Introduction
Gold is distributed unevenly in the Earth's crust and occurs mainly as concentrated anomalies in primary or secondary deposits (Falconer and Craw, Reference Falconer and Craw2009; Hough et al., Reference Hough, Noble, Hitchen, Hart, Reddy, Saunders, Clode, Vaughan, Lowe and Gray2008; Jaireth et al., Reference Jaireth, Hoatson and Miezitis2014). Within the latter deposits, gold occurs as millimetre-size particles to centimetre-size nuggets, both of which can be collected using simple tools, i.e. a pan and determination. This relatively easy acquisition instigated the North American and Australian gold rushes of the mid-19th Century. Although gold particles only have monetary value in large quantities, this form of gold has captivated the interest of historic prospectors and modern geomicrobiologists alike. From a biogeochemical perspective, gold particles are important for studying microbe–gold interactions using geological and microbiological techniques. Within the last few decades, many studies have involved the characterisation of gold particles from numerous locations around the world. In doing so, it has been determined that gold dissolution and reprecipitation processes constitute the biogeochemical cycle of gold which is catalysed, in part, by bacteria (Shuster and Reith, Reference Shuster and Reith2018 and reference therein). Using advanced electron microscopy techniques, the structure of gold particles down to the nanometre-scale have enabled geomicrobiologists to ‘see’ microenvironments from a bacterial perspective (Shuster et al., Reference Shuster, Reith, Cornelis, Parsons, Parsons and Southam2017; Shuster and Southam, Reference Shuster and Southam2015). In general, changes in gold particle morphology and surface textures reflect the physical and biogeochemical processes that acted upon gold particles through its ‘journey’ from a primary source (Rea et al., Reference Rea, Shuster, Hoffmann, Schade, Bissett and Reith2019a,Reference Rea, Wulser, Brugger, Etschmann, Bissett, Shuster and Reithb). More importantly, polymorphic layers (concavities on the surface of particles containing clays, organic material and pure secondary gold) are microenvironments where gold biogeochemical cycling occurs (Shuster et al., Reference Shuster, Johnston, Magarvey, Gordon, Barron, Banerjee and Southam2015; Shuster et al., Reference Shuster, Reith, Cornelis, Parsons, Parsons and Southam2017). Microbes can directly or indirectly contribute to gold cycling and their presence on gold particles has conventionally been inferred from amplified operational taxonomic units (OTUs) using high-throughput sequencing techniques. This type of characterisation provides insight on the types of microorganisms that could come in contact with a gold particle in the environment at some point (Rea et al., Reference Rea, Zammit and Reith2016; Reith et al., Reference Reith, Etschmann, Grosse, Moors, Benotmane, Monsieurs, Grass, Doonan, Vogt, Lai, Martinez-Criado, George, Nies, Mergeay, Pring, Southam and Brugger2009; Reith et al., Reference Reith, Falconer, Van Nostrand, Craw, Shuster and Wakelin2020; Reith et al., Reference Reith, Rogers, McPhail and Webb2006; Sanyal et al., Reference Sanyal, Reith and Shuster2020b; Shuster and Reith, Reference Shuster and Reith2018). More recent studies have confirmed the presence of Au-tolerant bacteria on particles and suggested that these bacteria could have a more important role in catalysing particle transformation and perpetuating gold biogeochemical cycling within natural environments including those impacted by anthropogenic activity (Sanyal et al., Reference Sanyal, Shuster and Reith2019b).
To date, Cupriavidus metalidurans CH34 and Delftia are considered to be ‘aurophilic’ microbes; however, other bacteria (e.g. Serratia sp. D1, Stenotrophomonas sp. D2, Pseudomonas sp. and Arthrobacter sp. BB-1) that are Au-tolerant and viable (metabolically active) have been isolated from various gold particles. Additionally, these microbes are known to have the physiological and genomic capacity to also be considered aurophilic (Sanyal et al., Reference Sanyal, Shuster and Reith2019a, Reference Sanyal, Brugger, Etschmann, Pederson, Delport, Dixon, Tearle, Ludington, Reith and Shuster2020a,Reference Sanyal, Reith and Shusterb). In light of this, there is validity in identifying and characterising other bacteria that could have an important role in gold particle transformation and gold biogeochemical cycling in general. Therefore, this study involves using classic microbiological techniques to isolate viable bacteria from gold particles and high-throughput molecular techniques to study their genomic and physiological capacity to survive on gold particles. In doing so, we aim to use viable bacteria from gold particles as proxies for understanding microbe–gold interactions.
