Introduction
The Hatrurim Formation, often referred to as the Mottled Zone, is the worlds largest pyrometamorphic complex (Gross, Reference Gross1977). The term ‘pyrometamorphism’ incorporates a suite of natural processes and environments which can provide high-temperature and low-pressure calcination and fusion of sedimentary lithologies (Grapes, Reference Grapes2011). The typical case is calcination of sediments entrapped by (or in contact with) igneous rocks (Reverdatto, Reference Reverdatto1970; Clark and Peacor, Reference Clark and Peacor1992; Souza et al., Reference Souza, Wang, Jin, Li, Yang, Botelho, Viana, Santos, Liu and Li2019). When the high-temperature processes are maintained at the expense of burning organic matter (bitumen, coal), the term ‘combustion metamorphism’ is often used (Grapes, Reference Grapes2011). Due to the inherently spatial nature, pyrometamorphism is preferentially a local phenomenon confined, e.g. to volcanic eruptions, subsurface magmatic chambers or natural coal fires (Burnham, Reference Burnham1959; Grapes, Reference Grapes2011). The Hatrurim Formation is a rare exception, as the eroded remnants of the Mottled Zone can be traced across an area of 150 × 200 km around the Dead Sea, both in Israel and Jordan (Gross, Reference Gross1977; Burg et al., Reference Burg, Starinsky, Bartov and Kolodny1992). The unprecedented scale of the Hatrurim Formation leads to the appearance of various pyrometamorphic lithologies with diverse mineral assemblages (Sharygin et al., Reference Sharygin, Sokol and Vapnik2008; Reference Sharygin, Britvin, Kaminsky, Wirth, Nigmatulina, Yakovlev, Novoselov and Murashko2021; Murashko et al., Reference Murashko, Chukanov, Mukhanova, Vapnik, Britvin, Polekhovsky and Ivakin2011; Galuskin et al., Reference Galuskin, Gfeller, Galuskina, Pakhomova, Armbruster, Vapnik, Wlodyka, Dzierżanowski and Murashko2015; Sokol et al., Reference Sokol, Kokh, Sharygin, Danilovsky, Seryotkin, Liferovich, Deviatiiarova, Nigmatulina and Karmanov2019; Khoury, Reference Khoury2020; Juroszek et al. Reference Juroszek, Krüger, Galuskina, Krüger, Vapnik and Galuskin2020; Britvin et al. Reference Britvin, Murashko, Vapnik, Vlasenko, Krzhizhanovskaya, Vereshchagin, Bocharov and Lozhkin2021a). Natural terrestrial phosphides are one such unique feature of these rare mineral assemblages (Britvin et al., Reference Britvin, Murashko, Vapnik, Polekhovsky and Krivovichev2015). The majority of phosphides discovered in the Hatrurim Formation belong to the system Fe–Ni–P (Britvin et al., Reference Britvin, Murashko, Vapnik, Polekhovsky and Krivovichev2017a; Reference Britvin, Murashko, Vapnik, Polekhovsky, Krivovichev, Vereshchagin, Vlasenko, Shilovskikh and Zaitsev2019a,Reference Britvin, Vapnik, Polekhovsky, Krivovichev, Krzhizhanovskaya, Gorelova, Vereshchagin, Shilovskikh and Zaitsevb; Reference Britvin, Murashko, Vapnik, Polekhovsky, Krivovichev, Krzhizhanovskaya, Vereshchagin, Shilovskikh and Vlasenko2020a,Reference Britvin, Murashko, Vapnik, Polekhovsky, Krivovichev, Vereshchagin, Shilovskikh, Vlasenko and Krzhizhanovskayab,Reference Britvin, Murashko, Vapnik, Polekhovsky, Krivovichev, Vereshchagin, Shilovskikh and Krzhizhanovskayac; Reference Britvin, Murashko, Krzhizhanovskaya, Vereshchagin, Vapnik, Shilovskikh, Lozhkin and Obolonskaya2022a). However, recent exploration has discovered a series of molybdenum-bearing phosphides (Britvin et al., Reference Britvin, Murashko, Vereshchagin, Vapnik, Shilovskikh, Vlasenko and Permyakov2022b). In this paper, we describe a new mineral, FeMoP, discovered on the Jordanian side of the Hatrurim Formation. The mineral has been named nickolayite, in honour of Dieter Nickolay (born 1941), the German mineral collector and mineralogist, for his contributions to the studies in systematic mineralogy. Dieter Nickolay studied mineralogy at the University of Freiburg in 1983; he is a member of the German mineral collectors’ community (VFMG) and one of the volunteers involved in preparation and verification of The New IMA List of Minerals (Pasero, Reference Pasero2022). Both the mineral and its name (symbol Nkl) have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2018-126, Murashko et al., Reference Murashko, Vapnik, Polekhovsky, Shilovskikh, Zaitsev, Vereshchagin and Britvin2019). The holotype specimen of nickolayite is deposited in the collections of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia, with the registration number 5290/1.
