I. INTRODUCTION
The copper–indium–gallium–diselenide (CIGS) tetragonal semiconductor has received more attention for its potential application as a prominent thin-film photovoltaic material because of its high optical absorption coefficient, wide absorbing spectrum, and long-term stability (Chandramohan et al., Reference Chandramohan, Velumani and Venkatachalam2010). CIGS solar cells with highest conversion efficiency of 20.3% have been successfully prepared by ZSW Company in Germany (Philip et al., Reference Philip, Dimitrios, Erwin, Stefan, Roland, Richard, Wiltraud and Michael2011). CIGS thin-film solar cells are of interest in the space power application because of the near optimum band gap for AM0 solar radiation in space. CIGS solar cells are expected to be superior to Si and GaAs solar cells for space missions especially in terms of the performance at the end of low earth orbit (LEO) mission (Dhere and Ghongadi, Reference Dhere and Ghongadi2001).
Cu(In,Ga)Se2 is a I-III-VI semiconducting material with a tetragonal chalcopyrite structure (Chun et al., Reference Chun, Kim and Yoon2005). Non-vacuum techniques have been used to prepare CIGS films by a process in which water-based inks formulated using nanoparticles of mixed oxides of Cu, In and Ga are used to deposit a precursor layer of fixed Cu/(In + Ga) ratio on a rigid or a flexible substrate of choice using printing techniques and form the CIGS film with an average thickness of 2.5 µm (Kapur et al., Reference Kapur, Bansal, Le and Asensio2003). Actually, the preparation methods for CIGS nanoparticles include solvothermal technique (Liu et al., Reference Liu, Kong, Li, Zhao, Chen and Brugger2012) and low-temperature colloidal technology (Ahna et al., Reference Ahna, Kim, Chun and Yoon2007). However, the application of the solvothermal technique is limited to large-scale production because of its long processing (Gu et al., Reference Gu, Shin, Yeo, Hong and Nahm2011). As for the low-temperature colloidal process, the toxic Na2Se had to be one of the starting materials for preparing CIGS nanoparticle. Comparing the two technologies, the mechanical milling method is employed for preparing CIGS with pure elements of copper, indium, gallium and selenium as raw materials for avoiding pollution in the air. This technology takes less time than that of the solvothermal technique. Thus, the mechanical milling technology could be considered as the appropriate method for preparing CIGS nanoparticles (Vidhya et al., Reference Vidhya, Velumani, Arenas-Alatorre, Morales-Acevedo, Asomoza and Chavez-Carvayar2010). Mechanical milling could induce physical or chemical changes during pulverization, friction and compression between the balls and CIGS powders under high mechanical energy (Bhojan et al., Reference Bhojan, Subramaniam, Alatorre and Asomaza2009). The grain sizes of agglomerated CIGS powders after ball-milling could be less than 100 nm (Liu and Chuang, Reference Liu and Chuang2012). CuIn0.5Ga0.5Se2 phase could be formed within a very short milling time of 30 min (Benslim et al., Reference Benslim, Mehdaoui, Aissaoui, Benabdeslem, Bouasla, Bechiri, Otmani and Portier2010).
In this study, CIGS semiconductors have been prepared by vacuum arc melting and the vacuum solid state reaction method, and CIGS nanoparticles were synthesized using a high energy ball mechanical milling. The influence of ball-milling speeds on phase structures in Cu(In,Ga)Se2 nanoparticles is studied. The goal of this study could supply valuable references for large-scale production of CIGS solar cells.
II. EXPERIMENTAL
The metals of copper granules (>99.95% Sigma Aldrich), indium powders (>99.999% Aldrich), fine chips of gallium (>99.99% Aldrich) and non-metal selenium (>99.9% Aldrich) were used as the starting materials. The precursor Cu–In and Cu–Ga binary alloys with nominal compositions CuX (X = In, Ga) were prepared using vacuum arc melting in an atmosphere of ultra-pure argon gas, and followed by sealing these precursors and nonmetallic Se in an evacuated quartz tube for solid state reaction in a furnace at 400–500 °C for several weeks.
