Introduction
Plant productivity is affected by Auchenorrhyncha (Hemiptera) hoppers throughout the world. These species transmit diseases (virus, phytoplasmas, spiroplasmas and bacteria) and also remove sap and debilitate the entire plant (Nault & Rodríguez, Reference Nault and Rodríguez1985). In fact, some of the main maize diseases in subtropical America are caused by maize-stunting pathogens transmitted in a persistent and propagative manner by leafhoppers and planthoppers, such as Dalbulus maidis (De Long) (Hemiptera: Cicadellidae) and Peregrinus maidis (Ashmead) (Hemiptera: Delphacidae). Despite the economic importance of delphacid and cicadellid vectors and considering that many of them are host-specific herbivores, there has been little attention paid to their olfactory sensitivity, which could be crucial in the development of new strategies for pest management. For leafhoppers and planthoppers, most studies suggest that olfaction is only supplementary to visual cues (Todd et al., Reference Todd, Phelan and Nault1990; Cook & Denno, Reference Cook, Denno, Denno and Perfect1994; Fereres & Moreno, Reference Fereres and Moreno2009; La Grange et al., Reference La Grange, Schröder, Glinwood, Ignell and Krüger2017; Zhang et al., Reference Zhang, Pengsakul, Tukayo, Yu, Fang and Luo2017), which are unlikely to provide host-specific information (Compton, Reference Compton, Bullock, Kenward and Hails2002). However, the role of olfaction in Auchenorrhyncha has not yet been fully clarified since it is known that some leafhoppers and planthoppers respond to olfactory cues in the absence of visual stimuli (Obata et al., Reference Obata, Koh, Kim and Fukami1981; Youn, Reference Youn2002; La Grange et al., Reference La Grange, Schröder, Glinwood, Ignell and Krüger2017). Also, the phototactic response to plant-reflected wavelengths may be modified by plant volatiles (Fereres & Moreno, Reference Fereres and Moreno2009).
Dalbulus maidis, a leafhopper, is considered a serious maize pest throughout most of the Americas, from southern USA to northern Argentina, primarily because it is the vector of corn stunt spiroplasma (CSS), maize rayado fino virus and maize bushy stunt mycoplasma (Carloni et al., Reference Carloni, Carpane, Paradell, Laguna and Gimenez Pecci2013; Virla et al., Reference Virla, Moya Raygoza and Luft Albarracin2013). Dalbulus maidis is a specialist herbivore feeding only on plants of the genus Zea, like maize (Zea mays L.) and its wild relatives, the teosintes (Zea spp.). In South America, it is the most prevalent leafhopper species associated with maize (Paradell et al., Reference Paradell, Virla and Toledo2001; Luft Albarracin et al., Reference Luft Albarracin, Paradell and Virla2008, Reference Luft Albarracin, Triapitsyn and Virla2017). CSS, one of the most important stunting diseases in maize, is caused by the mollicute Spiroplasma kunkelii (Spiroplasmataceae). The decrease in the yield of plants with CSS can be very high, from 12 to 100%, depending on the severity of symptoms (Virla et al., Reference Virla, Díaz, Carpane, Laguna, Ramallo, Gómez and Giménez Pecci2004a).
Peregrinus maidis is a broadly distributed planthopper, recognized as a maize pest, vector of other viral diseases, such as Maize Stripe Tenuivirus, Maize Mosaic Rhabdovirus, Maize Iranian Mosaic Virus, Maize Sterile Stunt Virus and also Mal de Río Cuarto Virus (MRCV) (Virla et al., Reference Virla, Miotti, Giménez Pecci, Carpane and Laguna2004b), one of the most prejudicial stunting pathogens in temperate South America (Lenardón et al., Reference Lenardon, March, Nome and Ornaghi1998; Argüello Caro et al., Reference Argüello Caro, Maroniche, Analía, Sagadín, Del Vas and Truol2013). MRCV is transmitted by planthoppers (Delphacidae), being Delphacodes kuscheli (Fennah) the main vector (Arneodo et al., Reference Arneodo, Guzmán, Conci, Laguna and Truol2002; Gimenez Pecci et al., Reference Gimenez Pecci, Laguna and Lenardón2012). Unlike Dalbulus leafhoppers, P. maidis apparently adapted to maize as a host in post-Columbian times (Nault, Reference Nault1983). It is a polyphagous insect, most frequently associated with Sorghum spp., but has also been found on Panicum spp. and other grasses (Tsai, Reference Tsai1996; Marino de Remes Lenicov & Paradell, Reference Marino de Remes Lenicov, Paradell, Giménez Pecci, Laguna and Lenardón2012; Diaz et al., Reference Diaz, Luft Albarracín and Alderete2016). Like many other planthoppers, adults have dual wing form, short wing (brachypterous) and long wing (macropterous). Brachypterous are unable to fly and occur when the environmental conditions are suitable for rapid population increase. In contrast, macropterous are produced when the population is too high to be sustained by the host plant, and thus migration to other hosts is favoured (Singh & Seetharama, Reference Singh and Seetharama2008).