Materials and methods
Thick polished sections containing nickolayite were prepared from the cut rock slices using conventional preparation techniques. The reflected-light study included visual description and reflectivity measurements performed in air by means of a MSF-2 spectrophotometer (LOMO, St. Petersburg), using Si as a reference material. After an optical investigation, the sections were coated with carbon film and underwent electron microprobe analysis, using an INCA WAVE 500 WDX spectrometer (20 kV, 15 nA) attached to a Hitachi S-3400N scanning electron microscope (SEM). Pure Ni, Fe and Co metals (Kα lines) and InP (PKα) were used as analytical standards. The analyses of rock-forming minerals were completed using the same instrumental setup, with oxides and silicates as reference standards. The microhardness measurements were performed after completion of microprobe studies, following the extraction of selected nickolayite grains for the X-ray examination.
The single-crystal X-ray studies of the holotype crystal were carried out using a four-circle Bruker APEX Kappa DUO CCD diffractometer equipped with a MoKα microfocus tube, operated at 50 kV and 0.6 mA. A single-crystal X-ray dataset for the low-Mo nickolayite was obtained by means of Bruker Smart APEX CCD diffractometer equipped with a MoKα tube, operated at 50 kV and 40 mA. Data collection and unit-cell refinements were performed with the Bruker APEX2 software package (Bruker Inc.), whereas subsequent data reduction and integration procedures were carried out with CrysAlisPro v. 171.41 software (Rigaku Oxford Diffraction, 2021). The crystal structure was solved and refined by the SHELX-2018 set of programs (Sheldrick, Reference Sheldrick2015) incorporated into the Olex2 v.1-5 graphical user interface (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). The details of data collection, reduction, structure solution and refinement can be retrieved from the crystallographic information files (CIF) which are included in the supplementary information. Powder X-ray diffraction data for the holotype material were obtained from the same grain that was used for the structure refinement, using a Gandolfi method implemented at the Rigaku RAXIS Rapid II imaging plate diffractometer. The instrument setup was as follows: CoKα radiation (rotating anode) with microfocus mirror optics, 40 kV, 15 mA, curved (semi-cylindrical) imaging plate (r = 128.4 mm), Debye-Scherrer geometry and exposure 60 mn. The data-to-profile conversion was performed using osc2xrd software (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017b). Unit-cell parameters refinement and calculation of theoretical pattern was carried out with STOE WinXPOW v.2.08 software (Stoe & Cie GmbH).
Results
Occurrence and mineral assemblages
Nickolayite was found in a small quarry operated for phosphorites, located in the Jizah District, Amman Governorate, Jordan (31°21′52″N, 36°10′55″E) (Britvin et al., Reference Britvin, Murashko, Vapnik, Polekhovsky and Krivovichev2015). The quarry exposes a suite of pyrometamorphic rocks belonging to the Hatrurim Formation, in general comprising severely metamorphosed chalks and marls. Phosphide assemblages, described previously, are confined to the fused, originally sedimentary lithologies, known in the literature under the term ‘paralava’ (Vapnik et al., Reference Vapnik, Sharygin, Sokol and Shagam2007) (Fig. 1). The paralavas containing nickolayite are dense rocks of dark-brown colour, consisting of submillimetre-sized elongated crystals of clinopyroxene and anorthite (Fig. 2). The mineral composition and texture of these paralavas resembles that of gabbro–dolerites, but they exhibit some specific features, such as a spatially high content of tridymite (confirmed by electron back-scatter diffraction - EBSD). Plagioclase crystals are chemically homogeneous and show compositions within a narrow range of Na/Ca ratios, approaching an anorthite end-member (Table 1). In contrast, the clinopyroxenes exhibit an extraordinary compositional zonation, varying from diopside (core) to hedenbergite (rim) within the same crystal (Table 2, Fig. 1) and can contain considerable amounts of Cr, V and Ti (Table 2). In addition, clinopyroxene can contain considerable amounts of Cr, V and Ti (Table 2). The accessory minerals are represented by baryte, chromite, hematite, trevorite, pyrrhotite, fluorapatite, titanite and powellite. The paralavas bear numerous submillimetre- to millimetre-sized cavities typically filled with secondary calcite (Fig. 1).

