Ceratopsid dinosaurs (the ‘horned dinosaurs’) are a ubiquitous component of Campanian and Maastrichtian terrestrial faunas in western North America. Our knowledge of the diversity of these animals has greatly expanded in recent years, with the discovery of derived non-ceratopsid neoceratopsians as well as new ceratopsids (Chasmosaurinae and Centrosaurinae) (Sampson Reference Sampson1995; Wolfe & Kirkland Reference Wolfe, Kirkland, Lucas, Kirkland and Estep1998; Ryan & Russell Reference Ryan and Russell2005; Ryan Reference Ryan2007; Wu et al. Reference Wu, Brinkman, Eberth and Braman2007; Currie et al. Reference Currie, Langston and Tanke2008; Kirkland & DeBlieux Reference Kirkland, DeBlieux, Ryan, Chinnery-Allgeier and Eberth2010; Loewen et al. Reference Loewen, Sampson, Lund, Farke, Aguillón-Martínez, de Leon, Rodríguez-de la Rosa, Getty, Eberth, Ryan, Chinnery-Allgeier and Eberth2010; McDonald & Horner Reference McDonald, Horner, Ryan, Chinnery-Allgeier and Eberth2010; Ott & Larson Reference Ott, Larson, Ryan, Chinnery-Allgeier and Eberth2010; Ryan et al. Reference Ryan, Eberth, Brinkman, Currie, Tanke, Ryan, Chinnery-Allgeier and Eberth2010; Sampson et al. Reference Sampson, Loewen, Farke, Roberts, Forster, Smith and Titus2010; Sampson & Loewen Reference Sampson, Loewen, Ryan, Chinnery-Allgeier and Eberth2010; Sullivan & Lucas Reference Sullivan, Lucas, Ryan, Chinnery-Allgeier and Eberth2010; Xu et al. Reference Xu, Wang, Zhao and Li2010; Farke et al. Reference Farke, Ryan, Barrett, Tanke, Braman, Loewen and Graham2011; Fiorillo & Tykoski Reference Fiorillo and Tykoski2012). The increased number of recognised ceratopsid taxa has also provided the opportunity for better phylogenetic reconstruction and character evolution mapping within the lineage.
It is generally accepted that craniofacial and frill morphology provides the most information for determining ceratopsid relationships. The pattern of nasal horns or bosses, supraorbital horns or bosses, and parietal and squamosal marginal ossifications and processes garners the greatest amount of attention when establishing and identifying ceratopsid taxa. A system of nomenclatural ‘standards’ has developed for identifying and describing potentially homologous frill processes (Sampson Reference Sampson1995; Farke et al. Reference Farke, Ryan, Barrett, Tanke, Braman, Loewen and Graham2011), which has allowed greater focus on cranial ornamentation as taxonomic identifiers in these fossil forms. As a result, there has been less of a concerted effort on other aspects of cranial anatomy that could provide phylogenetic and taxonomic data.
Another cranial region that exhibits potentially useful morphological information in many vertebrates is the braincase and endocranial cavity. The braincase in mature ceratopsids is a robust structure with a high fossilisation potential. Even so, few neoceratopsian braincases have been described relative to the number of known taxa, as pointed out by Witmer & Ridgely (Reference Witmer, Ridgely, Currie, Langston and Tanke2008). For many years, the only braincase or endocranial descriptions were for the basal neoceratopsian Protoceratops (Brown & Schlaikjer Reference Brown and Schlaikjer1940), and the chasmosaurine ceratopsids Anchiceratops (Brown Reference Brown1914) and Triceratops (Marsh Reference Marsh1891, Reference Marsh1896; Hatcher et al. Reference Hatcher, Marsh and Lull1907; Hay Reference Hay1909; Forster Reference Forster1996).
The first detailed description of a centrosaurine ceratopsid braincase was made by Langston (Reference Langston1975) in his seminal work on Pachyrhinosaurus canadensis. The lack of comparative material at the time was noted by Langston (Reference Langston1975, p. 1588) who stated, “Although the braincase of Triceratops has been extensively interpreted, and description of the brains of Anchiceratops and Protoceratops exist, there appears to be no useful account of a braincase from a short-faced ceratopsid in the literature, nor do I have a specimen for comparison.” A cursory description of this region of Centrosaurus apertus was only recently made (Dodson et al. Reference Dodson, Forster, Sampson, Weishampel, Dodson and Osmolska2004). A second Pachyrhinosaurus species, P. lakustai, was recently erected based upon numerous specimens from multiple individuals (Currie et al. Reference Currie, Langston and Tanke2008). Several braincases were preserved with the sample, and one of these was the subject of the first CT-based examination, visualization, and description of a ceratopsid braincase (Witmer & Ridgely Reference Witmer, Ridgely, Currie, Langston and Tanke2008). The exceptional 3-dimensional reconstructions of both external braincase and internal endocranial morphology in the latter work provided a detailed resource for additional comparative work. For a volume commemorating the career of Wann Langston, Jr., it is only fitting then that we take this opportunity to build on Langston's original work regarding the braincase anatomy of what has become an increasingly important ceratopsian.
Ceratopsid remains collected from the North Slope of Alaska in the 1980s were referred to Pachyrhinosaurus because of the presence of an enlarged nasal boss present on some specimens. A second locality along the Colville River, known as the Kikak–Tegoseak Quarry, later produced large numbers of ceratopsid specimens from a mono-dominant bonebed (Fiorillo et al. Reference Fiorillo, McCarthy, Flaig, Brandlen, Norton, Zippi, Jacobs, Gangloff, Ryan, Chinnery-Allgeier and Eberth2010a; Fiorillo & Tykoski Reference Fiorillo and Tykoski2012). Cranio-facial material from the site bore very large nasal and supraorbital bosses, leading to assignment of the Kikak–Tegoseak specimens to Pachyrhinosaurus as well (Fiorillo et al. Reference Fiorillo, McCarthy, Flaig, Brandlen, Norton, Zippi, Jacobs, Gangloff, Ryan, Chinnery-Allgeier and Eberth2010a). Incomplete parietal frill pieces preserved unique small horns that project anteriorly and slightly dorsally from the anterior margin of the transverse parietal bar, a locus unknown to give rise to frill ornamentation in any other ceratopsid. This, in combination with a mix of cranio-facial features shared by P. canadensis and P. lakustai, lead to the establishment of the Alaska material to a third Pachyrhinosaurus species, P. perotorum (Fiorillo & Tykoski Reference Fiorillo and Tykoski2012). A phylogenetic analysis of centrosaurine ceratopsids that treated the three species as separate operational taxonomic units was unable to fully resolve relationships between the three species (Fiorillo & Tykoski Reference Fiorillo and Tykoski2012). A minimum of ten individuals are represented in the quarry based upon partial braincases ranging from isolated, broken occipital condyles to an almost complete braincase preserved in articulation with a partial skull (DMNH 22558).
Here we provide a description of the braincase for Pachyrhinosaurus perotorum, based upon that in the skull DMNH 22558, as well as additional specimens that preserve anatomy not visible in the former. In addition, the braincase of DMNH 22558 was scanned at The University of Texas High-Resolution X-ray CT Facility at the University of Texas at Austin. The original purpose of the scan was to look for signs of relative ontogenetic development in the basicranial region, but it also provided an opportunity to compare internal and endocranial anatomy to that in the closely related species, P. canadensis and P. lakustai (Langston Reference Langston1975; Witmer & Ridgely Reference Witmer, Ridgely, Currie, Langston and Tanke2008). Comparison of braincase anatomy in the three species of Pachyrhinosaurus provides a test of whether braincase anatomy can be a tool to show species-level differences in ceratopsids, or whether we must remain reliant on craniofacial and frill characters to distinguish closely-related ceratopsid taxa.
