mean abundance of calling in the morning. Colombia males initiated pheromone emission at the beginning of the afternoon (13:00 h) and showed a steady increase in calling behaviour until late afternoon (17:00) followed by a reduction of activity.

Fig. 1. Pattern of calling behaviour in Anastrepha fraterculus populations from Tucumán, Piracicaba and Vacaria (Brazil-1 morphotype), Peru and Colombia (P < 0.05, generalized linear autologistic model – GLM). The standard deviation can be seen in Appendix A (Supplementary data).
Table 1. Generalized linear autologistic model of the data of the time of pheromone emission using Vacaria as reference population.

*Significant differences.
Male courtship sequences
Populations differed significantly in the frequency of male courtship behavioural units: some units were more frequent, while others were less frequent, or even non-existent in certain populations (fig. 2). Mobile (MO) was the most frequent behaviour in Colombia and Peru populations; within the Brazil-1 morphotype, it was more frequent for Vacaria than either Piracicaba or Tucumán (χ2 = 103.39; P < 0.0001). Calling (CALL) (χ2 = 91.753; P < 0.0001) and Flying (FL) (χ2 = 217.24; P < 0.0001) were also more frequent in the Colombia and Peru populations, while Tucumán showed the lowest levels. Transversal with pheromone emission (TR-call) was most frequent in Colombia, followed by Peru (χ2 = 67.92; P < 0.0001). Spin (SP-call) (χ2 = 233.12; P < 0.0001), Alignment (AL) (χ2 = 85.43; P < 0.0001), Arrowhead-2 (AH2-call) (χ2 = 65.03; P < 0.0001) and Contact (CO) (χ2 = 18.531; P = 0.0009) were less frequent in Colombia and Peru. Grooming (GR) was less frequent in Piracicaba and Tucumán (χ2 = 66.93; P < 0.0001). Hamation with pheromone emission (HA-call) (χ2 = 138.77; P < 0.0001.), Arrowhead-1 (AH1-call) (χ2 = 122.11; P < 0.0001) and Abdominal movement with pheromone emission (AB-call) (χ2 = 24.89; P < 0.0001) were less frequent, while Marking leaf (ML) was more frequent (χ2 = 9.73; P = 0.045) in Colombia. Enantion with emission of pheromones (EN-call) was less frequent in Piracicaba (χ2 = 56.86; P < 0.0001). Attempt (AT) (χ2 = 91.75; P < 0.0001), Enantion (EN) (χ2 = 144.7; P < 0.0001), Abdominal movement (AB) (χ2 = 16.55; P = 0.002359), Oscillation (OC) (χ2 = 18.03; P = 0.0012) and Stationary (ST) (χ2 = 105.4; P < 0.0001) were more frequent, while Fanning (FA-call) was less frequent (χ2 = 126.54; P < 0.0001) in Peru. For all comparisons, the degrees of freedom were 4.