Dalbulus maidis and P. maidis often coexist in subtropical maize fields, where two kinds of maize germplasms are cultivated by farmers; temperate germplasms are planted early in spring (off-season), and tropical germplasms are planted afterwards in the summer, soon after temperate maize harvest. Tropical germplasms are generally thought to be more resistant to maize stunting pathogens, because they have been developed from landraces obtained from area with high disease pressure. The coexistence of tropical and temperate maize in the beginning of the summer and the off-season maize harvest generates conditions under which CSS becomes a generalized problem in subtropical areas from South America (Oliveira et al., Reference Oliveira, Lopes and Nault2013). Apparently, part of the mechanism of resistance to CSS in maize germplasms involves less settling of the disease vectors (Carpane & Ingrassia, Reference Carpane and Ingrassia2012). In a field experiment, Virla et al. (Reference Virla, Casuso and Frias2010) demonstrated that Bt maize plots had significantly more D. maidis leafhoppers than the corresponding plots with a non-Bt maize isoline. Consequently, the authors observed that Bt maize were more affected by CSS than its non-Bt isoline (Virla unpublished). It is unknown if the preference for a particular germplasm involves phototactic responses, olfactive responses or both, but understanding the mechanism could be very important in the development of resistant hybrids.
Considering that Cicadellidae is a large family with at least 22,000 species (McKamey, Reference McKamey, Hoch, Asche, Homberg and Kessling2002), olfaction has been assessed in relatively few species (Saxena & Saxena, Reference Saxena and Saxena1974; Khan & Saxena, Reference Khan and Saxena1985; Todd et al., Reference Todd, Phelan and Nault1990; Bullas-Appleton et al., Reference Bullas-Appleton, Otis, Gillard and Schaafsma2004; Ranger et al., Reference Ranger, Winter, Backus, Rottinghaus, Ellersieck and Johnson2005; Patt & Setamou, Reference Patt and Setamou2007; Bento, Reference Bento2008; Mazzoni et al., Reference Mazzoni, Ioriatti, Trona, Lucchi, De Cristofaro and Anfora2009; Oluwafemi et al., Reference Oluwafemi, Bruce, Pickett, Ton and Birkett2011; La Grange, Reference La Grange2016 and Zhang et al., Reference Zhang, Pengsakul, Tukayo, Yu, Fang and Luo2017). In most leafhopper species, including D. maidis, the primary effect of host odour seems to be enhancing responsiveness to visual cues. To our knowledge, only two studies have proved that leafhoppers can discriminate between a preferred and non-preferred host species only based on the emitted volatile organic compounds (VOCs); Empoasca fabae (Harris) responded to leaf steam distillates of Medicago sativa L., discriminating between alfalfa varieties (Ranger et al., Reference Ranger, Winter, Backus, Rottinghaus, Ellersieck and Johnson2005) and Cicadulina storeyi China discriminated between headspace VOC samples of infested and uninfested maize seedlings (Oluwafemi et al., Reference Oluwafemi, Bruce, Pickett, Ton and Birkett2011). In a previous study with D. maidis, Todd et al. (Reference Todd, Phelan and Nault1990) found that there is an interaction between visual and olfactory stimuli during host finding. A strong orientation response of D. maidis towards yellow and green light plus maize volatiles was observed, but they observed no significant differences when a maize extract was assayed alone, without the green light stimulus, vs. a solvent control. Todd et al. (Reference Todd, Phelan and Nault1990) also proved that maize volatiles not only have an effect on orientation, but also on postcontact behaviours not associated with feeding.
In planthoppers, olfaction has been even less investigated, and selection of a host was largely thought to involve random settling of macropterous, and host plant specificity resulting because adults accumulate on favourable plants and selectively migrate from unsuitable hosts (Cook & Denno, Reference Cook, Denno, Denno and Perfect1994). Nevertheless, olfaction has been studied in three species (Obata et al., Reference Obata, Koh, Kim and Fukami1981, Reference Obata, Koh, Kim and Fukami1983; Liu et al., Reference Liu, Saxena and Wilkins1994; Youn, Reference Youn2002; Wang et al., Reference Wang, Zhou, Xin, Ji and Lou2015). Moreover, the genes involved in olfaction have been characterized in Nilaparvata lugens (Stål) (He et al., Reference He, Zhang, Liu, Zhang, Yang and Dong2011) and Sogatella furcifera (Horváth) (He et al., Reference He, Zhang and He2015). Nilaparvata lugens showed antennal responses in electrophysiological tests for several compounds, including the green leaf complex, monoterpenes such as linalool, sesquiterpenes and salicylates (Youn, Reference Youn2002). Also, the affinity of the odorant binding proteins in the antennae lumen of N. lugens was confirmed for several rice volatiles, including terpenes such as myrcene, α-pinene, limonene, β-caryophyllene and green leaf complex volatiles (He et al., Reference He, Zhang, Liu, Zhang, Yang and Dong2011). Sogatella furcifera preferred rice plants that released highest amounts of green leaf volatiles (Wang et al., Reference Wang, Zhou, Xin, Ji and Lou2015).