1. Materials and methods
1.1. Geological and palaeontological background
The Prince Creek Formation spans from the Cretaceous to the Palaeocene, and the Maastrichtian-aged rocks within the formation contain the densest known concentrations of dinosaur bones of any high latitude rock unit in both the northern and southern hemispheres (Rich et al. Reference Rich, Vickers-Rich and Gangloff2002). This rock consists largely of fluviatile sediments shed from the uplift of the Brooks Range during the Cretaceous with fossil soils that the floodplains were influenced by seasonally fluctuating water table levels (Fiorillo et al. Reference Fiorillo, McCarthy, Flaig, Brandlen, Norton, Zippi, Jacobs, Gangloff, Ryan, Chinnery-Allgeier and Eberth2010a; Flaig et al. Reference Flaig, McCarthy, Fiorillo, Davidson, Leleu and North2011). Whereas initial reports of Alaskan dinosaurs were of a general taxonomic nature (Brouwers et al. Reference Brouwers, Clemens, Spicer, Ager, Carter and Sliter1987; Parrish et al. Reference Parrish, Parrish, Hutchinson and Spicer1987), details of this dinosaur fauna are growing and now includes small and large theropods, hypsilophodontids, hadrosaurs, pachycephalosaurs, and ceratopsians (Davies Reference Davies1987; Parrish et al. Reference Parrish, Parrish, Hutchinson and Spicer1987; Fiorillo & Gangloff Reference Fiorillo and Gangloff2000, Reference Fiorillo and Gangloff2001; Gangloff et al. Reference Gangloff, Fiorillo and Norton2005; Fiorillo Reference Fiorillo2008a, Reference Fiorillo, Blodgett and Stanleyb; Fiorillo et al. Reference Fiorillo, Tykoski, Currie, McCarthy and Flaig2009, Reference Fiorillo, McCarthy, Flaig, Brandlen, Norton, Zippi, Jacobs, Gangloff, Ryan, Chinnery-Allgeier and Eberth2010a; Gangloff & Fiorillo Reference Gangloff and Fiorillo2010; Brown & Druckenmiller Reference Brown and Druckenmiller2011; Fiorillo & Tykoski Reference Fiorillo and Tykoski2012). In addition to the reports of these remains of fossil vertebrates, the Prince Creek Formation is now recognised to also contain a variety of invertebrate fossils and trace fossils, including commonly occurring horizons that exhibit trampling by dinosaurs (Flaig et al. Reference Flaig, McCarthy, Fiorillo, Davidson, Leleu and North2011).
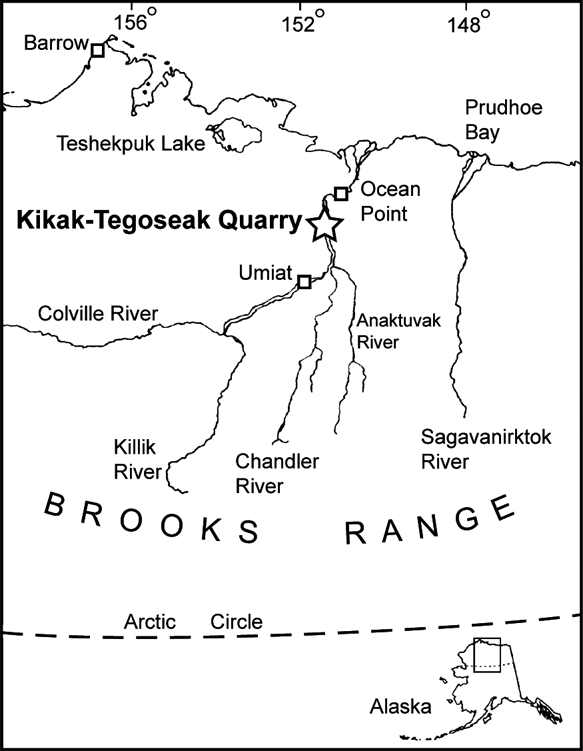
Though rich in terms of fossil yield, the dinosaur fauna from the Prince Creek Formation shows modest taxonomic diversity when compared to similarly aged faunas found in the lower latitudes (Fiorillo & Gangloff Reference Fiorillo and Gangloff2000; Fiorillo Reference Fiorillo2004). Additional elements of the fossil vertebrate faunas from the Prince Creek Formation include fossil fishes and mammals (Clemens & Nelms Reference Clemens and Nelms1993). The specimens described here are from the Kikak–Tegoseak Quarry, a site dominated by ceratopsian remains that has produced hundreds of bones from a bluff along the Colville River in northern Alaska (Fig. 1). The stratigraphic and sedimentologic setting of the quarry has been described elsewhere (Fiorillo et al. Reference Fiorillo, McCarthy, Flaig, Brandlen, Norton, Zippi, Jacobs, Gangloff, Ryan, Chinnery-Allgeier and Eberth2010a, Flaig et al. Reference Flaig, McCarthy, Fiorillo, Davidson, Leleu and North2011).

Figure 1 Map showing location of the Kikak–Tegoseak Quarry, North Slope, Alaska, USA. Quarry location indicated by large open star.
The fossil localities in this rock unit are compelling because not only are they at high latitudes today, but given the rotation of specific tectonic plates and the assembling of eastern Asia and northwestern North America, the Prince Creek Formation was deposited at latitudes similar to, or higher than the current geographic position (Witte et al. Reference Witte, Stone, Mull, Tailleur and Weimer1987; Lawver et al. Reference Lawver, Grantz, Gahagan, Miller, Grantz and Klemperer2002). Whereas the region now known as Alaska has been considered the gateway for faunal communication between Asia and North America (Russell Reference Russell1993; Sereno Reference Sereno, Benton, Shishkin, Unwin and Kurochkin2000; Fiorillo Reference Fiorillo2008a; Zanno Reference Zanno2010; Fiorillo & Adams Reference Fiorillo and Adams2012), and this gateway was probably established at least 108 Ma (Fiorillo et al. Reference Fiorillo, Decker, LePain, Wartes and McCarthy2010b), the Prince Creek Formation records this exchange in the greatest detail.
1.2. Specimens
The Kikak–Tegoseak Quarry has produced a large number of disarticulated Pachyrhinosaurus perotorum specimens. At least ten individuals are represented in the quarry, based upon the number of partial braincases and occipital condyles found to date (Table 1). There is some range in size between individuals, based upon the maximum diameter of the occipital condyles (Table 1). Even so, all the occipital condyles show complete fusion between the basioccipital and exoccipitals, indicating these specimens had all reached a degree of osteological maturity by the time of death.
Table 1 List of ceratopsid occipital condyles and partial braincases currently known from the Kikak–Tegoseak Quarry, North Slope, Alaska. Maximum diameter of the occipital condyle of each specimen is given where measureable.

1.3. CT scan specifications
The braincase of DMNH 22558, a partial skull missing right cheek region and all of the parietal-squamosal frill, was removed during preparation, reassembled, and then CT scanned at the University of Texas High-Resolution X-ray CT Facility at The University of Texas at Austin. Scan settings and parameters were: 1024×1024 16-bit TIFF images, 450kV, 3 mA, 1 brass filter, and maximum field of view of 270·9265 mm. Scan slice thickness was 0·75 mm, with inter-slice spacing of 0·7 mm, with 243 total slices. Correction applied to field of reconstruction. Streak- and ring-removal processing based on correction of raw sinogram data using IDL routines “RK_SinoDeStreak” with default parameters, and “RK_SinoRingProcSimul”. Our processing and viewing of the data was based upon 8-bit JPG versions of the original scan slices. The original scan data is currently archived in the collection of the Perot Museum of Nature and Science. The individual CT scan slices were initially packaged into simple ‘stacks’ of images using ImageJ, a free downloadable software available at the U.S. National Institute of Health (Rasband Reference Rasband1997–2011). This allowed easy scrolling through the stack of slices to better visualise the paths and internal features revealed by the scans. It was then decided that three dimensional renderings of the endocranial spaces were necessary in order to adequately assess the available data and compare them to other ceratopsids. Because of limitations of our available computing hardware and software, we utilized the 3D modelling plug-in capability of ImageJ to generate volumetric renderings of the endocranium and endosseous labyrinth.