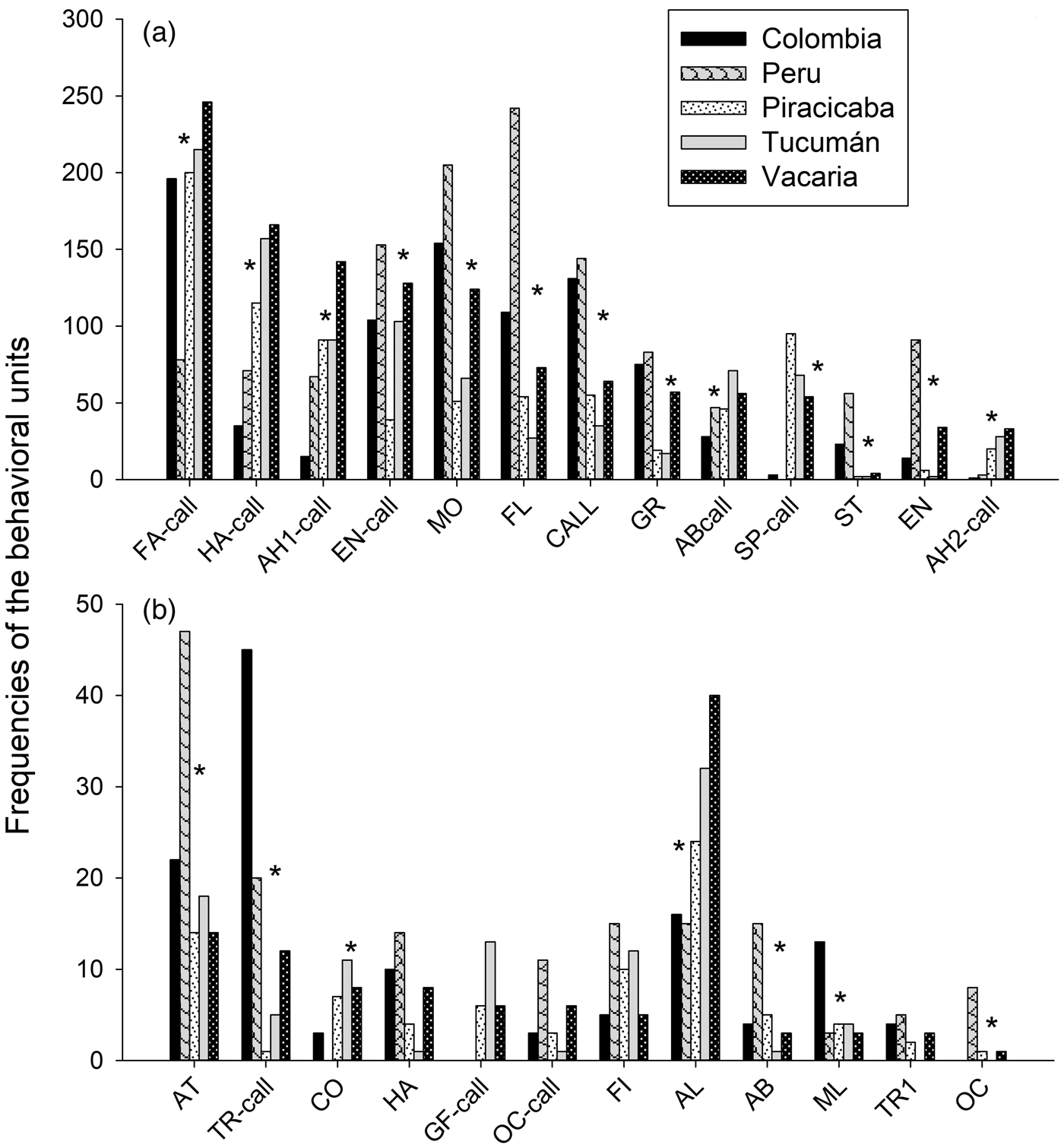
Fig. 2. Total frequencies of male courtship behavioural units in distinct Anastrepha fraterculus populations. Behavioural units with high (above 50) and low (below 50) frequencies are presented separately in plots a and b, respectively. Populations differ in the frequency of courtship units (P < 0.05, GLM).
Of the 14 highly frequent behavioural routines included in the analysis, nine entered in the final discriminant analysis model (Wilks’ λ = 0.065; F 36.159 = 4.74; P < 0.001). AH1-call>Al, CALL>FA-call, VO>CALL, SP-call>FA-call and EN-call>MO were the routines that most often contributed to the discrimination between the populations (table 2). The scatter plot indicated three separate groups, with the first being formed by the Vacaria, Tucumán and Piracicaba populations, the second by Colombia and the third by Peru (fig. 3 and table 3). The centroids of Vacaria, Tucumán and Piracicaba, all of them populations of the morphotype Brazil-1, were close to each other, while the morphotypes themselves were distant from each other in the discriminant space (table 4).

Fig. 3. Populations plotted within the discriminant function scatterplot obtained from behavioural routines. Ellipses confidence interval of 95%.
Table 2. Frequencies of the 14 routines that participated in the discriminant analysis. The variables presented in light grey were included in the final model, while those in dark grey were not.

Table 3. Discriminant functions, means of variables in the groups.

Table 4. Distances of Mahalanobis between the centroids of the populations obtained in the discriminant analysis.

The probabilistic trees generated (TGS) by EthoSeq indicated distinct behavioural paths to mate among the populations (fig. 4). In the Colombia population (fig. 4a), nine behavioural units preceded Attempt (AT) and the units with the highest frequencies were Flying (FL) (22.7%), Mobile (MO) (18.8%), Contact (CO) and Alignment (AL) (13.64%). In the Peru population (fig. 4b), six behavioural units preceded Attempt (AT) and the units with the highest frequencies were Flying (FL) (48.9%), Arrowhead-1 (AH1-call) (25.53%) and Calling (CALL) (10.92%). The three Brazil-1 populations (Piracicaba, Tucumán and Vacaria) showed the same units with the highest frequencies: Contact, Alignment and Arrowhead-1. The organization of these three units performed by Brazil-1 populations in the TGS was variable; also there were other units exclusive to one or another of these populations (figs 4c–e).
