Introduction
Salminus hilarii (Valencienes, 1850), commonly known as tabarana or dourado branco, is a rheophilic teleost fish, carnivorous, medium-sized (first maturation in captivity: 29.40 ± 2.40 cm for females and 23.80 ± 0.70 cm for males), which can be considered of great importance in the food chain in the upper Tietê river basin (São Paulo/SP, Brazil). This species can also be found in rivers of the upper Paraná, São Francisco and Tocantins, upper Amazonas and upper Orinoco basins. Factors such as: (i) pollution, mainly from the residential and industrial effluents, (ii) population growth, (iii) eutrophication, (iv) fishing, and (v) dam construction has significantly reduced the S. hilarii natural stocks in the upper Tietê basin (Honji et al., Reference Honji, Narcizo, Borella, Romagosa and Moreira2009, Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011, Reference Honji, Nóbrega, Pandolfi, Shimizu, Borella and Moreira2013; Araújo et al., Reference Araújo, Honji, Mello and Moreira2012).
As described by Agostinho et al. (Reference Agostinho, Gomes, Suzuki, Júlio, Carolsfeld, Harvey, Ross and Baer2003), S. hilarii has preference for small water bodies and this, therefore, makes the species more susceptible to local extinctions from pollutions and impoundments. Similarly, dam construction for energy production or water storage directly interferes in the migratory course of rheophilic fish species, preventing reproduction and consequently reducing their natural stocks (Honji et al., Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011; Araújo et al., Reference Araújo, Honji, Mello and Moreira2012). S. hilarii was classified in the 2008 and 2010 Red List of São Paulo state (Assembleia Legislativa do Estado de São Paulo, 2008; Bressan et al., Reference Bressan, Kierulff and Sugieda2009) as ‘almost threatened’, however S. hilarii captures are almost rare, suggesting ineffective survey and data collection of fauna by the competent agency. Production in captivity can be considered a viable strategy to reduce the anthropic pressure and collaborate with the conservation efforts for native rheophilic species (Silva et al. Reference Silva, Deus and Hilsdorf2006). However, basic information about biology, genetics, reproductive physiology and larval and embryonic development are essential to improve the productive performance in captivity.
Previous studies have been carried out with S. hilarii especially focused on reproduction (Honji et al., Reference Honji, Narcizo, Borella, Romagosa and Moreira2009, Reference Honji, Nóbrega, Pandolfi, Shimizu, Borella and Moreira2013), larval rearing (Araújo et al., Reference Araújo, Honji, Mello and Moreira2012), and population genetics (Silva et al., Reference Silva, Hallerman, Orfão and Hilsdorf2015) and providing essential information about this species production. Embryonic and larval studies are essential to understand fish development, mainly those performed with commercial interests or endangered species, not only to generate knowledge about their development characteristics, but also to have a comparison model to know when normal development is altered by external factors (Coelho et al., Reference Coelho, Costa, Bashiyo-Silva, Souza, Ribeiro, Senhorini, Veríssimo-Silveira and Ninhaus-Silveira2019). According to Morrison et al. (Reference Morrison, Miyake and Wright2001) knowledge about embryonic development in fish is essential to define adequate strategies to avoid high mortality rates influenced mainly by embryo deformities and bad formations. Due to this importance, several studies have been conducted in Brazil to describe the initial developmental phase of fish species with commercial interest or strong environmental appeal, such as Pseudoplatystoma corruscans (Cardoso et al., Reference Cardoso, Alves and Ferreira1995), Brycon cephalus (Romagosa et al., Reference Romagosa, Narahara and Fenerich-Verani2001), Brycon insignis (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001), Steindachneridion parahybae (Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012), among others.
Against this background, we investigated S. hilarii reproduction, embryonic and larval phase development to understand the reproductive biology and ontogenetic development to guide reproductive techniques and captivity production of this species. Our goals were: (i) to assess the performance of two different spawning techniques (dry stripping of gametes and natural spawning); and (ii) to describe the initial developmental stages, from activation of the oocyte to the initial larval period.