Institutional abbreviations. AK, University of Alaska, Fairbanks; AMNH, American Museum of Natural History; NMC, Canadian Museum of Nature; DMNH, Perot Museum of Nature and Science; TMP, Royal Tyrrell Museum of Paleontology.
2. Description
Description of overall braincase morphology is based mostly upon the articulated braincase of a partial skull (DMNH 22558), and a disarticulated specimen (DMNH 22194) with several broken surfaces that expose the traces of nerves and semicircular canals through the bones. The left side of the braincase in DMNH 22558 is hidden from view, and the right side is partially covered by an array of broken and incomplete bony elements of uncertain identity. As with other osteologically mature (i.e. ‘adult’) ceratopsids, the bones of the braincase are extensively fused in all specimens. This makes discerning bone to bone contacts difficult, and forces reliance on some assumptions of homology and association based on other taxa.
There is a range of different sized individuals represented in the sample, based upon measurements of the maximum diameter of the occipital condyle (Table 1), but none of the specimens exhibits discernible sutures between the exoccipitals and basiocciptial. Only DMNH 22558 preserves the braincase in articulation with a particular skull at this time (Fig. 2), although several large blocks from the quarry are yet to be prepared and may contain additional skulls. The occipital condyle of DMNH 22558 is not the largest in the sample, but the skull exhibits high degree of nasal and supraorbital boss development. Given hypotheses that ceratopsid craniofacial ornamentation is most dramatically expressed at or after the onset of reproductive maturity (Sampson et al. Reference Sampson, Ryan and Tanke1997; Currie et al. Reference Currie, Langston and Tanke2008), this suggests DMNH 22558 had reached reproductive maturity by the time of death.
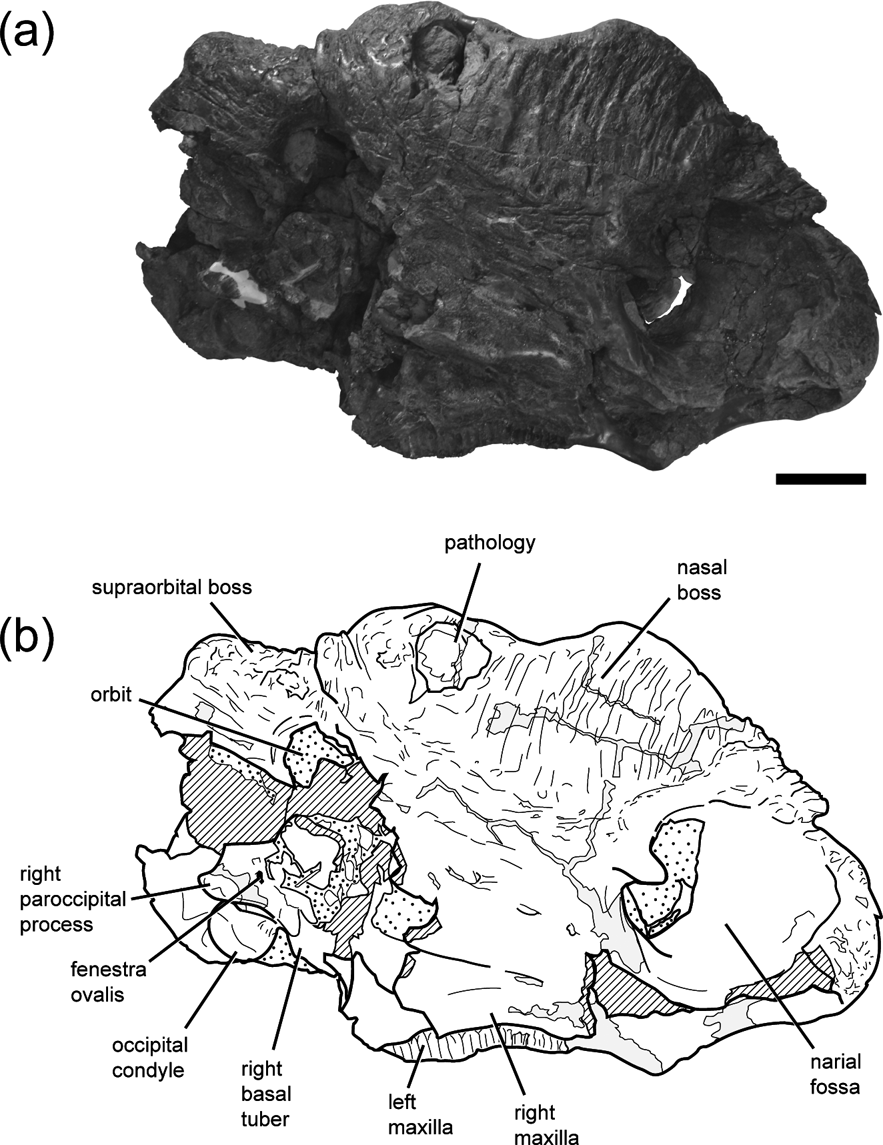
Figure 2 Photograph (a) and line drawing (b) of DMNH 22558, Pachyrhinosurus perotorum partial skull in right lateral view, showing orientation of the articulated braincase. Scale bar=10 cm. Cross-hatching indicates broken bone surfaces. Grey fill indicates reconstructed areas. Stippled areas indicate rock still visible in specimen.
2.1. External anatomy
2.1.1. Occipital region
The occipital condyle is large, subspherical, and set off from the occipital plate by a pronounced condylar neck. The neck and condyle angle posteroventrally approximately 45 degrees relative to the maxillary alveolar margin when the skull is oriented with the alveolar border at horizontal. This would tend to give the skull a neutral ‘nose-down’ attitude in life, and orients the planes of the occipital plate and maxillary alveolar margins nearly perpendicular to each other. The basal tubera of the basiocciptal are very large with a rugose texture on the ventral and ventrolateral surfaces (Fig. 3b, c, d). The width of the occipital condyle in DMNH 22194 is more than half the mediolateral width across the combined basal tubera, although the specimen has been crushed in an oblique dorsoventral manner.