Materials and methods
Gold particle acquisition and characterisation
Gold particles were obtained from the Prophet Mine (Stone Supplies) located in Queensland, Australia (26°06′51.0″S, 152°17′13.9″E). At the site, sediment was mechanically separated by size. The heavy, fine-grained sediment fraction was panned to separate and obtain ca. 250 gold particles in the field-sampling method outlined by Reith et al. (Reference Reith, Fairbrother, Nolze, Wilhelmi, Clode, Gregg, Parsons, Wakelin, Pring and Hough2010). Immediately after sampling, all particles were rinsed two times using 0.9% sterile NaCl solution to ensure that large pieces of soil debris were removed. Half of these particles were placed in 2.5%(aq) electron microscopy grade glutaraldehyde to preserve any bacterial cells and were incubated at 4°C for 24 hours. These fixed particles were prepared for scanning electron microscopy analysis in the method modified from Shuster et al. (Reference Shuster, Southam, Reith, Kenney, Veeramani and Alessi2019). Briefly, the fixed particles were transferred sequentially to ethanol solutions with increasing concentrations (25, 50, 75%(aq) and 3× 100%). The particles were incubated at 23°C for 15 minutes at each ethanol concentration. After the final incubation, the particles were transferred to a 100% ethanol:100% hexamethyldisilazane (HMDS) solution followed by 2× 100% HMDS solution; incubations were 30 minutes for each solution. The particles were removed from the HMDS solution, air-dried overnight and placed on aluminium stubs using carbon adhesive tabs. Particles were coated with a 10 nm thick deposition layer of either carbon or iridium. The surfaces of the particles were characterised in secondary electron (SE) and backscatter electron (BSE) modes using a JEOL JSM-7100 Field Emission Scanning Electron Microscope (SEM) or a Helios Nanolab 600 DualBeam SEM, both operating at 2 or 20 kV. The occurrence of cells on gold surfaces or on clay-mineral surfaces (polymorphic layers) were noted whilst imaging.
Culturing bacteria from gold particles and isolating Au-tolerant bacteria
Immediately after rinsing, the remaining samples (125 particles) were placed in a sterile saline solution and stored at 4°C until analysis. An aliquot of 10 rinsed gold particles were selected randomly and were placed into a test tube containing 10 mL of Tris Minimal Medium (TMM) to culture bacteria directly from these particles. This primary enrichment was incubated in a 25°C shaking incubator (100 rpm) for 120 hours. A test tube containing TMM and another test tube containing ten particles in sterile water were used as abiotic controls that were incubated under the same conditions previously described. Au-tolerant bacteria were selected by taking an aliquot (1 mL) of the primary enrichment and adding it to a test tube containing fresh TMM (9 mL) supplemented with 5 μM AuCl3. This concentration has been used in previous studies to select for Au-tolerant bacteria (see Sanyal et al., Reference Sanyal, Brugger, Etschmann, Pederson, Delport, Dixon, Tearle, Ludington, Reith and Shuster2020a). Additionally, this concentration could exist in microenvironments surrounding gold particles undergoing dissolution. This selective enrichment was incubated in the same shaking incubator for 72 hours. After incubation, the selective enrichment was serially diluted up to 10–4 fold. To obtain individual colonies of Au-tolerant bacteria, the dilutions were transferred to solid TMM Agar (1.5%(w/v)) plates amended with 5 μM AuCl3. These plates were incubated at 25°C for 48 hours during which bacterial colonies developed on the Agar surface. The most common colony occurred as beige, millimetre-size hemispheres. A Au-tolerant bacterial isolate was obtained by selecting a representative colony based on visual characterisation of colony size, colour and morphology. The selected colony was placed into a test tube containing fresh TMM (5 mL); in doing so, this Au-tolerant bacterial isolate enrichment was purified.