Materials and methods
Fish stocks, rearing conditions, and fish collection
Sexually adult wild S. hilarii females and males were collected from the Tietê River in the São Paulo States, Brazil (at intervals 23º32′45.3′′S, 46º08′03.2′′W and 23º34′36.5′′S, 45º54′23.9′′W) using artisanal fisheries (Fig. 1). These animals were transported individually to the Ponte Nova hatchery (23º35′33.8′′S, 45º58′09.1′′W), located in Salesópolis city (São Paulo, Brazil). S. hilarii broodstock were maintained in two ponds (300 m2 each) during the experimental period under a natural photoperiod and fed with commercial extruded diet for carnivorous fish (40% crude protein) with 2% of the biomass per day.

Figure 1. Map of the upper Tietê river basin and type locality of Salminus hilarii sampling sites. (a, b) Map of the Upper Tietê River Basin, São Paulo State (Brazil). (c) Upper Tietê River Basin with the collection site (1) (between the arrowhead) and Ponte Nova fish farm (2) (arrow). (d) Type locality of S. hilarii, Upper Tietê River in areas in which the water is clean, shallow and running. Source: Modified from DAEE (Departamento de Águas e Energia Elétrica), Brazil, and Gomes et al. (Reference Gomes, Tolussi, Boëchat, Pompêo, Cortez, Honji and Moreira2016).
Broodstock selection, hormonal induction and spawning
Salminus hilarii reproductive period normally occurs from October to February, with a peak in December and January (Honji et al., Reference Honji, Narcizo, Borella, Romagosa and Moreira2009, Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011). In December, artificial reproductions were performed using three females and three males (in each different groups explained below) that were selected by morphological characteristics of sexual ripeness, according to Honji et al. (Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011). Males were chosen according to colour (white) and running sperm were observed after a gently abdominal massage. Females were selected by the presence of a large, soft abdomen, and also by gonadal characteristics (biopsy procedure). Oocytes were collected to examine size, appearance, and diameter under a stereomicroscope, these are important criteria used to establish the spawning readiness of a total spawning S. hilarii broodstock (Honji et al., Reference Honji, Narcizo, Borella, Romagosa and Moreira2009, Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011).
After broodstock selection, animals were transferred to laboratory tanks (3.6 m2), and artificial reproduction induction was performed on S. hilarii using a routine protocol (Honji et al., Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011). Concisely, the females were induced using two intraperitoneal injections of carp pituitary extract (cPE; Fish Braz). Doses were 0.5 mg cPE/kg (first injection) and 5.0 mg of cPE/kg (second injection), diluted in 0.9% sodium chloride solution (considering 1.0 ml in each dose). The second injection (females) was administered 10 h after the first. Additionally, a single dose of cPE injection (3 mg cPE/kg also diluted in 0.9% sodium chloride solution) was applied to the males at the same time as the second cPE application in females.
After first hormone induction until second dose, the females were kept in separate tanks to the males, however under the same conditions. After final hormonal induction, males and females were kept in the same tank to allow natural spawning (Group 1 – G1) and consequently natural fertilization. Subsequently, water flow in the tanks was increased and the water temperature was monitored. The time for natural gamete releasing, counted from the second hormone administration until spawning, was calculated as accumulated thermal units (ATU), or degree-hours following Weingartner and Zaniboni-Filho (Reference Weingartner, Zaniboni-Filho, Baldisseroto and Gomes2005). The second group (G2) was established to compare the eggs and larval development using a standard protocol applied to other teleost species, in which males and females were stripped (from the results obtained by the ATU). The dry stripped oocytes from three females were mixed with sperm from three males. Subsequently, gametes from males and females were stripped in plastic containers and dry extrusion was carried out. Oocytes were weighed, sperm were added and then the mixture was gently homogenized. Water was added for hydration to allow fertilization (in accordance with the protocol described by Caneppele et al., Reference Caneppele, Honji, Hilsdorf and Moreira2009). In all groups, the estimated number of oocytes in S. hilarii was calculated indirectly by weighing the eggs and counting subsamples in triplicate (Caneppele et al., Reference Caneppele, Honji, Hilsdorf and Moreira2009; Honji et al., Reference Honji, Mello, Araújo, Rodrigues-Filho, Hilsdorf and Moreira2011). After spawning (natural or stripped), females were transferred back into their original ponds (100% survival). Additionally, data on broodstock weight were: G1 (871.33 ± 99.30 for females and 541.33 ± 24.30 for males) and G2 (674.67 ± 39.30 for females and 393.33 ± 45.00 for males).