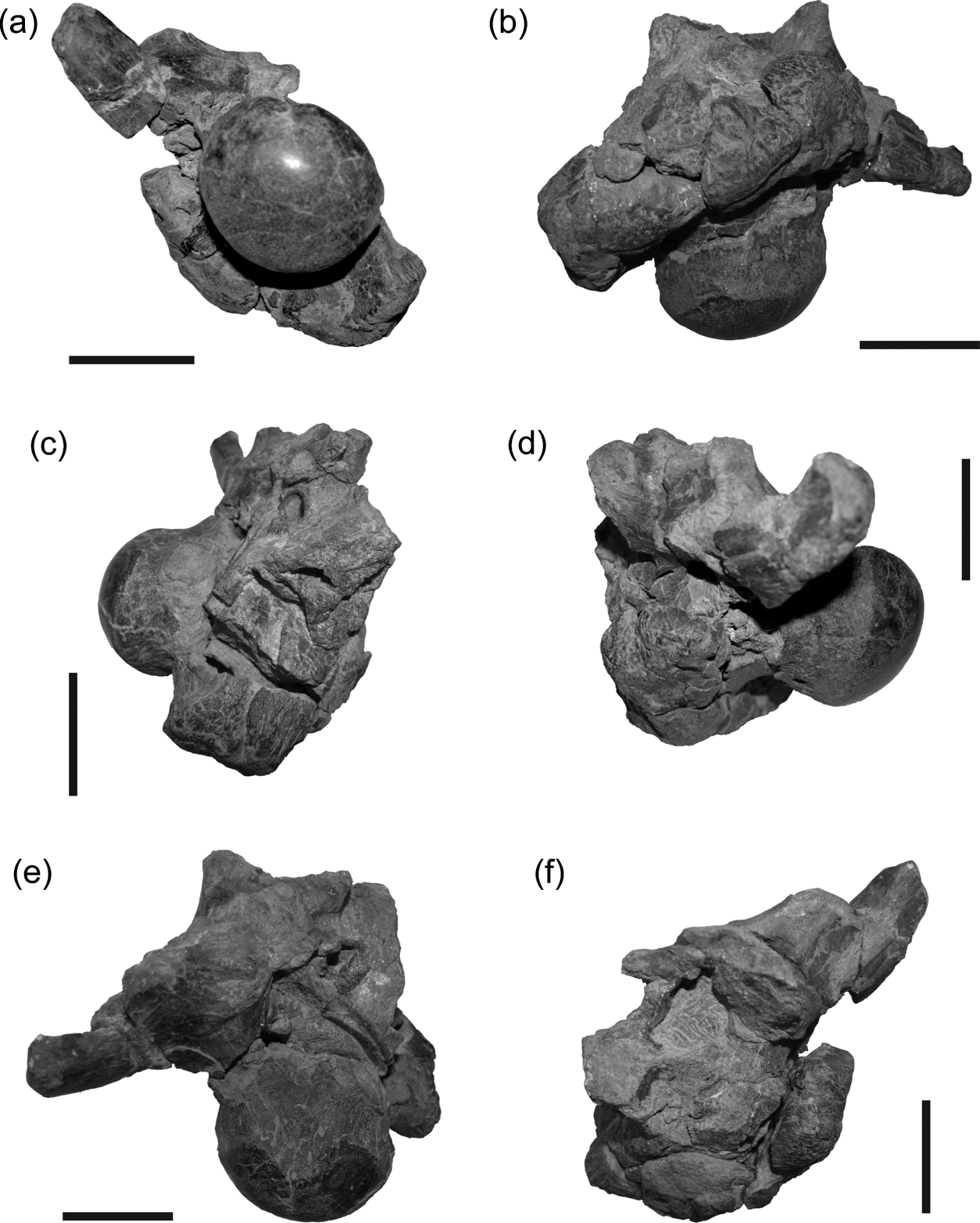
Figure 3 DMNH 22194, Pachyrhinosaurus perotorum, braincase in (a) posterior, (b) ventral, (c) right lateral, (d) left lateral, (e) dorsal and (f) anterior views. Scale bars=5 cm.
The exoccipital-opisthotics are indistinguishably fused as in dinosaurs generally, and appear to exclude the basioccipital and supraoccipital from the rim of the foramen magnum. Adult ceratopsid exoccipital-opisthotics form large, laterally expanded paroccipital processes that brace against the more lateral cranial bones. The base of the right parocciptial process is preserved in DMNH 22558 and the left in DMNH 22194. In both specimens the paroccipital processes are short, unexpanded, and terminate laterally in blunt, smoothly finished surfaces (Figs 3, 4). The interior surfaces of the left side of the cheek-region cranial bones were badly weathered in DMNH 22558, making it difficult to verify whether the left paroccipital process of this skull shares this blunt-tipped morphology, or if it has a more ‘normal’ ceratopsid form. A thick crista tuberalis (=metotic strut of Currie et al. Reference Currie, Langston and Tanke2008) connects the ventral margin of the paroccipital process and the dorsolateral margin of the basal tuber, dividing the occipital and lateral surfaces of the braincase. The base of each exoccipital is penetrated adjacent to the condylar neck by a pair of foramina, one dorsal to the other, for passage of cranial nerves X–XI (vagus, glossopharyngeal and accessory nerves) and XII (hypoglossal nerve) (Figs 3a, b, 4a, b). The supraoccipital is not well preserved in either specimen. Portions of it are preserved in DMNH 22558, but all the exterior bone surfaces were lost to erosion. The remaining parts offer only a hint of the gross morphology of the element, and do not provide additional useful data.
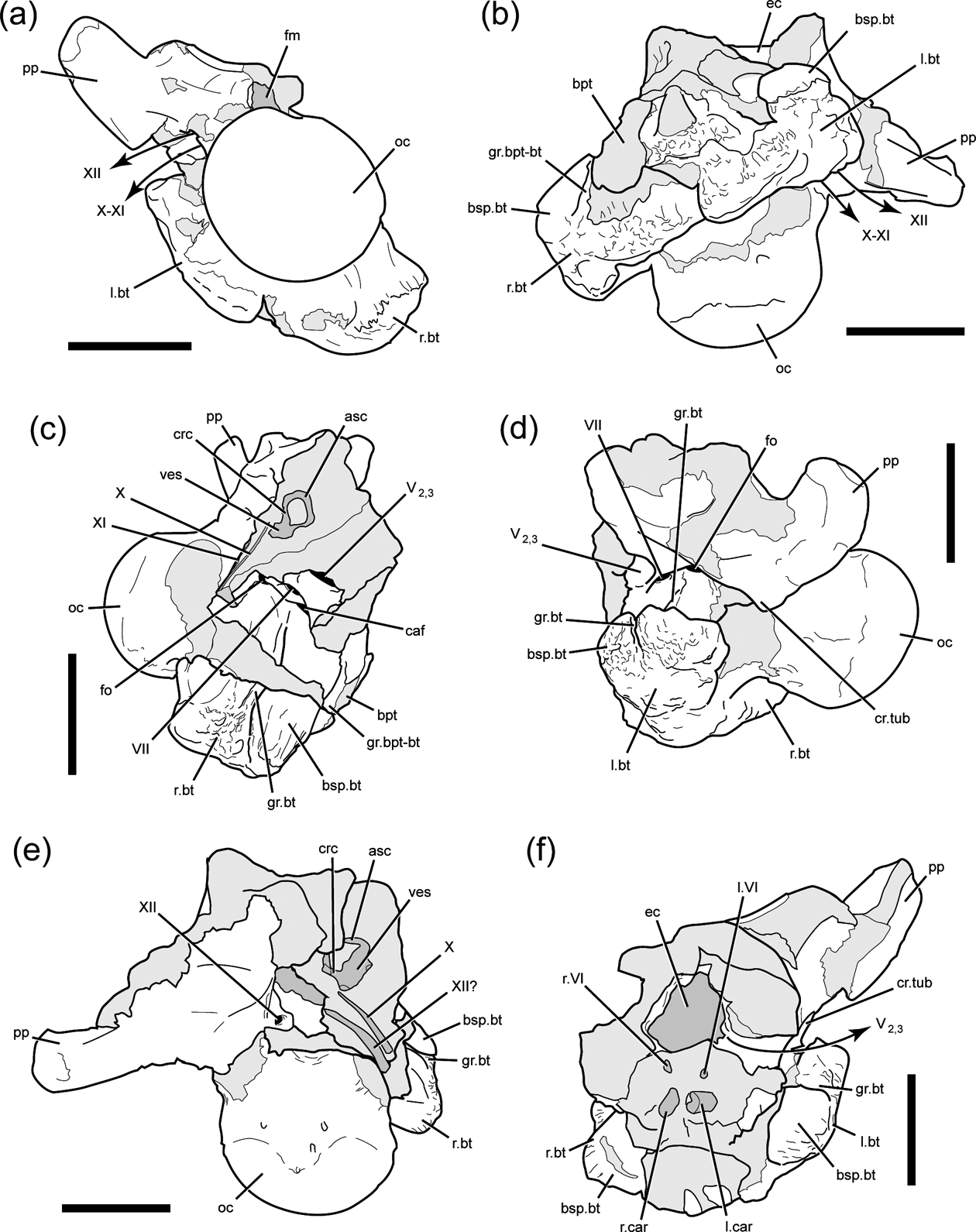
Figure 4 DMNH 22194, Pachyrhinosaurus perotorum, braincase line drawings of photographs in Figure 3. Braincase in (a) posterior, (b) ventral, (c) right lateral, (d) left lateral, (e) dorsal and (f) anterior views. Light grey indicates broken bone surface. Dark grey indicates matrix infill of internal space now visible in specimen. Scale bars=5 cm. Abbreviations: asc=anterior semi-circular canal; bpt=basipterygoid process base; bsp.bt=basisphenoid part of basal tuber; caf=carotid artery foramen; crc=crus communis; cr.tub=crista tuberalis; ec=endocranial cavity; fm=foramen magnum; fo=fenestra ovalis; gr.bpt-bt=groove separating basipterygoid process and basal tuber; gr.bt=groove on lateral surface of basal tuber; l.bt=left basal tuber; l.car=left carotid artery path; l.VI=left cranial nerve six (abducens nerve) path; oc=occipital condyle; pp=paroccipital process; r.bt=right basal tuber; r.car=right carotid atery path; r.VI=right cranial nerve six (abducens nerve) path; V2,3=cranial nerve five (trigeminal nerve) maxillary and mandibular branch path; VII=cranial nerve seven (facial nerve) foramen; ves=vestibule of inner ear; X=cranial nerve ten (vagus nerve) path; XI=cranial nerve eleven (accessory nerve) path; XII=cranial nerve twelve (hypoglossal nerve) path.