CIGS bulks for mechanical milling process were broken and followed by hand grinding in a glove box with high-purity nitrogen atmosphere. A wet milling technique was used to prepare CIGS nanoparticles by putting the mixtures of CIGS powders and anhydrous alcohol with a volume of 13 ml and milling balls into a grinding tank. Two sealed tanks in glove box were taken out and fixed in tanking holders for the milling process at room temperature. The milling time was 10 h and the rotational speeds were fixed at 460, 480, 500 and 520 r/min, respectively. In order to prevent the nanoparticles oxidation in air, the nanoparticles were taken out in the glove box.
The microstructures of CIGS nanoparticles were analysed by a scanning electron microscopy (SEM; JSM6010LA). X-ray diffraction intensity data used for crystal structure analysis were collected by a Rigaku diffractometer with rotating anode and an 18-kW X-ray generator CuK α1 radiation (λ α1 = 1.54056 Å) and a graphic monochromator for diffracted beams were used. A step-scanning mode was adopted with a scanning step of 0.02°/2 s and scan 2θ from 10 to 80°. The phases in CIGS were analysed with Jade5.0 and PDFWIN programs. The unit-cell parameters were determined using TREOR and a least-squares method. After normalization, the diffraction data were analysed by using Rietveld's powder diffraction profile-fitting technique to determine the atomic parameters.