Fig. 1. The dark green–grey paralava schlieren, along with white calcite veinlets dissecting metamorphosed chalk. Phosphorite quarry, Daba Siwaqa complex, West Jordan.

Fig. 2. The microstructure of nickolayite-bearing paralava. The cross-sections of euhedral clinopyroxene crystals (Cpx) exhibit sharp compositional zoning in back-scattered electrons (BSE), due to differences in Fe/Mg contents (cf. Table 2). Plagioclase (Pl) (Table 1) and tridymite (Trd), along with pyrrhotite (white inclusions) fill up the interstices between clinopyroxene crystals. Polished section, SEM BSE image.
Table 1. Representative chemical compositions of anorthite associated with nickolayite (wt.%).*
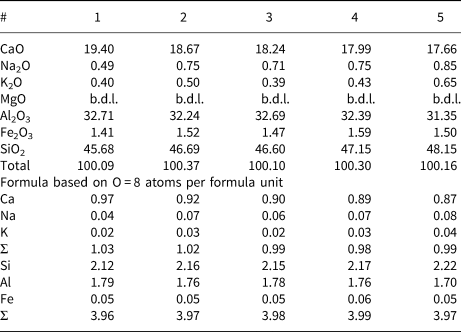
* b.d.l. – below detection limit
Table 2. Representative chemical compositions of clinopyroxene associated with nickolayite (wt.%).*

* b.d.l. – below detection limit
Appearance and physical properties of nickolayite
Nickolayite forms irregularly shaped rounded single-crystal grains up to 80 μm, disseminated in the host silicate matrix (Fig. 3). The grains show no signs of alteration (reaction) at the contact with the rock-forming minerals. In reflected light, the mineral has a purely white colour with no observable birefringence or pleochroism. It exhibits very weak anisotropy (ΔR 589 = 1.95%). Reflectance values are presented in Table 3. Loose nickolayite grains extracted from the rock matrix have metallic light-grey to greyish-white colour. The mineral is ductile and has no cleavage. The mean micro-indentation hardness (VHN load of 50 g) is 538 mm–2, ranging from 490 to 588 kg mm–2, which corresponds to a Mohs hardness of 5 to 6. The density of the holotype crystal calculated from the empirical formula and the single-crystal unit-cell parameters is 7.840 g cm–3.