2.1.2. Lateral braincase surface
The prootic forms a dorsal buttress, the crista prootica, over the otic cavity and encompasses the passages for several cranial nerves and vessels exiting the braincase. The fenestra ovalis is located just anterior to the paroccipital process (Figs 3c, d, 4c, d), and CT images of DMNH 22558 shows both stapes (=columella), are still preserved in apparent life position within the sediment that remains in both columellar canals (=otic canals) (Fig. 5a). The anterior boundary of the fenestra ovalis is defined by the edge of the pendant process of the prootic. Just anterior to the fenestra ovalis, the crest is penetrated by the foramen for CN VII (facial nerve), which opens ventrolaterally (Figs 3c, d, 4c, d). Ventral to CN VII is the large opening of the carotid canal, which is dorsally and anterolaterally roofed by the prootic and ventrally floored by the basisphenoid. Infillings of the two carotid canals at a point near their entry to the pituitary region are visible in the broken anterior surface of DMNH 22194 (Figs 3f, 4f). Anterior to the exit for CN VII is the largest foramen on the lateral braincase surface, the opening for CN V2-3 (trigeminal nerve, maxillary and mandibular branches). The left side of the braincase is broken in DMNH 22194, fully exposing the posterior wall of the canal for CN V2-3 (Figs 3d, f, 4d, f).
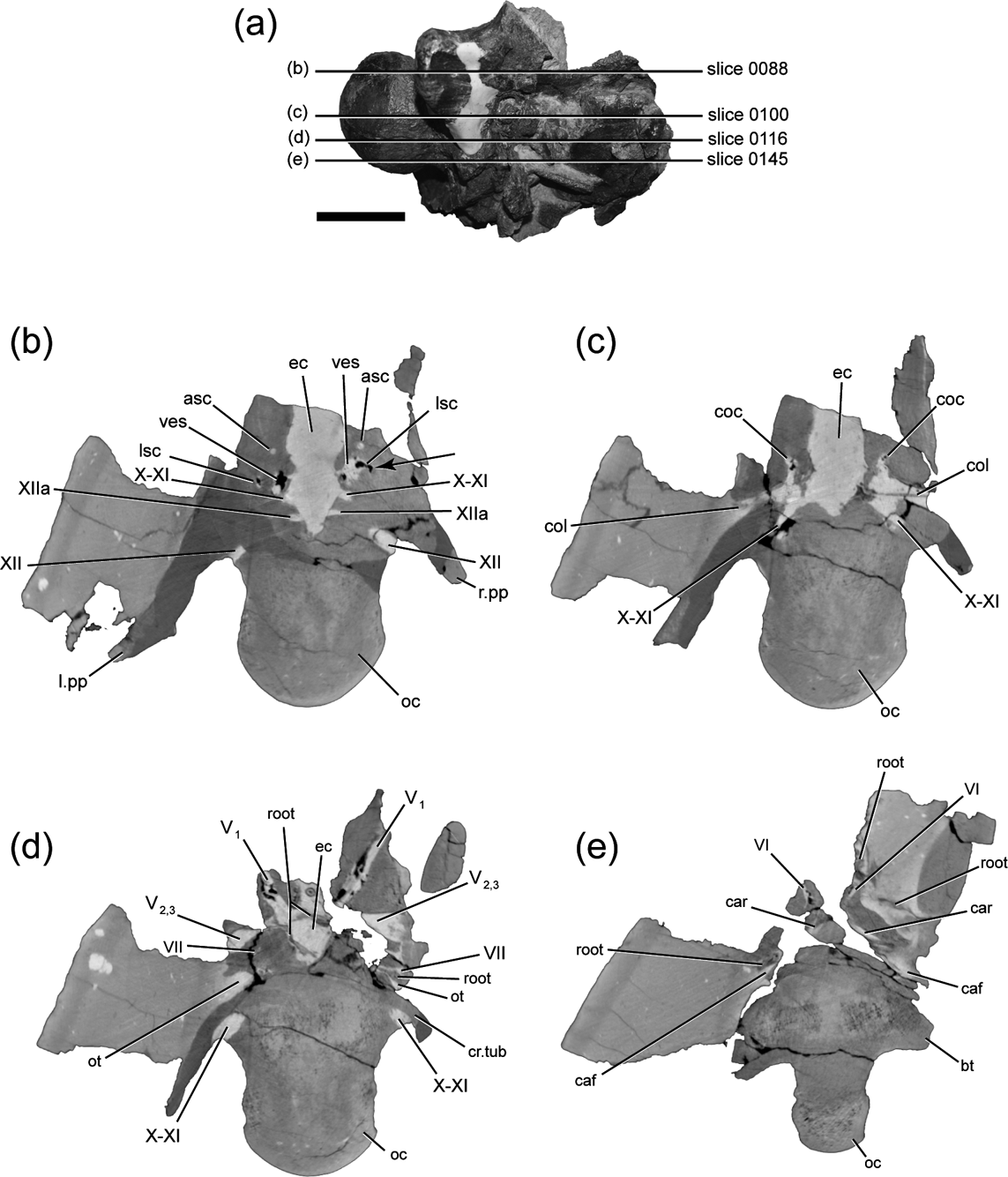
Figure 5 CT slices through braincase of DMNH 22558, Pachyrhinosaurus perotorum: (a) drawing showing relative position of scan slice planes through braincase in right lateral view, with slice numbers from CT scan data set indicated; (b) slice 0088. Arrow points to sharp, right-angle turn along anterior part of right horizontal semicircular canal; (c) slice 0100; (d) slice 0116; (e) slice 0145. Scale bar (a)=5 cm. Abbreviations: asc=anterior semicircular canal; bt=basal tuber; caf=carotid artery foramen; car=carotid artery path; coc=cochlea; col=columella (stapes); cr.tub=crista tuberalis; ec=endocranial cavity; ; l.pp=left parapophysis; lsc=lateral semicircular canal; oc=occipital condyle; ot=otic cavity; root=carbonised plant root; r.pp=right parapophysis; V1=cranial nerve five (trigeminal nerve) opthalmic branch path; V2,3=cranial nerve five (trigeminal nerve) maxillary and mandibular branch path; VI=cranial nerve six (abducens nerve) path; VII=cranial nerve seven (facial nerve) path; ves=vestibule of inner ear; X=cranial nerve ten (vagus nerve) path; XI=cranial nerve eleven (accessory nerve) path; XII=cranial nerve twelve (hypoglossal nerve) path; XIIa=anterior branch of cranial nerve twelve (hypoglossal nerve).