III. RESULTS AND DISCUSSION
A. SEM analysis
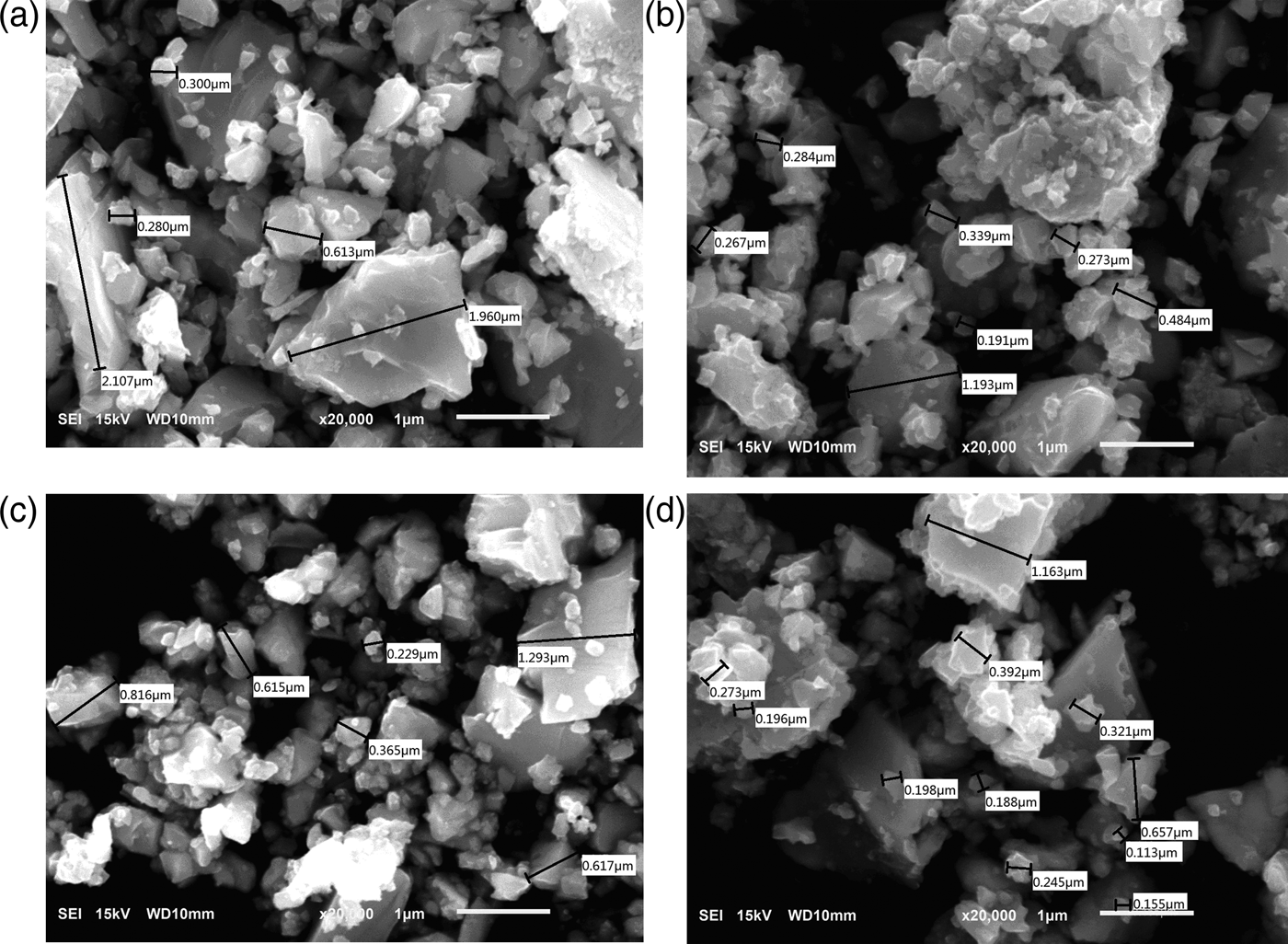
The main purpose of this work is to obtain CIGS nanoparticles by a simple process such as mechanical milling synthesis and to study the influence of ball-milling on the structure of the prepared CIGS nanoparticle. Figure 1 shows the morphologies of CIGS powders, the particle sizes with 200 nm are observed. The grain size of the CIGS powders decreases with increasing milling speed. However, the large agglomerates of CIGS nanoparticles present (see Figures 1(c) and (d)) with speed more than 500 r/min. It might be because of the high surface energy of the nanoparticles and strong van der Waals and Coulomb force between the grains. However, if there is obvious agglomeration among CIGS powders, the nanoscale of CIGS prepared will not be displayed.

Figure 1. SEM images of CIGS nanoparticles prepared at the milling speed of (a) 460, (b) 480, (c) 500 and (d) 520 r/min.
B. XRD analysis
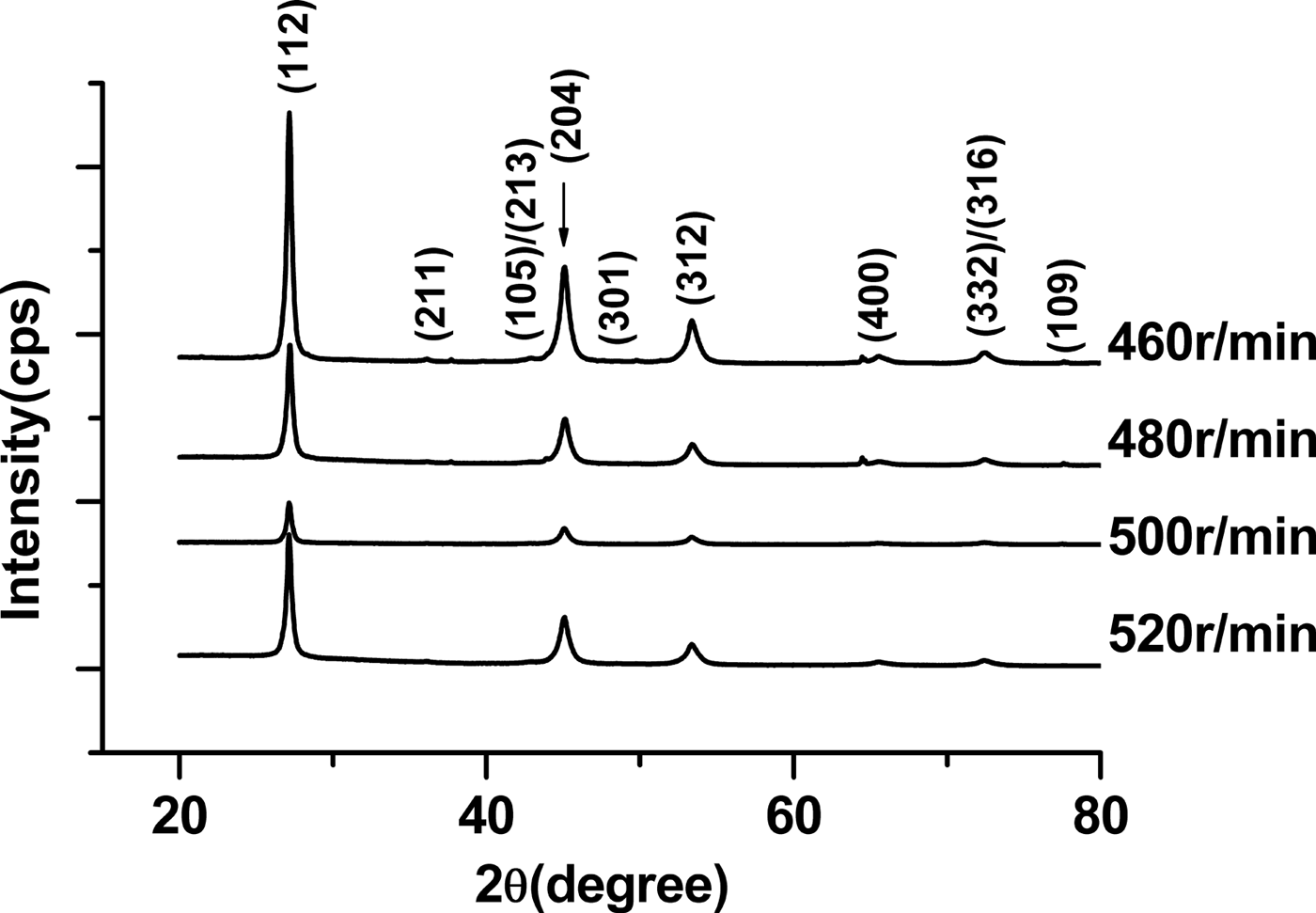