Au-tolerant bacterial isolate: Identification and whole-genome analysis
For identification of the isolate, genomic DNA (gDNA) was extracted using an ATP™ gDNA Mini Kit (Promega, USA) following to the manufacturer's instructions. To check whether the isolate contained any extra-chromosomal DNA, plasmid DNA was also extracted using the Wizard®Plus SV Minipreps plasmid DNA Purification kit (Promega, USA) following the manufacturer's instructions. The polymerase chain reaction (PCR) technique was performed using primers specific for the bacterial 16S rRNA gene (27F and 1492R) and followed by sequencing. This 16S rRNA gene sequence of the isolate was subjected to nucleotide BLAST analysis (http://www.ncbi.nlm.nih.gov/) to identify the species exhibiting the most significant homologies.
The isolate serves as a model to understand the genomic capabilities of the original ‘parent’ bacterium living on the surface of a gold particle. Therefore, extracted gDNA from the isolate was further analysed to understand its genomic capacity. As such, genes capable of conferring heavy-metal resistance and genes enabling nutrient cycling under nutrient-limiting conditions were targeted. The genome sequencing was performed using the Illumina HiSeq 2500 Sequencing System and the genome sequence was analysed following the methods described by Sanyal et al. (Reference Sanyal, Reith and Shuster2020b). This whole-genome shotgun project was deposited at NCBI GenBank under the accession JADKPY000000000. The version described in this paper is JADKPY010000000.
Au-tolerant isolate: Determining Au tolerance and reduction capacity
In terms of physiology, the isolate also serves as a model to understand how the ‘parent’ bacterium would interact with soluble Au during gold–silver dissolution, a process that contributes to enriched gold rims on particles. The isolate was grown in the presence of different Au concentrations using a 96 well microtiter plate-based assay in a method described by Sanyal et al. (Reference Sanyal, Reith and Shuster2020b) to determine the extent of Au tolerance. Briefly, aliquots (20 μL) of the purified isolate enrichment were placed into wells containing 130 μL TMM or TMM supplemented with AuCl3 (calculated 5, 10, 20, or 25 μM Au final concentration). The 96 well microtiter plate was incubated in a 25°C shaking incubator for 5 days. Optical density, a means of semi-quantifying turbidity, was measured using a BioTek SynergyTM Mx Microplate Reader. Measurements were taken at 2 hour intervals (between 0 and 18 hours of incubation), 12 hour intervals (between 24 and 96 hours of incubation) and at 120 hours of incubation.
The isolate's capacity to reduce soluble Au was assessed using the method described by Sanyal et al. (Reference Sanyal, Reith and Shuster2020b). Isolate enrichments were centrifuged (12,000 × g for 1 minute) forming a bacterial pellet and the supernatant of spent media was discarded. The pellet was re-suspended in a measured 168 μM of AuCl3 and mixed using a vortex. These bacterial–gold systems were incubated at 25°C for 0.5, 1, 2, or 4 hours. Note that aliquots of the Au solution were used as abiotic controls to determine if soluble Au could precipitate out of solution. All bacterial–gold systems and abiotic controls were performed in triplicate and were wrapped in aluminium foil to prevent any photocatalytic effects. The exposures were arrested by centrifugation to form a bacterial pellet and the supernatant containing any residual soluble Au was removed. The supernatant was analysed using an Agilent 8900x triple quad inductively coupled plasma mass spectrometer (ICP-MS) and gold standards with known concentrations. Reduction capacity, i.e. the percent of immobilised soluble Au, was calculated by subtracting the measured residual Au concentrations from the initial Au exposure concentration.