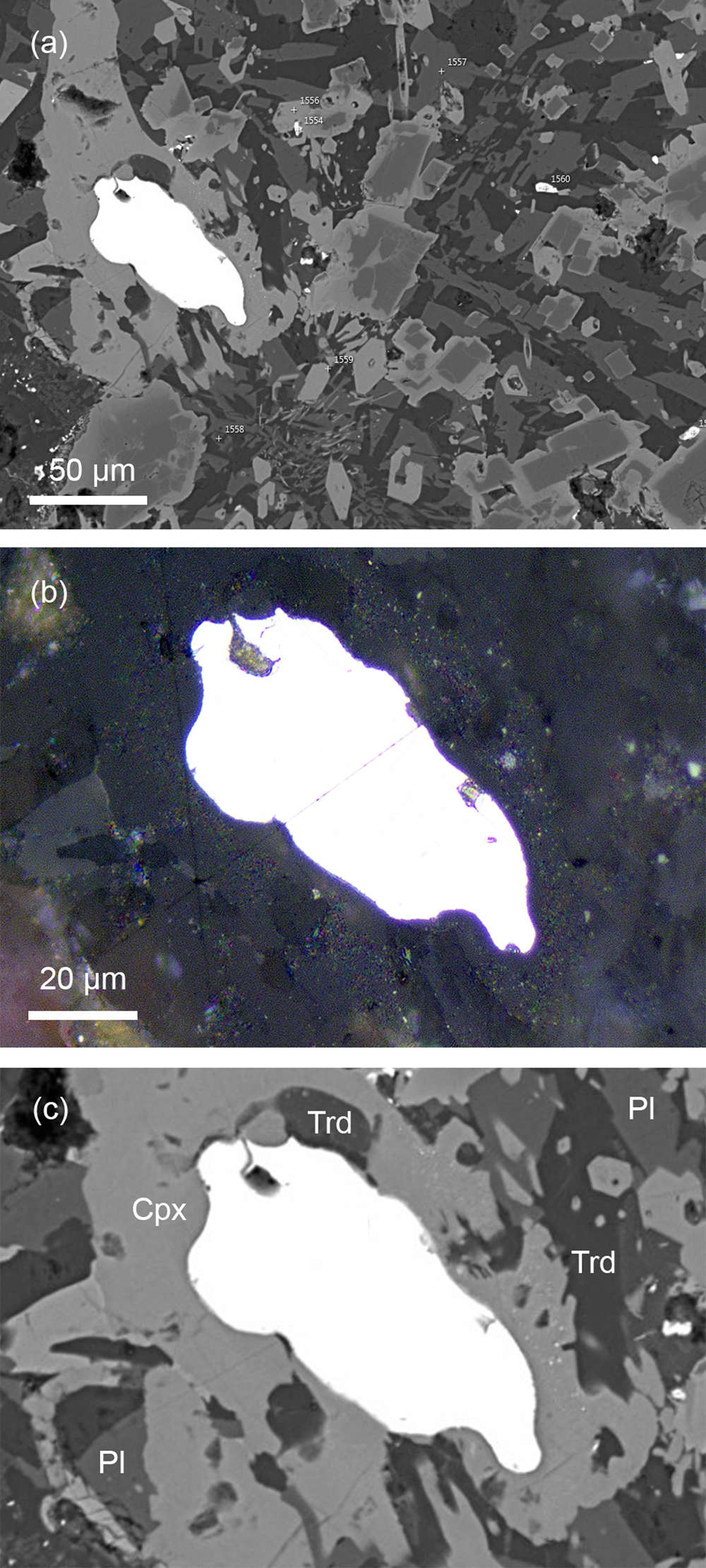
Fig. 3. The holotype nickolayite grain (specimen number 5290/1) in paralava. (a) General view. (b, c) The enlarged area, photomicrograph in reflected light (b) and BSE image (c). Abbreviations: Cpx – clinopyroxene; Trd – tridymite; and Pl – plagioclase.
Table 3. Reflectance values for nickolayite (%).*

* Data for wavelengths recommended by the IMA Commission on ore microscopy (COM) are marked in boldtype.
Chemical composition
The composition of three nickolayite grains was investigated (Table 4). The individual grains are homogeneous, with no chemical zoning. However, each one differs significantly in composition from the other two. The holotype crystal (#1 in Table 4) has the highest Mo content (0.87 atoms per formula unit), whereas grain #3 is depleted in Mo (0.44 Mo apfu) and, from the formal point of view, represents a composition intermediate between nickolayite, FeMoP, and Mo-rich allabogdanite, (Fe,Mo)2P (Fe>Mo). The crystal structure determinations performed for grains #1 and #3 (see below) confirm the results of electron microprobe analyses. The plot of the chemical compositions presented in Fig. 4 reveals that the change in the Mo contents linearly and inversely correlates with the change in the Fe contents, implying the substitution of 1 Fe atom for 1 Mo atom. At the same time, the Ni concentration remains almost constant: a feature which cannot be explained on the basis of the available data.