The XRD patterns of CIGS nanoparticles with various milling speeds are shown in Figure 2. According to ICDD cards of CuIn1−xGaxSe2 solid solutions (x = 0.3–0.5), the main phase of these samples prepared is CuIn1−xGaxSe2 with x < 0.5 with space group of ![]() $I\bar{4}2d$. At a low speed, small amount of impurity phase Cu7Se4 presents. With increasing milling speed, the content of impurity phase decreases. When the milling speed reaches 520 r/min, the Cu7Se4 phase disappears. As a result, the increase of rotational speed is beneficial for formation of a pure CIGS phase. It might be that fast rotation speed supplies high energy for pushing the chemical reaction for forming pure phase among copper granules, selenium and indium powders and fine chips of gallium.
$I\bar{4}2d$. At a low speed, small amount of impurity phase Cu7Se4 presents. With increasing milling speed, the content of impurity phase decreases. When the milling speed reaches 520 r/min, the Cu7Se4 phase disappears. As a result, the increase of rotational speed is beneficial for formation of a pure CIGS phase. It might be that fast rotation speed supplies high energy for pushing the chemical reaction for forming pure phase among copper granules, selenium and indium powders and fine chips of gallium.

Figure 2. X-ray diffraction patterns of CIGS powders prepared at the milling speed of 460, 480, 500 and 520 r/min.