Results
Gold particles: A mineral substrate for bacteria
As a population, the gold particles exhibited a range of morphologies (elongate, discoid or rounded) and surface areas containing concavities of varying volume that were filled with clay minerals, secondary gold nanoparticles and residual organic materials (Fig. 1a). Secondary gold nanoparticles occurred as euhedral crystals that appeared imbedded within clay. Although gold nanoparticles were observed within clay-filled concavities on all particles, the number of nanoparticles was variable (Fig. 1b). Of the 125 particles that were characterised, ~10% contained identifiable bacterial cells, based on the phenotypic qualities of cells (~1 μm cell size, rod-shape or cocci morphology, composed of carbon and the presence of extracellular polymeric substances (EPS)). These cells occurred individually or as small clusters (<5 cells) on clay-mineral surfaces within polymorphic layers. One gold particle contained a concavity with ~20 cocci cells that were attached to fine clay minerals and residual organics (Fig. 1c).

Fig. 1. (a) A back-scatter electron (BSE) micrograph of a gold particle from the Prophet Mine Australia. Note the nugget-like morphology and the presence of clay-filled concavities (arrow) on the particle surface. (b) A BSE micrograph of gold nanoparticles that occur as euhedral nanometre-scale crystals (left arrow) and micrometre-scale crystals (right arrow), both of which appear to be embedded within the clay ‘matrix’. (c) A high-magnification secondary electron micrograph of abundant cells occurring on the surface of one gold particle. Some cells appear to be attached to the mineral substrate by EPS (arrows)
Isolation of a viable bacterium and its identification
Positive bacterial growth in the primary enrichment was indicated by turbidity that developed in the fluid phase (TMM) during incubation. Turbidity was attributed to the increasing number of cells growing in TMM. Turbidity was not observed in the abiotic controls. Similarly, positive bacterial growth in the selective enrichment as well as the isolate were indicated by turbidity. Molecular analysis of the Au-tolerant isolate revealed that it shared significant sequence similarity (100%) with Acinetobacter junii strain ATCC17908. Based on this molecular identification, the isolate was named Acinetobacter sp. SK-43.
Acinetobacter sp. SK-43 genomic capabilities
The genome of Acinetobacter sp. SK-43 was ~3.3 Megabase pairs (Mbp) in size and contained 3316 functional genes. The genome contained six genomic islands and 12 mobile genetic elements, which could be integrated into the bacterial genome via lateral gene transfer. The isolate also contained a plasmid (pSK-43) that was 242 kilobase pairs (kbp) in length. Statistical information about Acinetobacter sp. SK-43's entire genome is available at https://www.ncbi.nlm.nih.gov/nuccore/JADKPY000000000.
In terms of capabilities, Acinetobacter sp. SK-43 contained diverse heavy-metal resistance genes and gene clusters. The chrAB, corA, NCCN, feoB and fbpABC genes were detected and would collectively allow for chromium (Cr), cobalt (Co), nickel (Ni) and iron (Fe) resistance when expressed (Table 1). Genomic analysis also confirmed the presence of genes clusters (operon) for heavy-metal resistance that were acquired laterally. These operons include copDCASRB, arsCRCBH and czcCBAF which enables copper (Cu), arsenic (As) and cobalt/zinc/cadmium (Co/Zn/Cd) resistance, respectively. Additionally, the genome contained other genes related to oxidative-stress tolerance, i.e. cellular damage repair caused by heavy metals. See Table 1 for full details on genes and genes clusters and their biological function related to mediating specific metals. In terms of nutrient-cycling genes, a total of 13 genes and operons related to the cycling of carbon (C), nitrogen (N), sulfur (S) and phosphorus (P) – essential elements for life – were detected. Genes for C-cycling under nutrient-limited conditions included csrA, cstA and YhgH whereas operons for N-, S- and P-cycling under nutrient-limited conditions included ntrBC, icsRSUA and pstSCAB, respectively (Table 2).
Table 1. A list of heavy-metal resistance genes and gene clusters from the genome of Acinetobacter sp. SK-43.
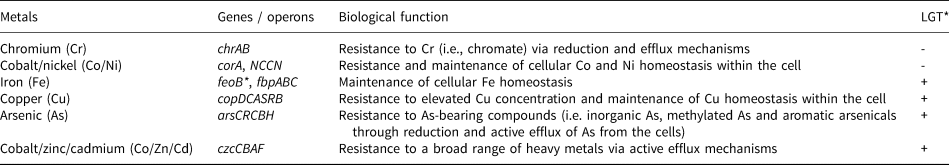
*LGT – Lateral Gene Transfer (+ detected, - not detected)
Table 2. A list of nutrient-cycling genes that function under nutrient-limiting conditions in Acinetobacter sp. SK-43.