In this study, we analysed the VOCs emitted constitutively by three maize germplasms that could act as odour guides determining host-selection. We studied a temperate, a tropical hybrid and a landrace that were reported to have different susceptibility to CSS and MRCV (Pionner, personal communication). We compared olfactory orientation of the corn leafhopper D. maidis and the corn planthopper, P. maidis, to the suite of three maize germplasms through free choice tests in an olfactometer. We chose to exclude the influence of vision in our trials, by covering the view of the plant, in order to analyse the role of volatile cues alone. Additionally, we tested if (±)-linalool, the compound dominating the volatile profile of the temperate germplasm, possesses a role in hoppers orientation. We predicted that (i) the three maize germplasms would have different volatile profiles, (ii) D. maidis and P. maidis would discriminate the germplasms based only on their emitted VOCs and (iii) (±)-linalool is one of the volatiles involved in host-selection by the studied hopper species.
Methods and materials
Insects and maize plants
Dalbulus maidis colony was established with individuals collected in Los Nogales, Tucumán, Argentina (26°42′S–65°13′W; 588 m a.s.l.). Peregrinus maidis colony was established with individuals from Cabeza de Buey, Salta, Argentina (24°48′S–65°02′W; 771 m a.s.l.). Both colonies have been maintained in the laboratory for several years and are periodically refreshed with wild insects to avoid inbreeding. Individuals used in the behavioural tests were obtained from pathogen free colonies that were reared under laboratory conditions for at least two generations.
Adult hoppers were placed in breeding cages (50 × 50 × 50 cm), made of aluminium with the lateral and upper sides covered with nylon mesh (organdy type). Potted maize plants were used as food source and for reproduction. The maize germplasm Leales 25 Plus was used for maintenance of the colonies, and they were reared in a greenhouse at San Miguel de Tucumán (26°4835.6′S–65°1424.6′W; 500 m a.s.l.) under the following conditions: temperature between 20 and 30°C, the natural photoperiod and no humidity control.
Two maize hybrids, P1780YR (temperate), P30B39HR (tropical) and the landrace sweet white maize ‘maizón’ (SWM) were selected because we were informed by the seed supplier they have different susceptibility to CSS (Pionner, personal communication). Maize plants were planted in pots (6.3 dm3) with commercial soil and left under greenhouse conditions as described above, isolated until analysis.
Volatile collection and chemical analysis
Headspace samples were taken by enclosing intact plants with two fully unfolded leaves (V2) into a 2 litres glass recipient. Charcoal-filtered air was pushed into the recipient with an aquarium air pump and then pulled using a suction pump at a constant rate of 0.5 litres min−1. Air leaving the recipient passed through a volatile collection trap (30mg HayeSep Q) where volatiles were collected. Plants were illuminated from above with blue and red LED lamps and a high-pressure metal halide lamp, light intensity 350 µmol m−1 s−1. Seven system blanks were also performed in order to exclude contaminant products from the list of volatiles. After a sampling period of 6 h (between 10:00 and 16:00 h), the volatile collection traps were eluted with 150 µl of dichloromethane containing 5 ng of dodecane as an internal standard.
Volatile samples were analysed by coupled gas chromatography-mass spectrometry (GC/MS) (Agilent 7890 instrument coupled with an Agilent 5977 selective mass detector). A DB5MS capillary column was used (0.25-mm i.d., film thickness 0.25 µm). Samples (1 µl) were injected at 240°C in a splitless mode. Helium was used as a carrier gas at 0.75 ml min−1. The column temperature was held at 35°C for 1 min, and then increased at a rate of 5°C min−1 until it reached 100°C, then 12°C min−1 until 230°C. Finally, the temperature was held at 230°C for 10 min. Compounds were identified by computer matching with commercial mass spectra libraries (NBS75K, NIST 98, WILEY275) and published data (Adams, Reference Adams2007); comparison of their kovats retention index on a DB5MS column and by comparison of retention times with authentic standards: (±)-linalool, d-limonene, β-myrcene, β-cis-ocimene, trans-β-caryophyllene, β-elemene, α-humulene, nerolidol and the alkane series. Data were collected with ChemStation software (Hewlett-Packard) and the detected volatiles were quantified on the basis of their peak area in comparison with the area of the internal standard.
Olfactometer bioassays
The attractiveness of D. maidis females (3–6 days old) and macropterous P. maidis females (3–6 days old) to volatile compounds released by V2 maize plants was evaluated in olfactory dual choice tests. Behavioural recordings were carried out using static olfactometers (no air flow), with the odour sources placed in opposite directions and releasing one female at a time. The system consisted of a central releasing chamber (5.5 × 5.5 × 2 cm) and two opposite side arms (9 cm long, 1 cm diameter) connected to glass containers (20 × 5 × 5 cm) that housed a whole plant of a given maize germplasm (Supplementary material). The pots with commercial soil were left out of the glass container, and that side of the container was closed with a plastic guillotine. The side of the glass container facing the central releasing chamber was white to avoid the use of visual cues. Bioassays were performed between 9 AM and 5 PM hours under laboratory conditions (25°C, 50–70% RH illuminated from above with LED tubes). Several tests were conducted in parallel, with blank experiments running simultaneously. Olfactometers were cleaned with acetone after each experiment and the maize plants were replaced for every new replicate. Dalbulus maidis females were starved for 2 h before the assays. Peregrinus maidis females were left overnight for the same purpose. Bioassays lasted 1 h for D. maidis (under continuous observation), and 8 h for P. maidis (checking the experiment every 2 h). The time spent in the central part of the olfactometer, where insects were released, was also recorded for D. maidis. The first choice of each female was recorded when it moved more than 4 cm towards the odour source in the olfactometer arm and did not return to the central part, otherwise it was scored as ‘no choice’. In both cases, insects could reach the plant if they passed the connecting tube, but D. maidis females were removed when they reached the end of the connecting tube, shortly after they made a choice, because they tended to move fast to the odour source. At least 40 replicates were performed for D. maidis and 30 replicates for P. maidis. A control experiment was also performed contrasting maize plants (SWM) vs. air, with 38 replicates for D. maidis and 23 replicates for P. maidis.