A deeply incised groove marks the contact between the basisphenoid and basioccipital ventral to the right fenestra ovalis in DMNH 22194 (Figs 3c, 4c, 6). The deep groove begins posterior to the opening to the carotid canal and continues posteroventrally to the edge of the basal tuber. The groove is so deep that it creates a distinct incision or notch on the distal surface of the basal tuber. Traces of the counterpart groove on the left side are present ventral to the fenestra ovalis and on the lateral margin of the dorsally displaced basal tuber. The groove does not mark the separation between the basal tuber and the basipterygoid process. The broken base of the right basipterygoid process is present anterior to the basal tuber, from which it is set off by a shallower groove. The latter groove is oriented approximately parallel to the deep groove below the otic cavity, with its dorsal end apparently terminating somewhere anteroventral to the foramen for CN V2-3.
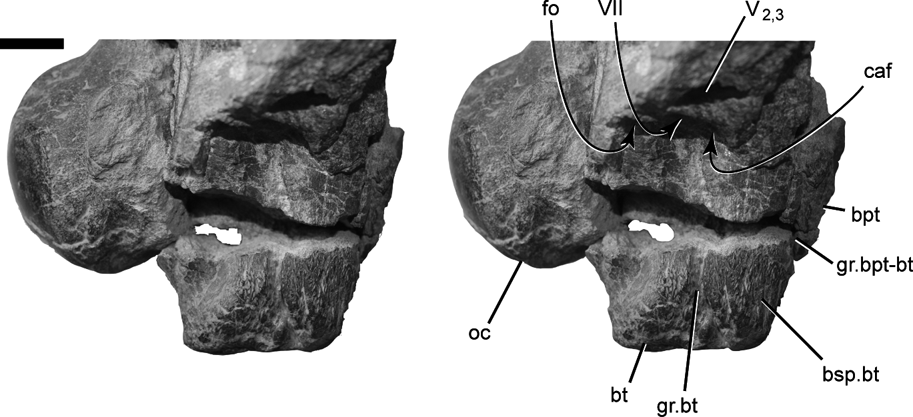
Figure 6 DMNH 22194, Pachyrhinosaurus perotorum, stereo photograph of right basal tuber and otic region in right lateral view, showing deep lateral groove marking the basioccipital and basisphenoid contact within the basal tuber. Scale bar=2 cm. Abbreviations: bsp.bt=basisphenoid part of basal tuber; bt=basal tuber; bpt=basipterygoid process broken base; caf=carotid artery foramen; fo=fenestra ovalis; gr.bpt-bt=groove separating basipterygoid process and basal tuber; gr.bt=groove on lateral surface of basal tuber; oc=occipital condyle; V2,3=cranial nerve five (trigeminal nerve) maxillary and mandibular branch foramen; VII=cranial nerve seven (facial nerve) foramen.
Only DMNH 22558 preserves portions of the braincase anterior to the opening for CN V, but these areas are hidden from direct view by surrounding bones. As noted above, the broken anterior surface of DMNH 22194 shows the matrix-filled passages for the carotids. Dorsal to the left carotid canal is another passage in transverse cross section, that for CN VI (abducens nerve) (Figs 3f, 4f). Other information regarding these more anterior regions is based upon the CT scans of DMNH 22558.
2.2. Internal anatomy
The CT images of the DMNH 22558 braincase and the resulting three-dimensional renderings of the endocranial cavity, neurovascular passages, and endosseous labyrinths (Figs 7, 8) reveal a great deal of information. The digital rendering of the endocranial region of Pachyrhinosaurus perotorum can also be compared to a similar reconstruction for P. lakustai (Witmer & Ridgely Reference Witmer, Ridgely, Currie, Langston and Tanke2008). The data seen in the CT slices is corroborated in some instances by the infillings of canals exposed in the broken parts of DMNH 22194. Descriptions are given here with the endocranial reconstruction positioned with the passage for the lateral semicircular canal oriented horizontally.

Figure 7 DMNH 22558, Pachyrhinosaurus perotorum, stereo images of digitally rendered endocranial reconstruction derived from CT scan of specimen in (a) right lateral, (b) left lateral, (c) dorsal, (d) ventral, (e) anterior and (f) posterior views. Scale bars = 4 cm. Abbreviations: car = carotid artery canal; cbl = cerebellum; cc = columellar canal; col = columella (= stapes); dls = dorsal longitudinal sinus; lab = endosseous labyrinth; l.V1 = left cranial nerve five (trigeminal nerve) ophthalmic canal; l.VI = left cranial nerve six (abducens nerve) canal; r.III = right cranial nerve three (occulomotor nerve) canal; r.V1 = right cranial nerve five (trigeminal nerve) ophthalmic canal; r.VI = right cranial nerve six (abducens nerve) canal; vls = ventral longitudinal sinus; III = cranial nerve three (occulomotor nerve) canal; V1 = cranial nerve five (trigeminal nerve) ophthalmic canal; V2–3 = cranial nerve five (trigeminal nerve) maxillary and mandibular branch path; VI = cranial nerve six (abducens nerve) canal; VII = cranial nerve seven (facial nerve) canal; VIII = cranial nerve eight (vestibulocochlear nerve) canal; IX? = cranial nerve nine (glossopharyngeal nerve) possible path; X–XI = canal for cranial nerves ten (vagus nerve) and eleven (accessory nerve); XII = cranial nerve twelve (hypoglossal nerve) canal.

Figure 7 (Continued)

Figure 8 DMNH 22558, Pachyrhinosaurus perotorum, stereo images of digitally rendered left endosseous labyrinth derivered from CT scan of specimen in (a) lateral, (b) posterior and (c) dorsal views. Arrow in (c) points to abrupt angle in anterolateral ‘corner’ of the path of the lateral semicircular canal. Scale bar=2 cm. Abbreviations: asc=anterior semicircular canal; asca=ampulla of the anterior semicircular canal; coc=cochlea (partial); crc=crus communis; lsc=lateral semicircular canal; lsca=ampulla of the lateral semicircular canal; psc=posterior semicircular canal; ve=vestibule of inner ear.