Acinetobacter sp. SK-43 interactions with soluble Au
On the basis of optical density measurements, Acinetobacter sp. SK-43 exhibited a bacterial growth curve with a typical sigmoid-shape when grown in aqueous TMM. The upper plateau of the sigmoid-shape curve represents a stationary growth phase when the rate of cellular growth slows down as cell numbers increase and nutrients in the growth medium is depleted. This stationary phase was reached after 60 hours of incubation for Acinetobacter sp. SK-43 grown in TMM or TMM with 5 μM Au. The stationary phase was reached after 84 hours of incubation for Acinetobacter sp. SK-43 grown in the presence of 10 or 20 μM Au. Little to no bacterial growth occurs when Acinetobacter sp. SK-43 was grown in the presence of 25 μM Au (Fig. 2a). From the reduction capacity experiment, the bacterial pellets appeared slightly pink and the reduction of 168 μM Au by Acinetobacter sp. SK-43 was rapid. An average 68% of soluble Au was reduced within 30 minutes of incubation and nearly 90% of soluble Au was immobilised after 4 hours (Fig. 2b). The Au concentration in the abiotic control remained unchanged.

Fig. 2. (a) Acinetobacter sp. SK-43 grown in Tris Minimal Medium (TMM) or TMM supplemented with varying gold concentrations. This bacterial isolate was tolerant up to 20 μM Au. The delay in reaching a stationary growth phase corresponded with increasing gold concentrations. (b) The experiment involving the gold reduction capacity of Acinetobacter sp. SK-43. The majority (68%) of the 168 μM Au was reduced within the first 30 minutes of exposure.
Discussion
Gold particles and viable bacteria
The overall morphology and surface textures of particles (Fig. 1a,b) were consistent with particles obtained from a creek located near the Prophet Mine (Reith et al., Reference Reith, Fairbrother, Nolze, Wilhelmi, Clode, Gregg, Parsons, Wakelin, Pring and Hough2010; Shuster et al., Reference Shuster, Reith, Cornelis, Parsons, Parsons and Southam2017), which further suggests that these particles have been subjected to punctuated ‘events’ of (bio)geochemical weathering. Although extremely rare, the abundant cells observed on one gold particle (Fig. 1c) meets the International Union of Pure and Applied Chemistry (IUPAC) definition of a biofilm. The presence of bacterial biofilms on other gold particles have been inferred from amplified OTUs (i.e. Rea et al., Reference Rea, Zammit and Reith2016; Reith et al., Reference Reith, Rea, Sawley, Zammit, Nolze, Reith, Rantanen and Bissett2018). In this study, the number of bacterial cells on the surface of particles observed by microscopy was low relative to the diverse types of bacteria interpreted from the molecular analysis in other studies. Fragments of bacterial DNA can occur within soil and can be amplified, thereby skewing microbial diversity estimates (Carini et al., Reference Carini, Marsden, Leff, Morgan, Strickland and Fierer2016). The culturing of Au-tolerant bacteria was first obtained from particles sourced from Colombia and included Sediminibacterium sp., Shewanella sp. and Nitrobacter sp. (Shuster et al., Reference Shuster, Johnston, Magarvey, Gordon, Barron, Banerjee and Southam2015). Additionally, it has been determined that viable bacteria represent a fraction of all microbes that may have come in contact with a particle at some point (Sanyal et al., Reference Sanyal, Shuster and Reith2019a). Bacteria receive no nutritional benefit from gold, but particles (like any other mineral) are a substrate that bacteria can colonise (Africa et al., Reference Africa, van Hille and Harrison2013; Mielke et al., Reference Mielke, Pace, Porter and Southam2003; Wakelin et al., Reference Wakelin, Anand, Reith, Gregg, Noble, Goldfarb, Andersen, DeSantis, Piceno and Brodie2012). If bacteria on gold particles are metabolically active, then they could have an impact on the biogeochemistry of the surrounding microenvironment, i.e. interactions occurring at the fluid–bacterial–mineral interface (Fig. 3). Therefore, characterising particle-associated bacteria that are both viable as well as tolerant to aqueous Au is important for understanding the bacterial contribution to particle transformation and gold biogeochemical cycling in natural environments.