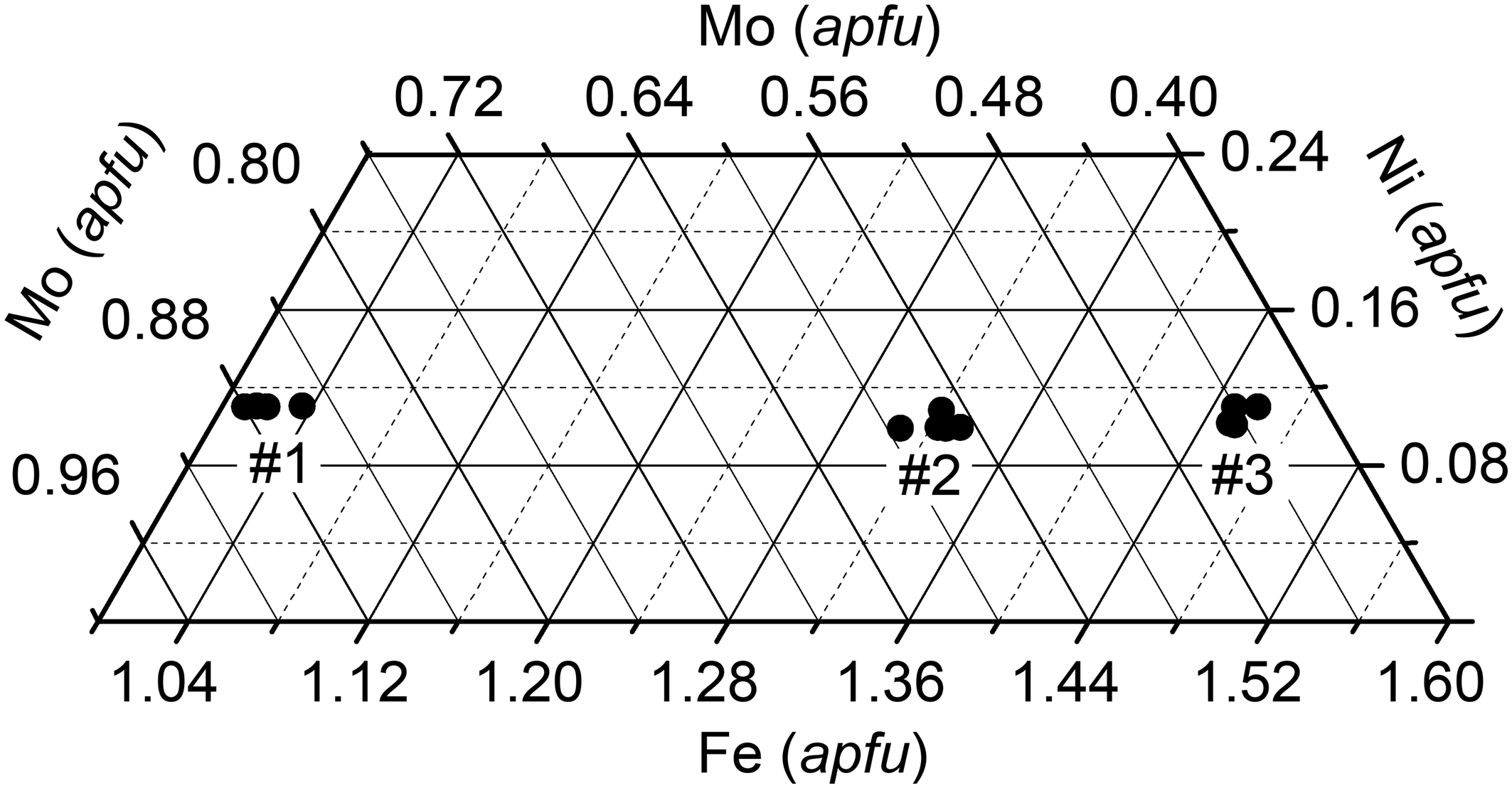
Fig. 4. Part of the Fe–Mo–Ni ternary diagram illustrating the chemical composition of the nickolayite crystals studied.
Table 4. Chemical composition of nickolayite (wt.%).

* The holotype material; S.D. – standard deviation.
Crystal structure and powder X-ray diffraction
X-ray structural studies were carried out on crystal #1 (the holotype one) and #3 (for the compositions, see Table 4). Their crystal parameters, data collection and structure refinement details are given in Table 5; atomic coordinates and thermal displacement parameters are listed in Table 6. Nickolayite, being an analogue of synthetic FeMoP, belongs to the structural type TiNiSi – a metal-ordered variant of the Co2Si framework (Rundqvist and Nawapong, Reference Rundqvist and Nawapong1966; Guérin and Sergent, Reference Guérin and Sergent1977). As a consequence, the nickolayite structure (Fig. 5) has the same site geometry and lattice metrics as the structure of the only known natural Co2Si-type phosphide, allabogdanite (Britvin et al., Reference Britvin, Rudashevsky, Krivovichev, Burns and Polekhovsky2002). There are two symmetrically independent metal sites: M(1), tetrahedrally coordinated by 4 phosphorus atoms, and M(2), which is five-fold coordinated by P, resulting in square–pyramidal coordination (Fig. 5). Phosphorus, is nine-fold coordinated by both metal and P atoms. The corner- and edge-sharing [M(1)P4] and [M(2)P5] polyhedra are arranged into infinite chains propagated along the b axis (Fig. 5). Both metal sites allow wide homogeneity ranges, where, in particular, Mo can be substituted by either Fe or Ni (Rundqvist and Nawapong, Reference Rundqvist and Nawapong1966; Guérin and Sergent, Reference Guérin and Sergent1977; Oliynyk et al., Reference Oliynyk, Lomnytska, Dzevenko, Stoyko and Mar2013).