Three branches of the hypoglossal nerve (CN XII) exit the endocranial cavity, with the largest and most posterior of them arcing posterolaterally through the exoccipital and exiting the exoccipital lateral to the neck of the occipital condyle through the more dorsally positioned of two foramina (Figs 5b, 7). Two smaller and more anteriorly located canals for CN XII exit the endocranial cavity and also arc posterolaterally a short distance, nearly paralleling each other, as seen in DMNH 22194 (Figs 3e, 4e). These branches of CN XII enter the larger vagus canal (=jugular canal, metotic canal), which is also the path for CN X (vagus nerve), CN XI (accessory nerve), and perhaps CN IX (glosspharyngeal nerve) (Fig. 7). The proximal portion of the vagus canal maintains a close association and connection with the proximal part of the otic cavity (Figs 5c, 7d). The vagus canal then splits away from the otic cavity and traces a nearly straight path posterolaterally to the occipital surface, where it exits the braincase through the exocciptial just ventral to the foramen for the most posterior branch of CN XII (Figs 4a, 7).
The otic region is well preserved in DMNH 22558, including the infillings of the osseous labyrinth, vestibule, and cochlea of the inner ear (Fig. 5b, c). Thin, rod-like stapes are preserved within the matrix filling both otic cavities (Figs 5c, 7a, b, c, d). Three-dimensional reconstructions of the endosseous labyrinth (sensu Witmer & Ridgely, Reference Witmer, Ridgely, Currie, Langston and Tanke2008), show some details of the inner ear structure (Figs 7, 8). The lateral (=horizontal) semicircular canal is much shorter than the other semicircular canals, and does not follow a smoothly continuous arc or curve. Instead, it passes laterally from the ampulla of the lateral semicircular canal, then makes a sharp, almost right-angle turn posteriorly (Figs 5b, 7c, 8c). The lateral canal then curves posteromedially in a gentle arc to rejoin the vestibule ventral to the confluence of the vestibule and the posterior semicircular canal. The broken surface on the right side of DMNH 22194 exposes a natural infilling of much of the vestibule and the entire path of the anterior semicircular canal and the crus communis (Figs 3c, e, 4c, e). A very small canal visible in the CT data between the endocranial space and the osseous labyrinth may be that of CN VIII (vestibulocochlear nerve) (Fig. 7a, b, d).
The passage for CN VII (facial nerve) exits the endocranial cavity just ventral to CN VIII (Fig. 7d). The canal for CN VII is very narrow for most of its path laterally and slightly posteriorly through the braincase wall. The canal greatly increases in diameter just before exiting the braincase (Figs 5d, 7a, b). The canal for each CN VI (abducens nerve) exits the endocranial cavity from the ventral side of the endocranial cavity near the midline. The canal for CN VI is long, passing anterolaterally through the bones flooring the endocranial cavity to emerge ventral and slightly anterior to the exit for CN V1 (Fig. 5e). The external aperture for the carotid canal is ventral to that of CN VII and traces a path anteromedially through the basisphenoid (Figs 5e, 7). In other vertebrates the carotid canals enter the pituitary (=hypophyseal) fossa, but this area is poorly preserved in the braincase of DMNH 22558 (Figs 3f, 4f), and only a hint of the convergence of both carotid canals is visible in the endocranial reconstruction (Fig. 7d, e).
The path of the trigeminal nerve (CN V) originates from the endocranial cavity as a single large canal, but itbifurcates almost immediately after exiting the endocranial cavity (Figs 5d, 7). One branch exits the braincase laterally as the path of the maxillary and mandibular branches of the trigeminal nerve (CN V2-3). The other branch angles anterolaterally and dorsally as the path of the ophthalmic branch of the trigeminal nerve (CN V1). No specimens adequately preserve the more dorsal parts of the anterior braincase, so we have no information regarding the path of CN IV (trochlear nerve). The external foramina for CN V1 and CN III (occulomotor nerve) are present on the right side of DMNH 22558 (Fig. 7b, c, d, e). The foramen for CN III is anteroventral to that for CN V1, but both open into a distinct fossa on the anterolateral surface of the braincase. The more medial part of the CN III canal was lost to weathering. A small part of the rim of the external foramen for CN II (optic nerve) is preserved medial to that of CN III, but nothing more can be inferred about the size or nature of the optic nerve canal.
3. Discussion
Pachyrhinosaurus candensis was named and initially described by Sternberg (Reference Sternberg1950), but it was Langston (Reference Langston1967, Reference Langston1968, Reference Langston1975) who first provided detailed descriptions of the cranial anatomy of this taxon and established the taxon as a centrosaurine ceratopsid. Importantly here, Langston (Reference Langston1975) also provided the first description and figures of the braincase of P. canadensis, and indeed the first such braincase description for a centrosaurine. The description of the braincase of P. lakustai by Currie et al. (Reference Currie, Langston and Tanke2008) and the valuable three-dimensional visualisations of the same elements by Witmer & Ridgely (Reference Witmer, Ridgely, Currie, Langston and Tanke2008) provided the opportunity to compare this region in all three species of Pachyrhinosaurus.
Overall braincase morphology is very similar in these three species. This is not unexpected given the close relationship between the taxa. Yet we were able to find at least two braincase details that at least have the potential to be informative and helpful in distinguishing P. perotorum from P. canadensis and P. lakustai. We acknowledge the reality of individual variation in the expression of anatomical details as fine as these, and in the absence of more robust sample sizes we emphasize that these are only potential characters.
3.1. Lateral groove on basal tuber
The first detail involves the deep groove on the lateral surface of the basal tuber in P. perotorum (Figs 3c, d, 4c, d, 6). The deep groove traversing the side of the basal tuber below the fenestra ovalis in DMNH 22194 appears to mark the boundary between the basioccipital and basisphenoid parts of the basal tuber. The groove is so deeply incised into the tuber that it forms a sharp lateral notch in the tuber's distal profile. Langston (Reference Langston1975) figured and described a similar groove in P. canadensis (NMC 9485), stating, “… a sulcus possibly marking the route of the stapedial artery may be followed along the basicoocipital-basisphenoid contact” (Langston Reference Langston1975, p. 1589).
The groove in P. canadensis originates posterior to CN VII and the carotid foramen, then turns ventral to the carotid foramen before continuing ventrally along the basiocciptial-basisphenoid contact. This resembles the lateral groove in P. perotorum, but in the latter the groove angles posteroventrally away from the foramen for the carotid, and does not curve ventral to it (Reference Currie, Langston and TankeFig. 6). If the groove accurately marks the basiocciptial–basisphenoid contact, then approximately the anterior one-third of the basal tuber is comprised of basisphenoid in P. perotorum. We suspect, based upon the lateral groove figured by Langston (Reference Langston1975), that the anterior third or more of the basal tuber is comprised of the basisphenoid in P. canadensis as well, and that the basipterygoid process is more anteriorly located as in P. lakustai and P. perotorum (Currie et al. Reference Currie, Langston and Tanke2008; Witmer & Ridgley Reference Witmer, Ridgely, Currie, Langston and Tanke2008).
This area is difficult to make out in the descriptions and illustrations of the braincase of P. lakustai. These are based mainly upon TMP 1989.55.1234, the specimen figured by Currie et al. (Reference Currie, Langston and Tanke2008), and CT-scanned and illustrated by Witmer & Ridgley (Reference Witmer, Ridgely, Currie, Langston and Tanke2008). The distal (ventrolateral) ends of the basal tubera are missing in the specimen, and much of the pertinent surface area ventral to the fenestra ovalis and posterior to the opening for the carotid canal is evidently damaged (Currie et al. Reference Currie, Langston and Tanke2008, fig 40B). Stereo-images of the same specimen (Witmer & Ridgely Reference Witmer, Ridgely, Currie, Langston and Tanke2008, figs 1A, D, 4A) show a faint line or groove ventral to the fenestra ovalis, but this also corresponds to the margin of the damaged area on the left side of the braincase. The broken edge of the basal tuber in TMP 1989.55.1234 shows no sign of the deeply incised groove cutting into surface the basal tubera such as that present in P. perotorum (DMNH 22194).