Eggs were transferred to four 60-litre fibreglass conical incubators, in which they were kept for 3 days until hatching and yolk-sac absorption. After this period, the larvae were transferred to horizontal fibreglass incubators (0.5 m2), in which they were fed with Artemia sp. nauplii. At 6 h after fertilization (AF), when the gastrula stage was well established, the fertility rate was calculated using the equation: F = (number of fertilized eggs ×100)/number of total eggs (Vazzoler, Reference Vazzoler1981, Reference Vazzoler1996), for both experimental groups.
Characteristics of the freshly spawned egg, terminology used for embryonic and larval development analysis and morphometric analyses
The main morphological features of each stage of embryonic and larval development of S. hilarii were described meticulously. Oocytes, eggs and larvae at each sampling were measured using a Leica EZ4 stereomicroscope (Leica Microsystems, Wetzlar, Germany) with coupled camera and using a computer image capture (Leica Application Suite v2.1.0). Every 5 min, fresh eggs samples were collected for analysis and a photographic record was taken until hatching. After hatching, the larvae were sampled every hour and a photograph was also recorded. The developmental stage was established when more than 50% of the specimens were at the same stage. This parameter was measured using five subsamples of the same group with 20 eggs and/or larvae samples. Additionally, at the end of each observation, characterization, and photographic record, the original volume of water and eggs/larvae was re-housed in the fibreglass conical incubators or horizontal fibreglass incubators. Embryonic and early larval development stages were identified according to descriptions established for others Characidae (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001; Reynalte-Tataje et al., Reference Reynalte-Tataje, Zaniboni-Filho and Esquivel2004; Nakaghi et al., Reference Nakaghi, Marques, Faustino, Ganeco and Senhorini2006; Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2009; Faustino et al., Reference Faustino, Nakaghi and Neumann2010a; Nakauth et al., Reference Nakauth, Villacorta-Correa, Figueiredo, Bernardino and França2016) and also by Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialtzki, Sanches, Makrakis and Pavanelli2001) and Richards (Reference Richards2006), who described the ontogenetic development of several fish species.
For morphometrical analysis of embryonic and larval development in S. hilarii during artificial reproduction in captivity, the following parameters were considered: arrangement of the development at the egg phase, total length of the larvae (Lt in millimetres), yolk sac length (YL) and yolk sac height (YH) (for both, in millimetres). YL and YH were measured shortly after hatching in this way: volumes were approximated by the formula for a prolate spheroid {V(mm3) = [π × l × (h2)]/6}, in which l is YL and h is YH (Bagarinao, Reference Bagarinao1986). For analysis of Lt and yolk volume, measurements were analyzed using Leica Application Suite Professional LAS v3.6 software.
Water quality analysis and additional information
Water quality, temperature (ºC) and dissolved oxygen (mg/l), during artificial reproduction (first and second doses; laboratory ponds), embryonic development (fibreglass conical incubators), and the annual mean (broodstock ponds) were monitored daily using an oximeter (YSI model 55) and are shown in Table 1. Additionally, all procedures used in the sampling of the broodstocks, eggs and larvae were in agreement with the Institutional Animal Care Protocols (Comissão de Ética em Manipulação e Experimentação Animal, CEMEA) of UMC University (protocol number and approval 0.28.04, 21 May 2004), and with the System of Authorization and Information on Biodiversity of the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) (protocol number and approval 125, 29 December 2004).