Fig. 5. Crystal structure of nickolayite. (a) General view along the b axis. Chains of edge- and corner-sharing tetrahedra [FeP4] (yellow) and square pyramids [MoP5] (red). (b) Projection onto (010) plane. (c) A fragment showing interconnections between tetrahedra [FeP4] (yellow) and square pyramids [MoP5] (red).
Table 5. Crystal parameters, data collection and structure refinement details for nickolayite.
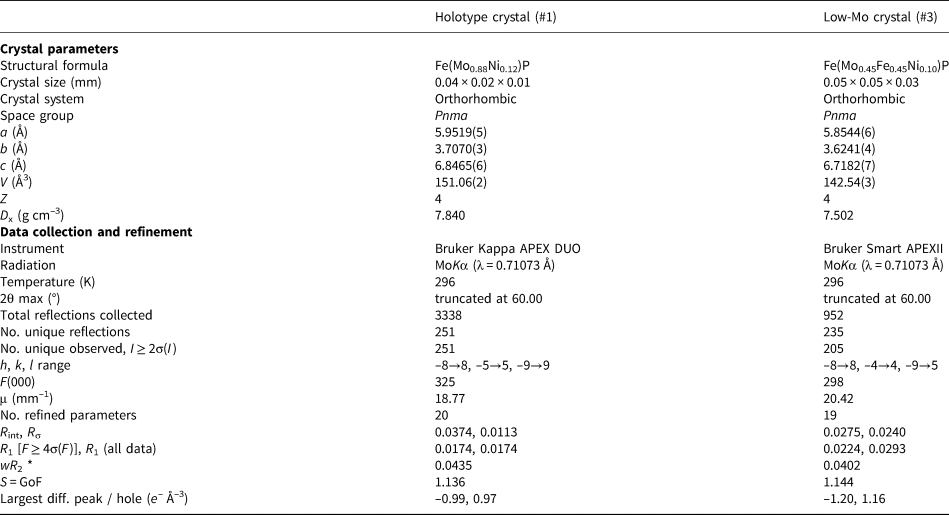
* SHELX weighting scheme applied: w –1 = [σ2(F o2) + (0.0255P)2 + 0.5924P] (crystal #1); w –1 = [σ2(F o2) + (0.0203P)2] (crystal #3), where P = [(F o2 + 2F c2)/3].
Table 6. Fractional atomic coordinates and displacement parameters (Å2) for nickolayite.*

* U 12 and U 23 displacement parameters are equal to zero owing to site symmetry.
** Occupancy of the M(1) site is Fe1.00 for both crystals. Occupancy of the M(2) site is Mo0.88Ni0.12 (crystal #1) and Mo0.45Fe0.45Ni0.10 (crystal #3).
The nickolayite crystals studied are distinguished by significantly different Mo contents; therefore they are of interest in view of Mo distribution across the structure. The free refinements of site populations reveal that Mo only incorporates into the square–pyramidal M(2) site. Taking into account that the atomic metal radius of Mo (1.39 Å) is greater than the corresponding radii of Fe and Ni (1.26 and 1.24 Å, respectively) (Pauling, Reference Pauling1960), the observed behaviour agrees well with the longer M–P distances at the M(2) site, in contrast to the considerably shorter M(1)–P bonds (Table 7). In addition, the M(2)–P bonds in the Mo-rich crystal #1 are longer than the corresponding bonds in the Mo-poor crystal #3 (Table 7). However, our data disagrees with the random distribution of Mo and Fe over the M(1) and M(2) sites as was determined in the synthetic (Mo1–xFex)2P solid solutions (Oliynyk et al., Reference Oliynyk, Lomnytska, Dzevenko, Stoyko and Mar2013). This discrepancy between natural nickolayite and synthetic (Mo1–xFex)2P may indicate that in the latter case, the synthesis time was insufficient for completion of the diffusion processes, so the metal site ordering seen in nickolayite was not achieved (cf. Britvin et al., Reference Britvin, Krzhizhanovskaya, Zolotarev, Gorelova, Obolonskaya, Vlasenko, Shilovskikh and Murashko2021c). The free occupancy refinements of the M(1) site show that the latter is populated solely by Fe. Nickel is thus incorporated into the M(2) position, leading to the structural formulae given in Table 5, which are consistent with the microprobe data (Table 4).