Bioassay with linalool
This compound was assayed alone since it is released in highest amounts by the temperate and SWM germplasms. (±)-Linalool was prepared from linalyl acetate according to Theodorou et al. (Reference Theodorou, Skobridis, Tzakos and Ragoussis2007). Briefly, 300 mg of linalyl acetate where dissolved in 6 ml of a solution of methylene chloride (CH2Cl2) and methanol (MeOH) 90:10. A solution of 3 N NaOH was added and left to react under continuous stirring. After 4 h, solvents were evaporated under vacuum, and the residue was resuspended in 5 ml of H2O and extracted four times with CH2Cl2. The organic extracts were reunited, filtrated and evaporated to yield 230 mg of (±)-linalool, confirmed by GC/MS analysis.
A 0.0001% solution of (±)-linalool was prepared in ethyl acetate and 100 µl were applied on filter paper disks for bioassay purposes. In a preliminary test, one of these filter paper discs was placed together with one plant of the tropical hybrid inside a 2 litre glass recipient to collect volatiles as previously described. Extracts were then analysed by GC/MS. This test confirmed that the release of linalool by filter paper was in accordance with the amounts of linalool released by the temperate maize plants (Supplementary material). Two kinds of assays were performed with this solution, linalool against solvent blanks and the tropical maize germplasm plus linalool vs tropical maize plus solvent. The two choice bioassays were conducted as previously described with both hopper species.
Statistical analyses
For VOCs analysis, peaks that appeared in the system blanks were discarded. VOC data means were compared with one-way analysis of variance (ANOVA) after normalization to log-normal distributions (P < 0.05). Homogeneity of variance and normality were checked using Bartlett's and Shapiro–Wilk tests, respectively. When a given compound was not detected in one of the germplasms, data were analysed by means of unpaired t-test between two germplasms. Mean pairwise comparisons were conducted using Tukey honestly significant difference. If ANOVA assumptions were not met, Kruskal–Wallis test was performed. All the compound classes (aromatic hydrocarbons, monoterpenes, sesquiterpenes, aliphatic aldehyde, ketone, alkanes, homoterpenes and salicylates) were used as variables to perform principal component analysis (PCA). Female choices in the olfactometer were compared by Chi-square goodness-of-fit test (χ2) (P < 0.05) using XLSTAT® 19.6.
Results
Chemical characterization
The volatile fraction collected by headspace sampling of V2 maize plants allowed the identification of several substances and the characterization of the VOC pattern of each maize germplasm. All three types of maize differed qualitatively and quantitatively in their constitutive volatile compounds. We identified 42 VOCs in detectable amounts in at least four samples of a germplasm (table 1). There were differences in the total amounts of volatiles released (F = 3.8, df = 218, P = 0.042). The temperate hybrid released significantly more VOCs than the tropical hybrid (P = 0.037). SWM was not significantly different from both hybrids (P > 0.050) (fig. 1). VOCs fell into seven different categories: aromatic hydrocarbons, monoterpenes, sesquiterpenes, aliphatic, alkanes, homoterpenes and salicylates. We found 22 common volatiles. In multiple comparisons between the three germplasms, the release of two VOCs was significantly different; (±)-linalool (F = 66.902, df = 218, P < 0.001) (fig. 1) and nerolidol (F = 4.008, df = 218, P = 0.036). Linalool was released in highest amounts by the temperate hybrid and SWM (table 1). The release of the following compounds was not significantly different between SWM and the temperate hybrid (t-test, P > 0.05), but they were not detected in the tropical hybrid: β-myrcene, methyl salicylate, decanal, unknown 6, tridecane, β-elemene, caryophyllene, β-gurjunene, geranyl acetone, α-humulene, ar-curcumene, α-muurolene, 2-isopropyl-5-methyl-9-methylenebicyclo[4.4.0]dec-1-ene, (3E,7E)-4,8,12-trimethyl-1,3,7,11-decatetraene (TMTT), cadalene, eicosane and unknown 7 (table 1).

Fig. 1. (a) Total VOCs emitted constitutively by SWM and the temperate and tropical maize hybrids. (b) Amount of linalool emitted by the three germplasms. Bars represent SD.
Table 1. Volatiles emitted constitutively by the three maize germplasms (N = 7).

DMNT, (3E)-4,8-dimethyl-13,7-nonatriene; TMTT, (3E,7E)-4,8,12-trimethyl-1,3,7,11-decatetraene.
Differences between germplasms were determined using one-way ANOVA after normalization to log-normal distributions. Mean pairwise comparisons were conducted using Tukey honestly significant difference. Means followed by the same letter within a line are not significantly different (P < 0.05) (±SE). Data expressed as ng h−1. RI = Kovats retention index determined according to n-alkanes on a DB-5MS capillary column. Identification based on mass spectra (MS), retention index and authentic standards (S).
a Coelution of another sesquiterpene alcohol (MW 220, C15H24O).
b Tentative identification.