Table 1. Data on water temperature and dissolved oxygen during artificial reproduction (first and second doses; laboratory ponds), embryonic development (conical incubators), and the annual mean (broodstock ponds) at the Ponte Nova hatchery (Departamento de Águas e Energia Elétrica, São Paulo, Brazil) during embryonic and larval development study. Data are presented as the mean ± standard error of the mean

Results
Broodstock selection, hormonal induction and spawning
All broodstock from G1 induced to the reproductive-specific protocol responded positively to artificial induction and naturally spawned in the tank (100% survival). Additionally, S. hilarii underwent external fertilization and produced spherical, transparent, pelagic eggs. Spawning occurred at 148.5 ±13.5 ATU (under 26ºC) with 127.10 g of spawned eggs and a fertilization rate of 65.64 ± 0.54%. By monitoring ATU for G1, it was possible to know the exact moment for extrusion of G2. In this way, G2 also responded positively (also 100% survival) but only 7.23 g of spawned eggs were obtained, and no fertilization was observed in this group.
Embryonic and larval developmental stages
Main embryonic events, such as zygote, cleavage, morula, blastula, gastrula, organogenesis, hatching and other phases, and some behavioural information such as cannibalism were observed and registered (Table 2). All stages were characterized in detail with the specific time of occurrence and a brief description (Table 2).
Table 2. Time at the main morphological observations and brief description during the embryonic and larval development of Salminus hilarii

As fertilization occurred in tanks, eggs were observed after hydration (Fig. 2a, b) and were characterized by the appearance of a cell region that was formed at the blastodisc division; it was possible to visualize a prominent cytoplasm layer. Activated eggs were composed of the chorion, perivitelline space and animal pole (Fig. 2c) that persisted until embryo development at the animal pole (more detail in Fig. 2d), in which the perivitelline space was large, clear and translucent.

Figure 2. Macroscopic aspects of embryonic development of Salminus hilarii. (a) Group 1 (G1 – natural spawning) egg hydrated soon after fertilization (AF) (time zero); egg cell (arrowhead). (b) Group 2 (G2 – stripped spawning) egg hydrated AF (time zero) egg cell (arrowhead). (c) Activated egg (G1) with animal pole (asterisk) (14 min AF). (d) More detail of activated egg (G1) with animal pole (asterisk) (14 min AF). (e) G1: two blastomeres (49 min AF). (f) G1: four blastomeres (57 min AF). (g) G1: eight blastomeres (1 h 10 min AF). (h) G1: 16 blastomeres (1 h 35 min AF). PS: perivitelline space. Bars: 2000 µm (a, c); 200 µm (b, d–h).
First cleavage was observed for fertilization at 49 min AF, and was characterized by the appearance of a cellular region above the yolk sac and division of the blastodisc into two blastomeres of the same size (the first cleavage, 49 min AF; Fig. 2e). Second and third cleavages occurred, generating four blastomeres (second cleavage, 57 min AF; Fig. 2f) and eight blastomeres (third cleavage, 1 h and 10 min AF; Fig. 2g), respectively. Fourth and fifth cleavages produced 16 blastomeres (fourth cleavage, 1 h and 35 min AF; Fig. 2 h) and 32 blastomeres (fifth cleavage, 1 h and 42 min AF; Fig. 3a). Following development, the sixth cleavage give rise to 64 blastomeres (sixth cleavage, 1 h and 54 min AF; Fig. 3b), at which time the number of blastomeres was still evident. After this stage cell cycles lost synchrony and it became difficult to observe the number and the pattern of cleavage.

Figure 3. Macroscopic aspects of embryonic development of Salminus hilarii. (a) G1 (Group 1 – natural spawning): 32 blastomeres (1 h 42 min AF). (b) G1: 64 blastomeres (1 h 54 min AF). (c) G1: gastrula stage: epiboly of 25% (3 h 56 min AF). (d) G1: gastrula stage: epiboly of 50% (4 h 26 min AF). (e) G1: gastrula stage: epiboly of 90% (6 h 16 min AF). (f) G1: final gastrula stage and blastopore closure (8 h 31min AF). (g) G1: differentiation of embryonic layers, head (arrowhead), tail (arrow), and yolk sac (asterisk) (9 h 1 min AF). (h) G1: distinguish between the cephalic (arrowhead) and caudal (arrow) regions, somites (asterisk), and optic vesicle (arrowhead) (9 h 31 min AF). Bars: 200 µm (a–h).