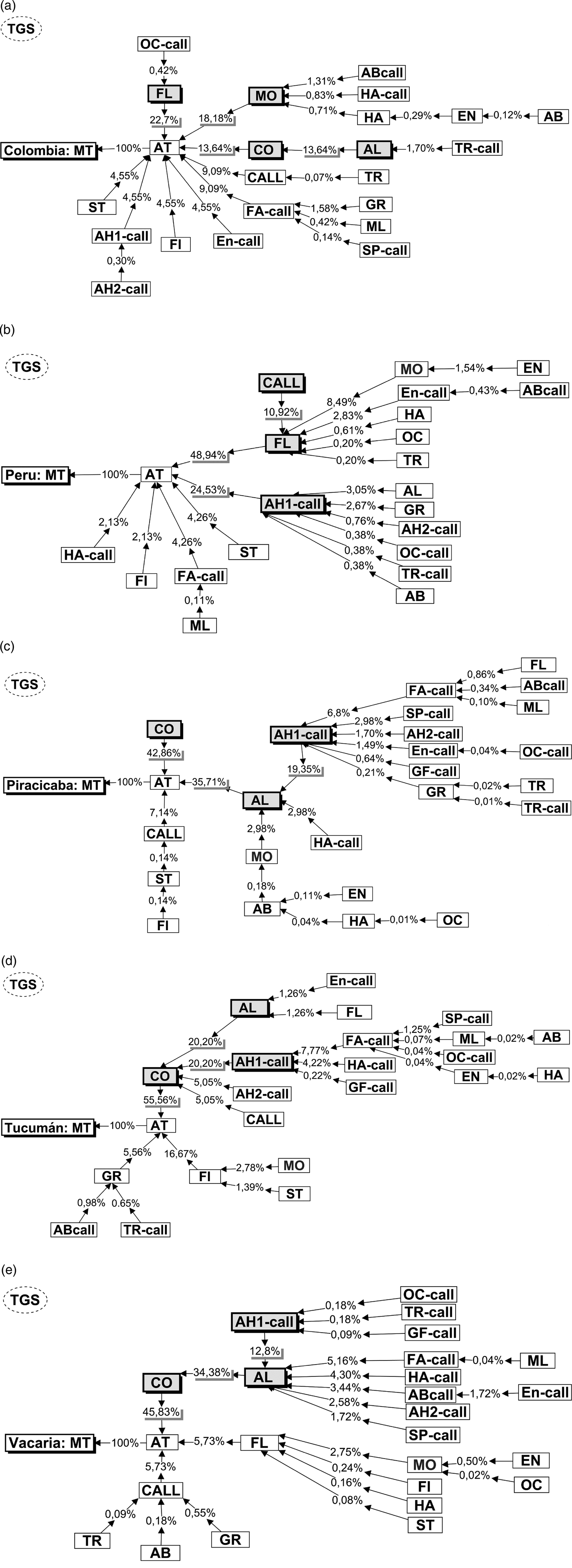
Fig. 4. Behavioural probabilistic tree of male courtship that resulted in mating (MT) in the populations (a) Colombia, (b) Peru, (c) Piracicaba, (d) Tucumán and (e) Vacaria. The arrows point to the next behavioural unit in sequence with the respective percentages of occurrence of each specific routine. The percentages are the probabilities of each path. The farther from the copulation (MT), the lower the percentages of occurrence of behavioural routines: the probability of FL/AT/MT is 22.7%, while that of OC-call/FL/AT/MT is 0.42% (a). The units highlighted were the most frequent ones in the tree generated sequences (TGS) of the courtship. The abbreviations of the behavioural units are described in the Appendix B.
Discussion
In the present study, we found differences on the time of pheromone emission (fig. 1) and the sequence of courtship behaviours (table 3, fig. 4) between Brazil-1, Peruvian and Andean morphotypes but the trend of both parameters assessed have shown similarity within populations of the same morphotype (Brazil-1). This implies that differences in mating behaviours are relevant parameters for distinguishing lineages within the A. fraterculus cryptic species complex.