PCA explained 81.9% of the total variation (fig. 2). Temperate maize was positively correlated with a greater abundance of monoterpenes, homoterpenes and methyl salicylate, whereas SWM was positively correlated with sesquiterpenes, alkanes and aromatic compounds. Tropical maize, on the other hand, was not related to monoterpenes, sesquiterpenes or homoterpenes (fig. 2). The first axis of PCA accounted for 56.2% of the total variation (eigenvalue 3.37) and was positively correlated with all the compound classes. The second component explained 25.7% of the variation (eigenvalue 1.54) and was related to monoterpenes, homoterpenes and salicylates, and negatively related to alkanes, sesquiterpenes and aromatic compounds.
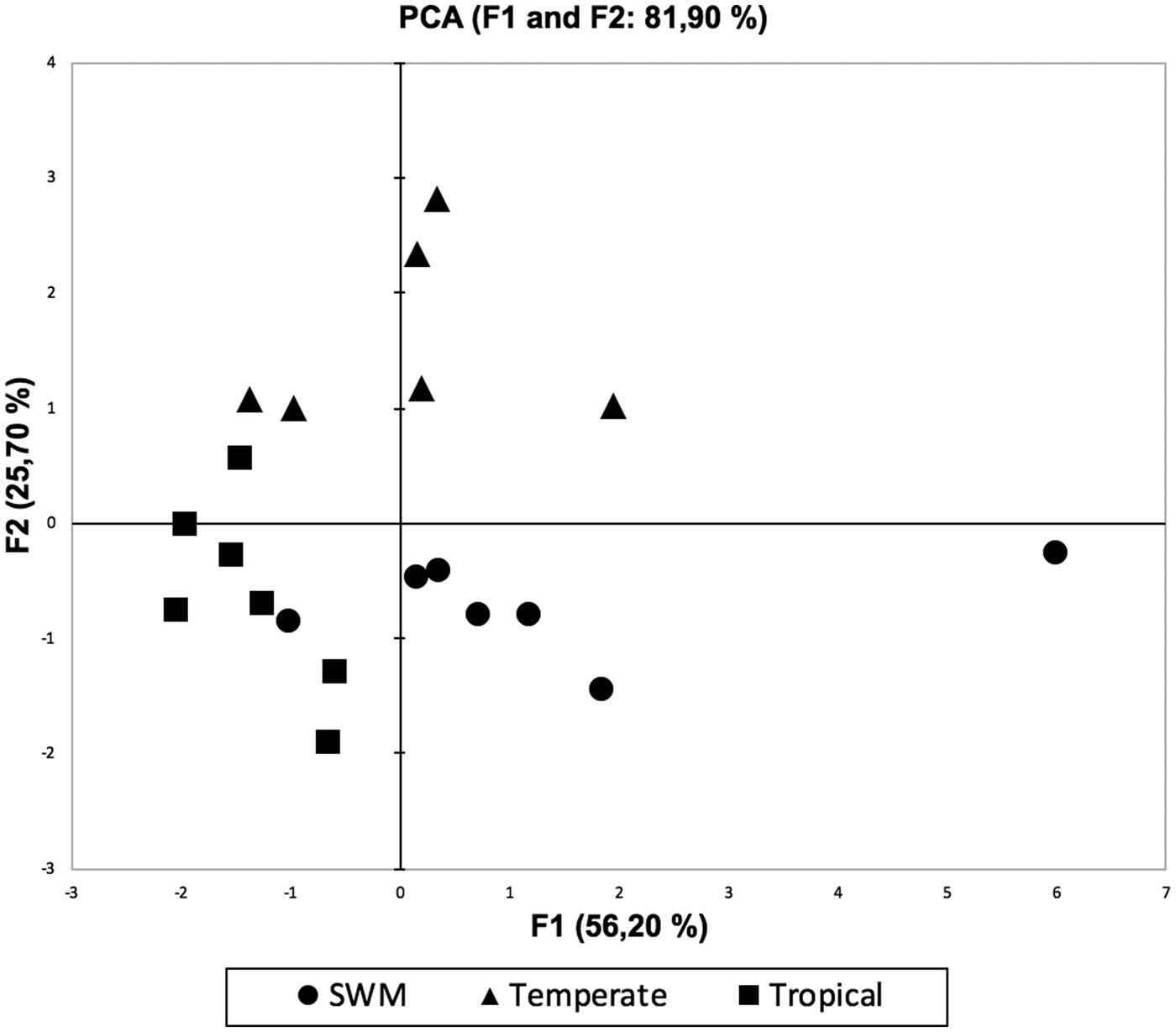
Fig. 2. PCA representing the association among the 21 cases belonging to three maize germplasms on relative amounts of odours emitted. The first two axes account for 56.2 and 25.7% of the total variation.