Table 7. Selected bond lengths (Å) in nickolayite.

Powder X-ray diffraction data for crystal #1 are given in Table 8. The unit-cell parameters refined from the XRD pattern are: a = 5.942(1), b = 3.703(1), c = 6.841(2) Å and V = 150.52(4) Å3.
Table 8. Powder X-ray diffraction data for holotype nickolayite (d in Å).*
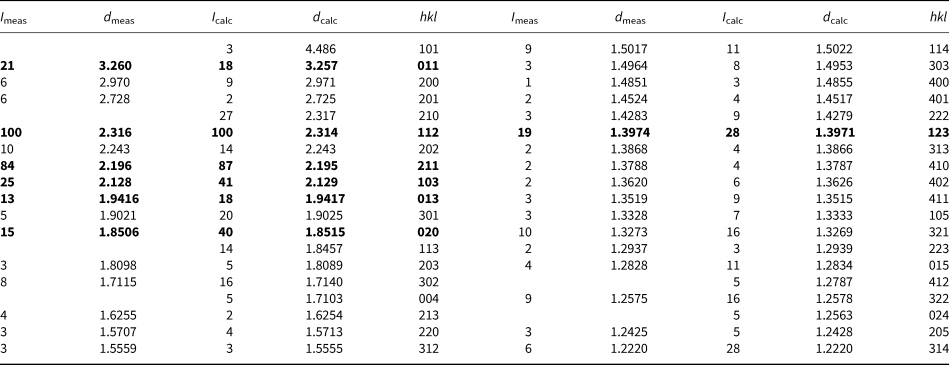
* The theoretical pattern was calculated on the basis of atomic coordinates obtained from the structure refinement and unit-cell parameters refined from powder diffraction data. The intensities of seven strongest lines are highlighted in bold. Calculated lines with intensity <2 have been omitted.
Discussion
Relationship to other phosphides and arsenides
Nickolayite is the second terrestrial Mo-bearing phosphide, after polekhovskyite, MoNiP2 (Britvin et al., Reference Britvin, Murashko, Krzhizhanovskaya, Vereshchagin, Vapnik, Shilovskikh, Lozhkin and Obolonskaya2022a). The mineral expands the series of natural phosphides belonging to the NiTiSi or Co2Si structure type (Table 9). From the chemical point of view, nickolayite is an Fe analogue of monipite, MoNiP (Ma et al., Reference Ma, Beckett and Rossman2014) (Table 9). However, the latter crystallises in the barringerite structure type (the low-pressure hexagonal Fe2P) (Buseck, Reference Buseck1969; Carlsson et al., Reference Carlsson, Goelin and Rundqvist1973), and its synthetic analogue is not known to adopt the Co2Si structure type (Guérin and Sergent, Reference Guérin and Sergent1977). With the exception of nickolayite, all minerals listed in Table 9 were originally discovered in meteorites. Allabogdanite, the high-pressure modification of (Fe,Ni)2P (Britvin et al., Reference Britvin, Shilovskikh, Pagano, Vlasenko, Zaitsev, Krzhizhanovskaya, Lozhkin, Zolotarev and Gurzhiy2019c), has been described recently in terrestrial pyrometamorphic rocks of the Hatrurim Formation in Israel (Britvin et al., Reference Britvin, Vereshchagin, Shilovskikh, Krzhizhanovskaya, Gorelova, Vlasenko, Pakhomova, Zaitsev, Zolotarev, Bykov, Lozhkin and Nestola2021b), however the others remain solely of meteoritic origin. Besides phosphides, two arsenide minerals belong to the Co2Si structure type: these are rhodarsenide, (Rh,Pd)2As (Tarkian et al., Reference Tarkian, Krstic, Klaska and Ließmann1997), and palladodymite, (Pd,Rh)2As (Britvin et al., Reference Britvin, Rudashevsky, Bogdanova and Shcherbachov1999).
Table 9. Comparative crystallographic data for nickolayite and some related minerals.