It is tempting to speculate that the obvious lateral groove on the basal tuber present in both P. canadensis and P. perotorum could be a unifying synapomorphy indicating a closer relationship between these two species to the exclusion of P. lakustai. The damaged surface of this area in TMP 1989.55.1234 (P. lakustai) and the fact that the distal ends of the basal tubera are missing, leaves open the chance that a groove was present here but was destroyed. Also, the braincase of Centrosaurus apertus, figured by Dodson et al. (Reference Dodson, Forster, Sampson, Weishampel, Dodson and Osmolska2004), shows a groove or sulcus in almost the same place as in P. canadensis. That opens the likelihood that this character has a greater distribution among centrosaurines. The posteroventral angle taken by the groove in P. perotorum differs from the path of the groove in P. canadensis and C. apertus, so it is yet possible that aspect of the feature could be used to distinguish P. perotorum.
3.2. Lateral semicircular canal
There is a potential difference of inner ear anatomy between P. perotorum and P. lakustai which is only discernable from CT-based imagery. The left endosseous labyrinth was digitally extracted and reconstructed for this study (Fig. 8), although the cochlea and more ventral vestibular regions of the structure were difficult to discern in the dataset and were not fully rendered in time for this study. The lateral semicircular canals in both P. perotorum and P. lakustai are both relatively short. The stereo-image renderings of the endosseous labyrinth of TMP 1989.55.1234 by Witmer & Ridgely (Reference Witmer, Ridgely, Currie, Langston and Tanke2008, fig. 3C) show the lateral semicircular canal exits the ampulla of the lateral semicircular canal in a posterior, and only slightly lateral direction. Just posterior to the mid-point of the canal's path, the canal makes a sharp, medial-ward turn and rejoins the vestibule. This medial turn of the canal creates a sharp, almost ninety-degree posterolateral corner in the path of the lateral canal.
As we describe above, the lateral semicircular canal in Pachyrhinosaurus perotorum (DMNH 22558) also has a pronounced angle along its path. In contrast to that in P. lakustai, the angle is at the anterolateral corner of the canal (Figs 5b, 8c). The anterior end of the lateral canal exits the ampulla in a lateral direction, then makes a sharp posteriorward turn that results in an anterolateral corner in the path of the canal. The lateral canal then curves posteromedially to smoothly rejoin the vestibule, without the sharp posterolateral corner present in P. lakustai. This endosseous labyrinth morphology is present and symmetrical on both right and left sides of DMNH 22558 (Fig. 7c). It is, therefore, unlikely that the sharp anterolateral corner in the lateral semicircular canal is a result of postmortem distortion of the specimen.
Unfortunately, the sample size of reconstructed or recovered neoceratopsian inner ear labyrinths is very small. The endocranial cast of the chasmosaurine Anchiceratops ornatus (AMNH 5259) also preserves the traces of the osseous labyrinth (Brown Reference Brown1914). The lateral canal traces a simple, continuous arc out of and back into the vestibule, with no hint of an abrupt angulation along its path. Likewise, the path of the lateral canal traces a simple arc in the cranial endocast of Protoceratops andrewsi (AMNH 6466) (Brown & Schlaikjer Reference Brown and Schlaikjer1940).
3.3. Usefulness of braincase anatomy in species differentiation
Much as Langston (Reference Langston1975) voiced frustration at the lack of literature and specimens of centrosaurine braincases in his description of Pachyrhinosaurus canadensis, we are also faced with too small a sample of specimens from too few taxa to make confident statements about the usefulness of this region in ceratopsid species determination. The existence of well-described braincases for three species of Pachyrhinosaurus presented us the best opportunity to test whether useful, species-level differences could be found to aid in distinguishing the species from one another. Also, it provided the chance to find additional phylogenetic data that could resolve relationships between the three species, which are otherwise based mostly upon craniofacial and frill ornamentation characters (Fiorillo & Tykoski Reference Fiorillo and Tykoski2012).
The deep lateral groove on the lateral surface of the basal tuber is easily visible in Pachyrhinosaurus perotorum (DMNH 22194), P. canadensis, and perhaps other centrosaurines (Dodson et al. Reference Dodson, Forster, Sampson, Weishampel, Dodson and Osmolska2004). The more posteroventral orientation and apparent depth of the groove in P. perotorum may be useful for distinguishing it from other centrosaurines. The incomplete preservation of this area in published specimens of P. lakustai renders this feature uninformative for the taxon at this time. Further examination of additional P. lakustai specimens may be able to provide additional clarity regarding the distribution and variation of this character.
The different morphology of the lateral semicircular canal in P. lakustai and P. perotorum is another character with potential for differentiating these two species. Each species has a lateral canal with a pronounced corner along its path, but the corner is on opposite ends of the canal (anterior end of the canal in P. perotorum, posterior end of the canal in P. lakustai). While intriguing and potentially informative, the utility of this character is limited because of the small sample of individuals (n=1) of each species for which an osseous labyrinth, or its reconstruction, is known. We cannot rule out the possibility that the morphological difference in the lateral semicircular canal between these specimens could be attributed to individual variation. There is nothing known about the osseous labyrinth of P. canadensis. It would obviously be informative if this data could be obtained, so as to compare it to the other two species of Pachyrhinosaurus. Unfortunately, this feature is also almost impossible to observe without CT scans of available specimens, or matrix removal and endocast creation by highly skilled fossil preparators.
We conclude that braincase morphology is not currently a useful tool for distinguishing species in the centrosaurine Pachyrhinosaurus. The reasons for this are: (1) the small number of potentially useful characters we found to differentiate the braincase of Pachyrhinosaurus perotorum from the other Pachyrhinosaurus species; (2) the small sample size of individuals and taxa for which comparable elements are available, opening the possibility that differences we see are the result of individual and not species-level variation; and (3) the difficulty in obtaining endocranial data either via CT scanning of specimens, or by extensive and time-consuming manual preparation and endocast fabrication. Hopefully, over time a ‘critical mass’ of ceratopsian braincases across a broad spectrum of taxa can be sampled and processed, and at least broad patterns of variation and useful phylogenetic data can be coaxed from this complex region. At this time though, our study reinforces the importance of, and our reliance upon, craniofacial and frill ornamentation as the most informative means of determining lower-level affinities and phylogenetic relationships of ceratopsid taxa.
4. Acknowledgments
We are grateful to the Guest Editors of this volume for the invitation to contribute a paper that, given his earlier important contributions on the subject, is on a subject matter that we hope is near and dear to the heart of Wann Langston. We thank Wann for his enthusiastic conversations early in the preparation of some of the Pachyrhinosaurus material used in this paper. We also thank the numerous members of the field crews that contributed to the data collecting for this project, particularly David Norton, Paul McCarthy, Peter Flaig, Kent Newman, Thomas Adams, Christopher Strganac and Jason Petula. The Barrow Arctic Science Consortium (BASC) and CH2M Hill (formerly Veco Polar Resources) provided logistical support for the field component of this project. Funding was provided by the National Science Foundation Office of Polar Programs (OPP 0424594). We also thank Matthew Colbert of The University of Texas High-Resolution X-ray CT Facility at The University of Texas at Austin who performed the CT scan of DMNH 22558, and Jesse Maisano of the same facility who performed the post-scan processing of the data. Discussions with Mike Polcyn of Southern Methodist University greatly aided our efforts to produce our three-dimensional endocast reconstructions. Lastly, the Arctic Management Unit of the Bureau of Land Management provided administrative support. The specimens we collected under BLM permit number AA-86367.