Fig. 3. A schematic diagram highlighting the interactions occurring at the fluid–bacteria–mineral interface (e.g. Acinetobacter sp. SK-43 living on particles). It is important to remember the three-dimensional ‘architecture’ of microenvironments to understand the structure-function relationship of microbe–mineral interaction. Arrows represent the impact of one constituent on another.
Genomic insights on the capabilities of viable bacteria from gold particles
Bacterial genomes are considered to be a molecular ‘blueprint’ for studying the possible strategies for adapting to different environmental conditions (Banfield et al., Reference Banfield, Tyson, Allen, Whitaker, Banfield, Cervini-Silva and Nealson2005; Newman and Banfield, Reference Newman and Banfield2002). Therefore, understanding the genome of viable bacteria could provide insight on how these microorganisms survive living on gold particles under nutrient-limited conditions within microenvironments. In this study, the Au-tolerant Acinetobacter sp. SK-43 was isolated from the gold particles. From this isolate, the presence of laterally acquired heavy-metal resistance genes, especially operonic clusters, demonstrates an evolutionary adaptation to potentially toxic metal concentrations within the environments (Table 1). It has been demonstrated that cop genes are important for synergistic Cu and Au resistance (Bütof et al., Reference Bütof, Wiesemann, Herzberg, Altzschner, Holleitner, Reith and Nies2018; Wiesemann et al., Reference Wiesemann, Bütof, Herzberg, Hause, Berthold, Etschmann, Brugger, Martinez-Criado, Dobritzsch and Baginsky2017). Therefore, the laterally acquired copper resistance gene clusters (copDCASRB) detected in Acinetobacter sp. SK-43 could be an essential genetic determinant for living on gold particles and surviving gold/silver dissolution. Similar cop clusters have been detected individually in viable Pseudomonas sp. and Arthrobacter sp. cultured directly and isolated from gold particles from South Africa (Sanyal et al., Reference Sanyal, Brugger, Etschmann, Pederson, Delport, Dixon, Tearle, Ludington, Reith and Shuster2020a). Therefore, it is reasonable to suggest that copper resistance gene clusters in other microbes could enable survival on gold-bearing mineral substrates. Additionally, C- and N-metabolising genes, such as cstA, csrA and ntrBC, are known to regulate cellular metabolism under nutrient-deficient conditions by enabling the utilisation of cellular peptides or DNA as the alternative source of carbon for energy (Dubey et al., Reference Dubey, Baker, Suzuki, Jones, Pandit, Romeo and Babitzke2003) (Table 2). The products of phosphate transporter gene cluster (pstSCAB) are known to enable the accumulation of phosphate as intracellular inclusions under phosphate-limiting conditions (Vuppada et al., Reference Vuppada, Hansen, Strickland, Kelly and McCleary2018). In general, bacterial communities catalyse the cycling of major elements (C, N and S) that are linked to the biogeochemical cycling of gold (Falkowski et al., Reference Falkowski, Fenchel and Delong2008; Sanyal et al., Reference Sanyal, Shuster and Reith2019b). Indeed, the expression of genes is temporal (Laub et al., Reference Laub, McAdams, Feldblyum, Fraser and Shapiro2000). However, the detection of heavy-metal resistance and nutrient-cycling genes and gene clusters highlights the genomic capacity of how Acinetobacter sp. SK-43 could interact with gold within microenvironments whilst remaining viable (Fig. 3).