Thus, courtship difference could explain previously reported mating incompatibility within this cryptic species complex (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014). Our results make evident that male courtship behavioural units assessed serve to attract the female of the same population or morphotype, allowing the female to choose a potential partner (Aluja et al., Reference Aluja, Piñero, Jácome, Díaz-Fleischer, Sivinski, Aluja and Norrbom1999; Gomez Cendra et al., Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011). The reproductive incompatibilities found between the Brazil-1 morphotype and the populations from Colombia and Peru (Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014) and between the Brazil-1 and Peruvian morphotypes (Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Rull et al., Reference Rull, Abraham, Kovaleski, Segura, Mendoza, Liendo and Vera2013; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014) may be consequence of this distinct courtship behaviour. Other pre-zygotic factors, such as variations in pheromone composition (Lima et al., Reference Lima, House and do Nascimento2001; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009) and cuticle hydrocarbons (Vaníčková et al., Reference Vaníčková, Virgilio, Tomčala, Břízová, Ekesi, Hoskovec, Kalinová, Do Nascimento and De Meyer2014, Reference Vaníčková, Břízová, Pompeiano, Ferreira, de Aquino, Tavares, Rodriguez, Mendonça, Canal and do Nascimento2015b, Reference Vaníčková, Břízová, Mendonça, Pompeiano and do Nascimentoc), are probably also involved in conspecific recognition within this at cryptic species complex.
The differences in the time of pheromone emission between morphotypes is directly associated with differences in their mating times, as pheromone emission necessarily precedes mating (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Segura et al., Reference Segura, Vera, Rull, Wornoayporn, Islam and Robinson2011; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014; Dias et al., Reference Dias, Silva, Lima, Petitinga, Hernandez-Ortiz, Laumann, Paranhos, Uramoto, Zucchi and Joachim-Bravo2015). Based on the time of pheromone emission, we confirmed the occurrence of time-based isolation in terms of mating activity between the Brazil-1 morphotype and the Andean morphotype (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014). Also, we proved that there are overlapping periods of pheromone emission between Peru and the remaining populations. The lack of complete time-based isolation and the mating incompatibilities detected previously (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006; Cáceres et al., Reference Cáceres, Segura, Vera, Wornoayporn, Cladera, Teal, Sapountzis, Bourtzis, Zacharopoulou and Robinson2009; Devescovi et al., Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014), particularly between the Brazil-1 and Peruvian morphotypes, indicate that pheromone emission is not the only pre-zygotic factor to influence reproductive isolation. In all, the pattern of pheromone emission was observed to be an important factor for classifying the biological lineages of A. fraterculus, but should be not considered as the unique factor involved in the reproductive isolation found between morphotypes.
The frequency of some behavioural units showed some peculiarities. The unit Spin (SP-call) was infrequent in the Peruvian and Colombian populations, and more frequent in the Vacaria, Tucumán and Piracicaba (Brazil-1) populations (fig. 2), thus seemingly representing a Brazil-1 courtship characteristic/peculiarity as reported previously (Gomez Cendra et al., Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011; Passos Roriz et al., Reference Passos Roriz, Japyassú and Sordi Joachim-Bravo2018). Other behavioural units that allow morphotype distinction were the behavioural unit Graceful (GF-call), which was not observed in the Colombian and Peruvian populations and the behavioural unit Flying (FL), which presented high frequency in these two populations and low frequency in the remaining three populations. Behavioural units that were not observed in certain populations (such as Oscillation in Colombia and Tucumán and Spin in Peru) may reflect intrinsic characteristics of courting in those populations rather than linage characteristics. Yet, this hypothesis needs confirmation.
Courtship routines allowed segregating the Peruvian and Colombian populations into two distinct groups, corroborating the observations of Devescovi et al. (Reference Devescovi, Abraham, Roriz, Nolazco, Castaneda, Tadeo, Cáceres, Segura, Vera, Joachim-Bravo, Canal and Rull2014) and Hernández-Ortiz et al. (Reference Hernández-Ortiz, Bartolucci, Morales-valles, Frías and Selivon2012). Furthermore, the courtship routines allowed/enable grouping the populations representing the Brazil-1 morphotype (Vacaria, Piracicaba and Tucumán) (fig. 3). In contrast, other workers have reported reduced pre-zygotic compatibility between Piracicaba and Vacaria (Dias et al., Reference Dias, Silva, Lima, Petitinga, Hernandez-Ortiz, Laumann, Paranhos, Uramoto, Zucchi and Joachim-Bravo2015) and between Piracicaba and Tucumán (Vera et al., Reference Vera, Cáceres, Wornoayporn, Islam, Robinson, De La Veja, Hendrichs and Cayol2006) as well as morphometric differences between Piracicaba and Vacaria populations (Dias et al., Reference Dias, Silva, Lima, Petitinga, Hernandez-Ortiz, Laumann, Paranhos, Uramoto, Zucchi and Joachim-Bravo2015). Although the present work did not identify strong differences among the three populations of the Brazil-1 morphotype, the behavioural routine MO>EN appears to differentiate Vacaria from the other two populations in the same way that the AH1-call>AL routine appears to separate Tucumán from the other two populations (table 2). Therefore, when one examines these populations in more detail, small differences can be seen between them. The probabilistic trees generated detected heterogeneous behavioural sequences among the populations. Nonetheless, the behavioural units that occurred most often in the behavioural probabilistic tree (TGS) of courtship that resulted in mating were the same in the biological lineages of this cryptic species complex. The behavioural units that occurred most in TGS of the Piracicaba, Vacaria and Tucumán (Brazil-1 morphotype) populations were the same (CO; AL; AH1-call), with some of the units being similar to those of the Colombia population (FL; MO; CO; AL) and with the Peru population (FL; AH1-call; CALL). In addition, the behavioural units Contact (CO) and Alignment (AL) were very important in both the Andean and the Brazil-1 morphotypes, where they were more frequent (fig. 2).