Morula stage was identified as the successive cell division of more than 64 blastomeres and was arranged in a half-berry shape; the periblast and blastoderm became visible. Blastula stage was characterized by the presence of a cup-like shape with spaces between blastomeres at the animal pole; the beginnings of cell movement started at the gastrula stage. At this stage it was possible to observe morphogenetic movement (epiboly), which produced rearrangement of blastoderm to the yolk mass and originated the germinative follicles and embryonic axis. Epiboly was detectable at three different times, 25% epiboly (3 h 56 min AF; Fig. 3c), 50% epiboly (4 h 26 min AF; Fig. 3d) and 90% epiboly (6 h 16 min AF; Fig. 3e) and finally, the final gastrula stage and blastopore closure (8 h 31 min AF; Fig. 3f).
Next, organogenesis started and the process was divided into early segmentation phase and late segmentation phase. At the early segmentation phase it was possible to observe the cephalic and caudal regions and yolk sac (9 h 01 min AF; Fig. 3g), in which the tail was very short and attached to the yolk mass, and it was clearly possible to distinguish the optic vesicle and the somites that posteriorly originated the muscle tissue (9 h 31 min AF; Figs 3h and 4a, b). This subdivision ended when the tail detached and the embryo start growing with the tail well extended and completely free from the yolk sac (11 h 51 min AF; Fig. 4c). After this stage, the embryo started conspicuous muscular contractions (Fig. 4d) with a heterogenic egg wall (Fig. 4e), followed by hatching (Fig. 4f). At 12 h after hatching (HAH), an initial cannibalistic behaviour was observed (Fig. 4g, h) and at 33 HAH this behaviour clearly became more intense.

Figure 4. Macroscopic aspects of embryonic development of Salminus hilarii. (a) G1 (Group 1 – natural spawning): distinguish between the cephalic with optic vesicle (arrowhead) and caudal (arrow) regions, somites details (asterisk), and yolk sac (YS) (10 h 1 min AF). (b) G1: detail of cephalic region with optic vesicle detail (arrowhead) and yolk sac (YS) (10 h 1 min AF). (c) G1: complete development of larvae (arrowhead), the tail release (arrow), and homogeny egg wall (asterisk) (11 h 51 min AF). (d, e) G1: complete development of larvae (arrowhead) with movement, beginning (d), advanced (e) of heterogenic of egg wall (arrows), and next (e) to hatching (asterisk) (20 h 46 min AF). (f) Hatching (time zero after hatching – AH). (g, h) G1: cannibalism (g) and detail (h) among the S. hilarii larvae (12 h 23 min AH). Bars: 200 µm (a–f); 2000 µm (g); 1000 µm (h).