Olfactometer bioassays
A majority of the females from both species, D. maidis and P. maidis, made a choice for the olfactometer arm with the odour source consisting on a maize plant. When offered a maize plant vs. clean air, D. maidis females spent an average of 20.5 min (±14.8) on the central part of the olfactometer, where insects were released, and more than 70% preferred the side with the maize plant (χ2 = 6.125, df = 1, P = 0.013). If no such odour is offered, most D. maidis females remained in the central releasing chamber during the 60-min test period. Peregrinus maidis females also preferred the side with the plant (χ2 = 4.545, df = 1, P = 0.033), but they spent a much longer time in the centre of the olfactometer until they made a choice for an olfactometer arm; 50% remained in the central part after 4 h, and by 8 h, 72.7% have moved to the odour plant source, while 27.3% had moved to the side with no plant.
When offered two different germplasms in each side of the olfactometer, D. maidis preferred seedlings with temperate genetic background (73.7%) instead of tropical background (χ2 = 8.526, df = 1, P = 0.003), and did not discriminate between SWM and tropical maize (χ2 = 0.105, df = 1, P = 0.746) or SWM and the temperate hybrid (χ2 = 0.947, df = 1, P = 0.330) (fig. 3).
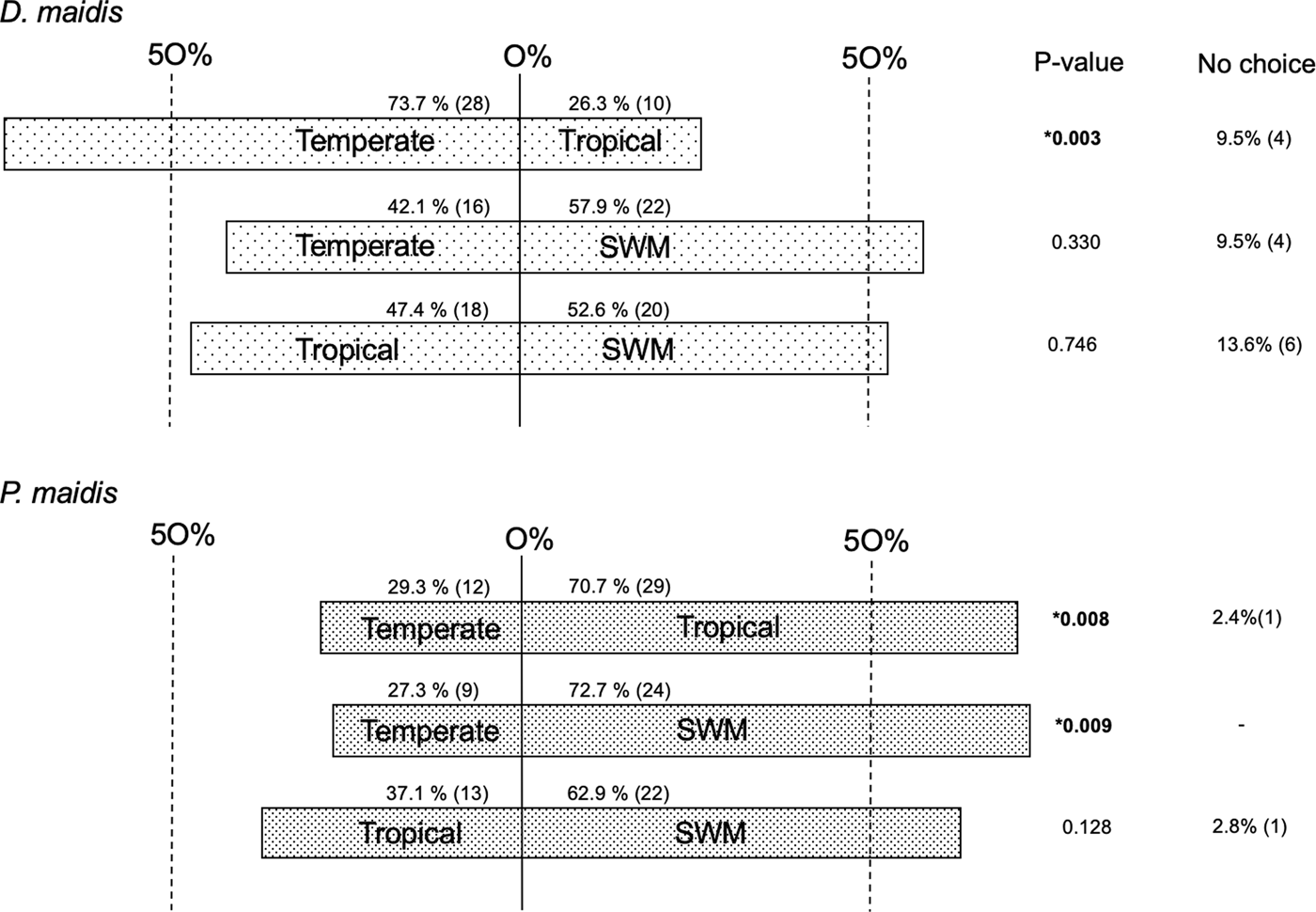
Fig. 3. First choice (%) between different germplasms in olfactometer. Chi-square goodness-of-fit test (χ2) *P < 0.05.
On the other hand, P. maidis chose seedlings with tropical genetic background (70.7%) over temperate maize (χ2 = 7.048, df = 1, P = 0.008); significantly chose SWM (72.7%) over temperate (χ2 = 6.818, df = 1, P = 0.009) and there was a slight preference for SWM (62.9%) over tropical maize although not significant (χ2 = 2.314, df = 1, P = 0.128) (fig. 3).