Contact (CO) and Alignment (AL) may present important functions in the courtship behaviour of A. fraterculus. In Ceratitis capitata, they were found to favour male mating success (Briceño & Eberhard, Reference Briceño and Eberhad2002; Briceño et al., Reference Briceño, Eberhad, Vilardi, Cayol and Shelly2007) and are probably linked to female choice/evaluation during courtship. The behavioural unit Arrowead-1 (AH1-call), most often in populations belonging to the Brazil-1 and the Peruvian morphotype, may reflect the power of the male in a certain territory (Dodson, Reference Dodson1982). This behaviour unit has already been reported as important for A. fraterculus male courtship in a population from Argentina (Gomez Cendra et al., Reference Gomez Cendra, Calcagno, Belluscio and Vilardi2011) and a population from Bento Gonçalvez from Brazil (Passos-Roriz et al., Reference Passos Roriz, Japyassú and Sordi Joachim-Bravo2018), both belonging to the Brazil-1 morphotype. It is possible that the behavioural unit Flying (FL) most often in the Andean and Peruvian morphotypes has important function in fleeing from predators (Boller et al., Reference Boller, Katsoyannos, Remund and Chamber1981; Cayol, Reference Cayol, Aluja and Norrbom1999). During courtship, flying could be a sign of male aptitude, although it could also be an exploratory behaviour of the male to gain the attention of the female.
Despite the recent advances in describing the sexual behaviour and related traits of the different morphotypes, more studies are needed that link the analysed attributes of the morphotypes to different ecological factors. The eight morphotypes described were classified into three non-connected phenotypic lineages that evolved separately in three biogeographic regions: Meso-Caribbean, Andean and Brazilian (Hernández-Ortiz et al., Reference Hernández-Ortiz, Canal, Tigrero Salas, Ruíz-Hurtado and Dzul-Cauich2015). An important assumption is that the ecological niche of each of three lineages differed in terms of environment which could explain the different pheromone emission times.
In conclusion, in this work, it was possible to perceive that behavioural factors are distinct among the morphotypes, which likely reflects different selective pressures in the A. fraterculus cryptic species complex. Attributes of the males can be selected and remain in the population by female's preference (Andersson, Reference Andersson1994; Ritchie, Reference Ritchie1996; Yamada et al., Reference Yamada, Tomaru, Matsuda and Oguma2008). Perhaps, female's preferences in this cryptic species complex are directing the evolution of the courting behaviour specifically in each lineage. The results of this work indicate a tendency to behavioural isolation of courting among the analysed distinct lineages, and therefore abiotic factors that force to each linage to have different courtship behaviour may be contributing to the evolution of the divergences between them. Studies that compare courtship behaviour in heterotypic mating in this cryptic species complex and analysis of ecological factors that drive behavioural trends will contribute to deepen our understanding of the ongoing speciation process.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318000846.
Acknowledgements
We are grateful to CAPES (Coordenação of Aperfeiçoamento of Pessoal of Nível Superior) for the financial support granted to the first author. Thanks to the International Agency of Atomic Energy (IAEA/FAO, Austria, Vienna) for financial support and all support for the realization of the experiments in Vienna given through the ‘Coordinated Research Project on Resolution of Cryptic Species Complexes of Tephritid Pests to Overcome Constraints to SIT and International Trade (Research Contract No. 16060)’: for Jorge Hendrichs, Marc Vreysen, Ulysses Santo Tomas, Thilakasiri Dammalag, Sohel Ahmad and Elena Cancio. We thank Dr Rui Pereira Cardoso and Amirul Islam for the support offered to the first author. We are grateful to all technicians who supported the maintenance of colonies of flies and experiments in the Unit of Insect Pest Control in the laboratory of Entomology, FAO/IAEA (Seibersdorf, Austria).