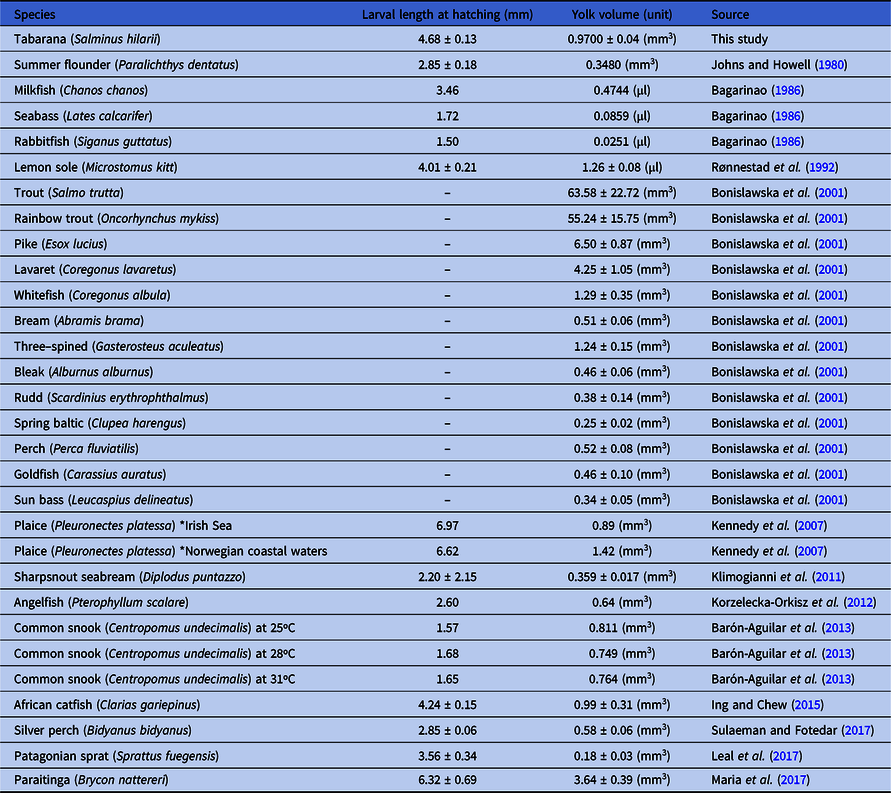
Additionally, other morphological aspects of S. hilarii larvae were measured, such as larval length and yolk sac volume at hatching, and were compared with aspects of other teleost fish larvae (Table 3). S. hilarii larvae hatched with a 4.68 ± 0.13 mm length and yolk sac volume was 0.97 ± 0.04 mm3.
Table 3. Overview of larval length, yolk volume and/or yolk diameter measurements at hatching in several teleost species. Data are presented as the mean ± standard error of the mean
Discussion
In general, S. hilarii-induced reproduction using the cPE technique is recommended followed by natural spawning in the tank. This species presented similar embryogenesis and larval development compared with a congeneric species (S. brasiliensis), with larvae containing a large yolk sac and strong pigmentation in the body and eyes, and with a very strong cannibalistic behaviour that generally can be minimized by selection by size during development.
Water quality parameters observed in this study were similar to those observed in previous trials carried out with S. brasiliensis. In the same way, reproductive protocols used for this congeneric species were successfully applied for S. hilarii (Weingartner and Zaniboni-Filho, Reference Weingartner, Zaniboni-Filho, Baldisseroto and Gomes2005; Zanandrea et al., Reference Zanandrea, Weingartner and Zaniboni-Filho2016). In contrast, animals from the G2 group released a low amount of eggs and we failed to obtain fertilized eggs by manual striping. This failure is probably related to either water volume at the moment of fertilization, oocyte quality, or synchronization of the micropyle opening time (Mylonas et al., Reference Mylonas, Fostier and Zanuy2010). Compared with G2, fertilized eggs were observed in animals from G1, nevertheless compared with the other Characidae species, fertilization rates were considered lower (65%) (Luz et al., Reference Luz, Reynalte-Tataje, Ferreira and Zaniboni-Filho2001; Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Narahara and Fenerich-Verani2002; Narahara et al., Reference Narahara, Andrade-Talmelli, Kavamoto and Godinho2002; Weingartner and Zaniboni-Filho, Reference Weingartner, Zaniboni-Filho, Baldisseroto and Gomes2005; Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012). Additionally, S. hilarii ATU in both groups were close to those of previous studies performed with Characidae, with only a few slight variations observed according to species. Therefore, natural spawning after hormonal induction is highly recommended for S. hilarii.