Viable bacteria as models for gold biogeochemical cycling
Metabolically active bacteria, if occurring as biofilms on particles, could influence the biogeochemistry within a given microenvironment, which could promote either gold dissolution or precipitation processes (Reith and McPhail, Reference Reith and McPhail2007; Reith et al., Reference Reith, Rogers, McPhail and Webb2006). In terms of particle dissolution ‘events’, a fundamental question regarding fluid–bacterial–mineral interactions arises. What happens to Au-tolerant bacteria residing on particles when gold dissolves from particles? In this study, Acinetobacter sp. SK-43 was tolerant up to 20 μM Au (Fig. 2a), a concentration that can be toxic for most microbes. This tolerance is attributed to the presence of heavy-metal resistance and oxidative stress genes (Zammit et al., Reference Zammit, Weiland, Brugger, Wade, Winderbaum, Nies, Southam, Hoffmann and Reith2016). While the gold concentrations used in this study were high in comparison to measured values in the natural environment (Mann, Reference Mann1984; Webster and Mann, Reference Webster and Mann1984), the amount of gold and bacteria used in the experiments was proportional to the number of bacteria within microenvironments surrounding gold particles.
The reduction of soluble Au by Acinetobacter sp. SK-43 highlights (1) biomineralisation as a mechanism of survival (Shuster et al., Reference Shuster, Bolin, MacLean and Southam2014; Shuster et al., Reference Shuster, Johnston, Magarvey, Gordon, Barron, Banerjee and Southam2015) and (2) the possible contribution to the occurrence of pure gold nanoparticles within polymorphic layers and potentially gold-enriched rims (Reith et al., Reference Reith, Falconer, Van Nostrand, Craw, Shuster and Wakelin2020 and references therein). For the former, the extracellular surface and intracellular material can serve as reducing agents for metals including Au, thereby reducing the soluble metal concentration to a less-toxic level and enabling other bacteria to survive (Kenney et al., Reference Kenney, Song, Bunker and Fein2012; Langley and Beveridge, Reference Langley and Beveridge1999; Southam and Beveridge, Reference Southam and Beveridge1994). The reduction of soluble Au by organic material results in the precipitation of nanoparticles, which can appear pink when ca. 20 nm size nanoparticles are suspended (Kalabegishvili et al., Reference Kalabegishvili, Kirkesali, Rcheulishvili, Ginturi, Murusidze, Pataraya, Gurielidze, Tsertsvadze, Gabunia and Lomidze2012; Turkevich et al., Reference Turkevich, Stevenson and Hillier1951). Interestingly, the bacterial pellet from the reduction capacity experiment appeared pink. It is reasonable to suggest that soluble Au was probably reduced forming nanoparticles (Fig. 2b), a non-toxic form of gold (Correa-Llantén et al., Reference Correa-Llantén, Muñoz-Ibacache, Castro, Muñoz and Blamey2013). Therefore, viable bacteria from gold particles could be used to provide insight on the possibility of preferable microenvironments (or niches) where fluid–bacteria–mineral interactions occur (Fig. 3).
Conclusions
In this study, the physiology and genomic capabilities of a viable bacterium were identified using microanalytical and molecular techniques. Collectively, this study provides a comprehensive overview of how gold-tolerant bacteria are capable of residing on gold particles, mediate the toxicity of soluble Au and catalyse particle transformation. Acinetobacter sp. SK-43 could be used as a model organism to understand how other gold-tolerant microbes could colonise particles and subsequently contribute to particle transformation and the development of gold-enriched rims on particles over geological time. By connecting the fields of microbiology and geology, this study also highlights that gold particles and their associated bacteria can be used to understand the bacterial–gold interaction during a particles’ ‘journey’ through the biogeosphere. The experimental evidence supports the potential development of new geobiological tools for gold exploration and processing. Therefore, fundamental research on gold geomicrobiology should continue to identify other types of Au-tolerant and viable bacteria from gold particles and their genomic, physiological and potentially proteomic capabilities.
Acknowledgements
This research was and supported, in part, by the Australian Research Council (ARC) Future Fellowship (FT100150200) awarded to the late Frank Reith (c/o Jeremiah Shuster). This study is dedicated in memory of Associate Professor Frank Reith. We thank J. Parsons for acquiring gold particle samples from the Prophet Mine and R. Tearle, A. Ludington, S. Pederson and S. Gilbert for their technical support at the University of Adelaide.