Bioassays with linalool
(±)-Linalool was assayed alone since it is released in differential amounts for all three genotypes (higher for temperate, lower for SMW and almost null for tropical) (table 1, fig. 1). A pure 0.0001% linalool solution failed to produce a behavioural effect both on D. maidis (χ2 = 0.133, df = 1, P = 0.715), and on P. maidis (χ2 = 0.676, df = 1, P = 0.411) (fig. 4). But, when both species were offered the 0.0001% linalool solution plus the tropical hybrid, providing other maize contextual cues, D. maidis chose the tropical hybrid plus linalool (χ2 = 4.000, df = 1, P = 0.045), whereas P. maidis had a slight preference, although not significant, for the tropical hybrid plus solvent (χ2 = 2.000, df = 1, P = 0.157) (fig. 4).
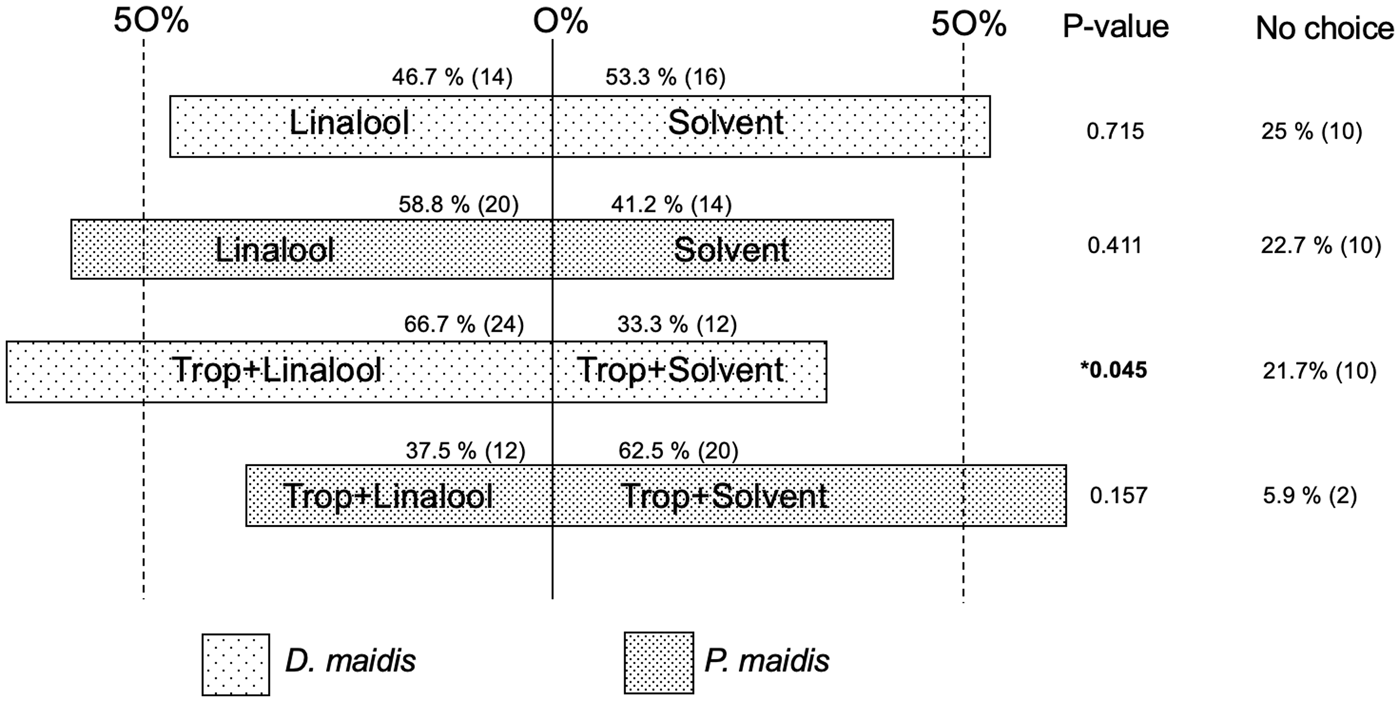
Fig. 4. First choice (%) in olfactometer between 0.0001% linalool vs. solvent and tropical hybrid plus 0.0001% linalool vs. tropical hybrid plus solvent. Chi-square goodness-of-fit test (χ2) *P < 0.05.
Discussion
Analysis of the VOCs collected from the three maize germplasms showed quantitative and qualitative variations in the composition of the blend. Both hopper species, D. maidis and P. maidis were able to discriminate between the two maize hybrids by the use of volatile cues in the absence of supplementary visual cues.
The temperate and the tropical hybrid differed significantly in the total amount of volatiles released. SWM was not significantly different in terms of VOCs production from both hybrids. All the identified VOCs were previously cited for maize (Krokos et al., Reference Krokos, Konstantopoulou and Mazomenos2002; Oluwafemi et al., Reference Oluwafemi, Birkett, Caulfield and Pickett2012; Robert et al., Reference Robert, Erb, Hiltpold, Hibbard, Gaillard, Bilat, Degenhardt, Cambet-Petit-Jean, Turlings and Zwahlen2013; Molnár et al., Reference Molnár, Tóth, Fejes-Tóth, Dekker and Kárpáti2015; Potter et al., Reference Potter, Olson, Ni and Rains2015). The temperate hybrid was very different from the tropical hybrid in qualitative volatile composition, particularly because the tropical hybrid lacked many compounds that were present in the temperate one.