In general, embryogenesis is characterized mainly by fertilization to zygote formation, when it is possible clearly distinguish the blastodisc from the vegetative pole (Richards, Reference Richards2006; Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialtzki, Sanches, Makrakis and Pavanelli2001; Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011). In S. hilarii this stage was characterized from the moment of fertilization up to 14 min AF, while for others Characidae species this process occurred later, as observed for B. cephalus (25 min at 26.8ºC; Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2009), Brycon gouldingi (45 min at 26.4°C; Faustino et al., Reference Faustino, Nakaghi and Neumann2010a), Prochilodus lineatus (75 min at 24°C; and 34 min at 28°C, Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006). Previous studies performed with B. cephalus (Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2009), and S. brasiliensis (Nakagui et al., Reference Nakaghi, Marques, Faustino, Ganeco and Senhorini2006) showed a large perivitelline space in the egg similar to that observed for S. hilarii (937.89 ± 107.19 μm). According to Nakatani et al. (Reference Nakatani, Agostinho, Baumgartner, Bialtzki, Sanches, Makrakis and Pavanelli2001) the perivitelline space is described as ‘large’ when it reaches an area between 20 and 29.9% of total egg volume; this characteristic is considered an advantage as could prevent possible damage in the eggs, and consequently improves the survival rate (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001).
Cleavage stage is characterized by successive cell divisions that result in a blastomere volume increase, consequently decreasing the cytoplasm volume (Gilbert, Reference Gilbert2003; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008). In S. hilarii, the 2–64-blastomeres stage was observed between 49 min AF and 1 h 42 min AF; this phase is considered critical mainly due to a high deformity incidence and consequently higher mortality (Morrison et al., Reference Morrison, Miyake and Wright2001; Ninhaus-Silveira et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006). Morula and blastula stages are mainly distinguished by differentiation in the blastoderm (embryonic cells) and periblast (yolk syncytial layer) (Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008). The blastoderm stage presented more than 64 blastomeres arranged in two or more layers, and separated from the blastocoele periblast (large space between the yolk and the blastomeres). Additionally, the periblast plays an essential role during the period of endogenous feeding, as it is directly related to nutrient absorption processes. In S. hilarii these stages particularly occurred from 1 h 54 min AF, whereas for B. cephalus it occurred from 1 h 30 min (26.8°C; Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2009), in L. macrocephalus and P. corruscans from 2 h 0 min AF (29°C; Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011; 27ºC; Marques et al., Reference Marques, Nakaghi, Faustino, Ganeco and Senhorini2008 respectively) and in P. lineatus from 3 h 0 min AF (24°C; Ninhaus-Silveira, et al., Reference Ninhaus-Silveira, Foresti and Azevedo2006). These differences showed a distinct pattern according to species specificity and temperature.
Gastrula phase is initially characterized by the first cellular movements in an event called epiboly, and is finished by closure of the blastopore, resulting in blastoderm rearrangement in relation to yolk mass (Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012; Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011; Nakagui et al., Reference Nakaghi, Marques, Faustino, Ganeco and Senhorini2006; Mello et al., Reference Mello, Araújo, Campos, Rodrigues-Filho, Garcia and Moreira2018). Gastrula stage in S. hilarii was initiated at 3 h 56 min to 9 h 1 min, this time was considered late and no longer compared with other species such as P. maculatus (2 h 15 min to 5 h 0 min at 29ºC; Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011), B. cephalus (1 h 45 min to 7 h 45 min at 26.8°C; Alexandre et al., Reference Alexandre, Ninhaus-Silveira, Veríssimo-Silveira, Buzollo, Senhorini and Chaguri2009), and S. parahybae (2 h 40 min to 8 h 40 min at 24°C; Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012).
Initially, organogenesis is marked by notochord development, which will set the caudal cephalic corporeal axis, allowing distinction between the embryonic axis and the yolk sac (Nakatani et al., Reference Nakatani, Agostinho, Baumgartner, Bialtzki, Sanches, Makrakis and Pavanelli2001). The main features during this phase were optic vesicle maturation, a marked increase in somites, and pigmentation in distinct body regions, which occurred at 9 h 1 min to 20 h 45 min AF. At the end of organogenesis, muscular segmentation was observed, and the tail was completely released from the yolk sac (Andrade-Talmelli et al., Reference Andrade-Talmelli, Kavamoto, Romagosa and Fenerich-Verani2001; Faustino et al., Reference Faustino, Nakaghi and Neumann2010a,b; Chalde et al., Reference Chalde, Fernández, Cussac and Somoza2011; Honji et al., Reference Honji, Tolussi, Mello, Caneppele and Moreira2012). At approximately 20 h 46 min AF, an intense pigmentation in S. hilarii was observed in the cephalic and ventral posterior regions, this verified an intense muscular activity that consequently resulted in chorion rupture, initiating the hatching process.