* References: [1] this work; [2] Ivanov et al. (Reference Ivanov, Zolensky, Saito, Ohsumi, MacPherson, Yang, Kononkova and Mikouchi2000), Rundqvist and Nawapong (Reference Rundqvist and Nawapong1966); [3] Zolensky et al. (Reference Zolensky, Gounelle, Mikouchi, Ohsumi, Le, Hagiya and Tachikawa2008), Kumar et al. (Reference Kumar, Krishnamurthy, Bipin, Srivastava, Daa and Paranjpe2004); [4] Britvin et al. (Reference Britvin, Rudashevsky, Krivovichev, Burns and Polekhovsky2002); [5] Ma et al. (Reference Ma, Beckett and Rossman2014), Guérin and Sergent (Reference Guérin and Sergent1977).
** Structural data for natural florenskyite, andreyivanovite and monipite are not available; the unit cell parameters have been assigned on the basis of an EBSD pattern match with the corresponding synthetic analogues.
Some remarks on the origin of nickolayite
The enrichment in Mo minerals is a known feature of pyrometamorphic lithologies of the Hatrurim Formation. Since the 1960s it has been documented that the sedimentary strata – the protoliths of pyrometamorphic rocks – are enriched in Ni and Mo (Issar et al., Reference Issar, Eckstein and Bogoch1969; Ilani et al., Reference Ilani, Kronfeld and Flexer1985; Gilat, Reference Gilat1994; Bogoch et al., Reference Bogoch, Gilat, Yoffe and Ehrlich1999; Ryb et al., Reference Ryb, Erel, Matthews, Avni, Gordon and Anbar2009; Fleurance et al., Reference Fleurance, Cuney, Malartre and Reyx2013), and these elevated concentrations are retained in the marbles and paralavas of the Hatrurim Formation. Powellite, CaMoO4, an accessory mineral in the rocks of the Mottled Zone, was found both in Israel and Jordan (Khoury, Reference Khoury2020; Sokol et al., Reference Sokol, Kokh, Seryotkin, Deviatiiarova, Goryainov, Sharygin, Khoury, Karmanov, Danilovsky and Artemyev2020). Phosphides of the barringerite–transjordanite series, Fe2P–Ni2P, occurring on both sides of the Dead Sea, may contain up to 3 wt.% Mo (Britvin et al., Reference Britvin, Murashko, Vapnik, Polekhovsky, Krivovichev, Krzhizhanovskaya, Vereshchagin, Shilovskikh and Vlasenko2020a). Allabogdanite, the high-pressure modification of (Fe,Ni)2P, also bears up to several wt.% Mo (Britvin et al., Reference Britvin, Vereshchagin, Shilovskikh, Krzhizhanovskaya, Gorelova, Vlasenko, Pakhomova, Zaitsev, Zolotarev, Bykov, Lozhkin and Nestola2021b). Prior to the discovery of nickolayite, the most Mo-rich phosphide in this area was polekhovskyite, MoNiP2 with 44 wt.% Mo (Britvin et al., Reference Britvin, Murashko, Vereshchagin, Vapnik, Shilovskikh, Vlasenko and Permyakov2022b). The likely pathway for the formation of Mo-bearing phosphides is co-reduction of oxygen-bearing Mo and Fe–Ni minerals and phosphates, which has been observed experimentally in corresponding synthetic systems (Burns et al., Reference Burns, Hargreaves and Hunter2007). Natural methane, hydrogen or bitumen are the likely candidates for the role of reductants (Novikov et al., Reference Novikov, Vapnik and Safonova2013; Galuskin et al., Reference Galuskin, Galuskina, Vapnik and Murashko2020). The temperatures reached in pyrometamorphic processes (up to 1400°C) are more than sufficient for the initiation and maintenance of reduction reactions. Powellite might be a possible source of Mo. The accessory mineral, trevorite, NiFe2O4, could provide sufficient Ni supply, whereas fluorapatite is a widespread phosphate in the pyrometamorphic rocks of the Hatrurim Formation. It is thus quite likely that nickolayite will not be the last Mo-phosphide discovered.
Acknowledgements
We are thankful to the Principal Editor Stuart Mills and Associate Editor Mike Rumsey for editorial handling and linguistic correction of the manuscript, and to Peter Leverett, the Structural Editor, for careful examination of crystallographic data. We are grateful to Igor Pekov and an anonymous referee for many valuable corrections and suggestions. This study was carried out with the financial support of the Russian Science Foundation, grant 18-17-00079. The authors thank the Centre for X-ray diffraction studies, Geomodel Resource Centre, and Centre of Microscopy and Microanalysis of St. Petersburg State University for the access to instrumental and computational resources.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.52
Competing interests
The authors declare that they have no conflict of interest.
