As expected, tropical maize plants presented low variability between individuals in terms of VOCs quantities and the identity of the compounds released. SWM, on the other hand, had more variability between individuals, since landraces are crop cultivars that are usually genetically diverse and have been selected by local environments and farmers over many generations (Davila-Flores et al., Reference Davila-Flores, DeWitt and Bernal2013). Surprisingly, the temperate hybrid presented considerable variability between individuals. Hybrids are the result of selective breeding programmes and are usually unable to produce viable seeds after a couple of generations. It is possible that this hybrid has retained a high degree of genetic variability during its breeding programme.
The corn leafhopper, D. maidis, has a long history of coevolution with maize ancestors, the teosintes and maize landraces in tropical environments (Nault, Reference Nault1983). Tropical hybrids are developed from these landraces that have been subjected to high CSS and herbivore pressures and they are supposed to be more resistant to CSS, which was associated, at least partially, to lower incidence of disease vectors (Carpane & Ingrassia, Reference Carpane and Ingrassia2012). On the other hand, for the more polyphagous corn planthopper, P. maidis, sorghum would be the ancestral host and P. maidis would have adapted only in post-Columbian times to maize as a host (Nault, Reference Nault1983).
Based on the origin of the germplasm and also on information from the seed supplier, we expected the tropical hybrid to be the less preferred by the corn leafhopper. Accordingly, D. maidis chose the temperate hybrid over the tropical hybrid but failed to discriminate between the temperate hybrid and SWM and the tropical hybrid and SWM. The most abundant monoterpene released by the temperate hybrid is (±)-linalool. Other monoterpenes such as α-pinene and camphene are known to be active towards the leafhopper Amrasca devastans (Distant) (Saxena & Saxena, Reference Saxena and Saxena1974). Dalbulus maidis was not attracted by a pure 0.0001% linalool solution, in the order of magnitude released by the temperate hybrid. This result suggested that other contextual cues could be necessary to obtain a response, so the tropical hybrid plus linalool and the tropical hybrid plus solvent were contrasted, and this time the tropical hybrid plus 0.0001% linalool was significantly preferred. The temperate hybrid could be more attractant to the leafhopper than the tropical hybrid because of a blend of compounds, that includes linalool. In that case, the tropical hybrid would be less preferred because of decreased attraction, that could be an adaptation in an environment with high D. maidis pressures.
Peregrinus maidis females, chose the tropical hybrid and SWM over the temperate hybrid. As mentioned before, this last hybrid releases higher amounts of monoterpenes (β-myrcene, β-cis-ocimene and linalool) and also homoterpenes. Many terpenoids are bitter defence compounds. Particularly, (E)-caryophyllene, TMTT and methyl salicylate, all present in the temperate hybrid but not in the tropical hybrid, were previously identified as volatile semiochemicals involved in plant defence against other sucking insect pests, including the leafhopper C. storeyi (Oluwafemi et al., Reference Oluwafemi, Bruce, Pickett, Ton and Birkett2011). The more generalist P. maidis would choose the less defended tropical hybrid. SWM might also attract P. maidis being chemically similar to the tropical hybrid. When the 0.0001% linalool solution in combination with the tropical hybrid was offered to P. maidis, the side with no added linalool was slightly preferred, but other compounds could also be responsible for making the temperate hybrid less attractive to the planthopper.
Most P. maidis females responded after at least 4 h in the olfactometer, even after being starved for at least 14 h. This behaviour could be related to the ecological role of macropterous females that can spend several hours without feeding, in search for a suitable host. D. maidis females made their choices faster, usually about 20 min after being released. The lack of a visual stimulus could be responsible for the long time both insects spent in the olfactometer.
Although most of the literature suggest that the role of olfactory cues in long-range orientation within Auchenorrhyncha is likely to be supplementary to visual cues (Todd et al., Reference Todd, Phelan and Nault1990; Fereres & Moreno, Reference Fereres and Moreno2009; La Grange et al., Reference La Grange, Schröder, Glinwood, Ignell and Krüger2017; Zhang et al., Reference Zhang, Pengsakul, Tukayo, Yu, Fang and Luo2017), our results suggest that olfaction might play a role in host selection within short distances, where responsiveness to other host plant cues might arise. Therefore, both D. maidis and P. maidis could also select their host plants based on the emitted VOCs. Ultimately, there is potential to develop a behavioural manipulation method in agronomic practices, such as trap plants or a push–pull strategy, where volatile cues are used to push insects away from important crop plants and attract them to a trap or trap crop (Cook et al., Reference Cook, Khan and Pickett2007; Hassanali et al., Reference Hassanali, Herren, Khan, Pickett and Woodcck2008), combining visual and olfactory cues.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S000748531900004X.
Acknowledgements
The authors wish to thank Miss Barbara Salina, who helped collecting behavioural data. This research was supported by ANPCyT (Prestamo BID PICT 2015-1147 and partially by PICT 2014-0607).