After 21 h 17 min AF at 26°C, 100% of S. hilarii larvae were hatched, while in S. brasiliensis total hatching was observed after 18 h 0 min AF at 23.4°C (Nakaghi et al., Reference Nakaghi, Marques, Faustino, Ganeco and Senhorini2006; Vega-Orellana et al., Reference Vega-Orellana, Fracalossi and Sugai2006), and in B. orbignyanus after 14 h 0 min AF at 27.9°C (Nakatani et al., 2011). Based on these events, we can suggest that S. hilarii presented a late development. After hatching, S. hilarii larvae presented a large yolk sac (0.9700 ± 0.04 mm3) this characteristic is considered an important advantage, as fish species with this profile have more energy-containing substrates and consequently more time to complete their development and find food. Additionally, in this shift phase, high mortality rates are normally observed, mainly influenced by the transition event from endogenous to exogenous feeding (Bagarinao, Reference Bagarinao1986; Jaworski and Kamler, Reference Jaworski and Kamler2002; Yufera and Darias, Reference Yufera and Darias2007; Jaroszewska and Dabrowski, Reference Jaroszewska, Dabrowski and Holt2011; Barón-Aguilar et al., Reference Barón-Aguilar, Rhody, Brennan, Main, Peebles and Muller-Karger2013; Sulaeman and Fotedar, Reference Sulaeman and Fotedar2017).
After hatching, S. hilarii larvae presented a large yolk sac, subtly pigmented eyes, and an adhesive gland present in the frontal cephalic region that have the main function to keep the larvae adhered to different substrates. S. hilarii larvae showed an intense cannibalistic behaviour, which was observed from 33 h AF and continued throughout development; this was similarly observed for the congeneric species S. brasilienses (Nakaghi et al., Reference Nakaghi, Marques, Faustino, Ganeco and Senhorini2006; Vega-Orellana et al., Reference Vega-Orellana, Fracalossi and Sugai2006). Therefore, to avoid high mortality rates by cannibalism, S. hilarii larvae should be fed intensively (especially using Artemia sp. nauplii) associated with size grading. This technique is highly effective in decreasing cannibalism and consequently the mortality rate (Buzollo et al., Reference Buzollo, Veríssimo-Silveira, Oliveira-Almeida, Alexandre, Okuda and Ninhaus-Silveira2011).
In conclusion, the most effective protocol to artificially reproduce S. hilarii is hormonal induction using cPE, followed by spontaneous spawning in the tank, and then oocyte collection. The results clearly showed that manual extrusion followed by dry fertilization of gametes could not be applied for this species, or needs to be adapted, as no fertilized eggs were obtained. Ontogeny and embryonic development were very similar to that of other teleost species, especially compared with S. brasiliensis. However, the time at which the events occurred were largely influenced by water temperature. The results obtained in this study could be considered fundamental to improving reproduction and consequently enhancing the survival of S. hilarii in captivity, allowing their massive production and directly contributing to an effective conservation programme in the upper Tietê river basin. However other studies mainly related to their basic biology, reproductive physiology and first stage nutrition are still essential to this species production.
Acknowledgements
The authors would like to thank the local fishermen (Milton Nunes de Santana) and LAMEROA team (Laboratório de Metabolismo e Reprodução de Organismos Aquáticos, Instituto de Biociências, Universidade de São Paulo) for their help with sample collection of animals.
Financial Support
This research was supported (Technical and Financial) by the following grants: FAPESP 2001/10483-1, 2008/57687-0, 2014/16320-7 and 2017/06765-0 (Fundação de Amparo à Pesquisa do Estado de São Paulo) and a Master’s student fellowship CNPq 134451/2005-8 (Conselho Nacional de Desenvolvimento Científico e Tecnológico). AWSH and RGM are recipients of CNPq productivity scholarships.
Conflicts of Interest
None.
Ethical Standards
Not applicable.