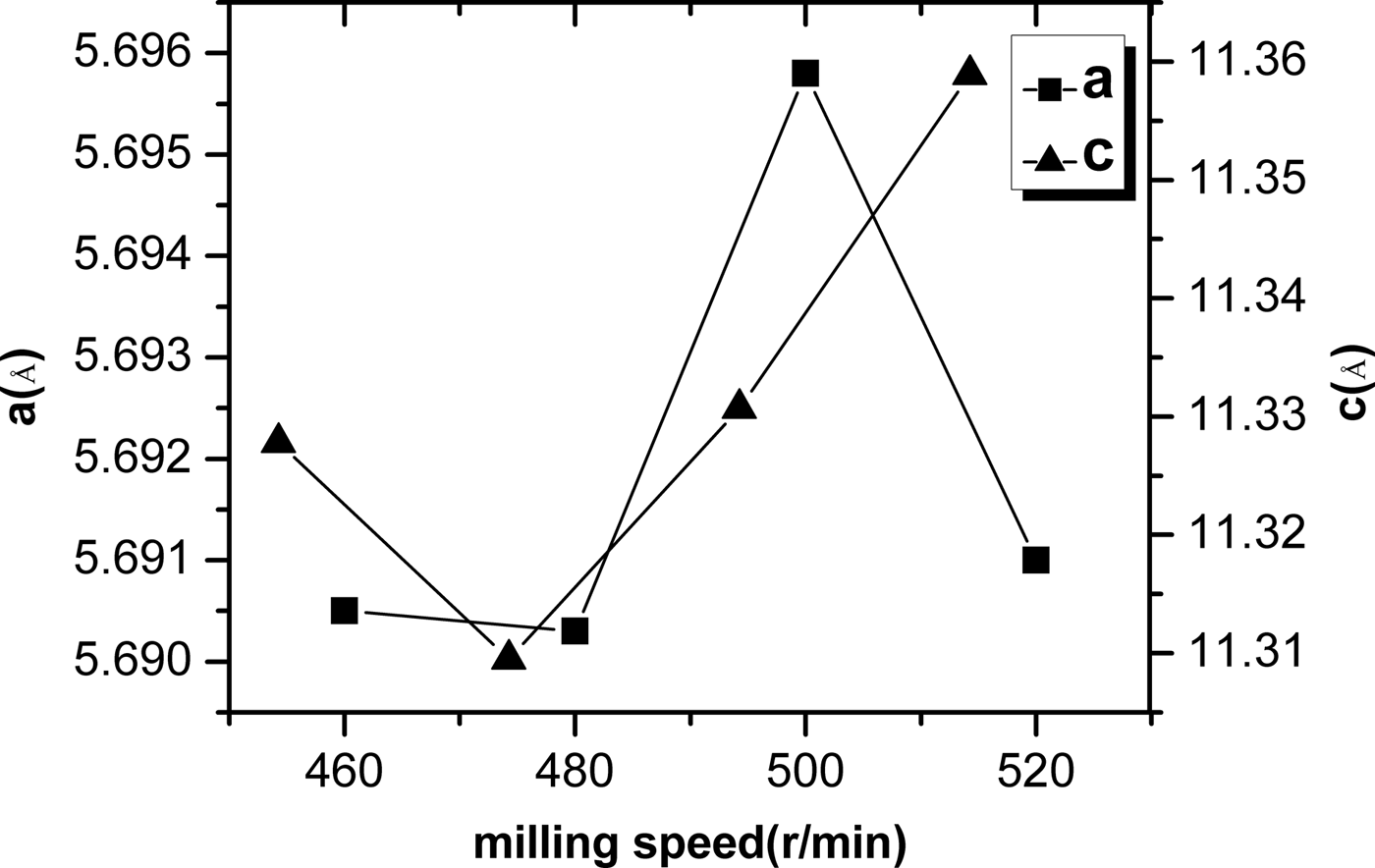
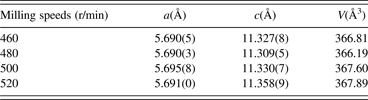
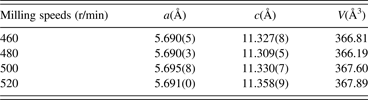
TREOR and a least squares method were used to determine the planar indices and lattice parameters. The results are listed in Table I. The unit-cell parameters (a and c) dependence of milling speeds are shown in Figure 3. It is noticed that the lattice parameters and cell volume are sensitive to the milling speeds. The relation between the unit-cell parameters and milling speeds is not linear. The unit-cell parameters are minimum at a speed of 480 r/min. The diffraction characteristic peaks of (1 1 2), (2 0 4), (3 1 2), (4 0 0) and (3 3 2)/(3 1 6) planes in the chalcopyrite structure appear for the CIGS samples at various milling speeds. By increasing the milling speed, the diffraction peak of (1 1 2) shows a disordered change, and its 2θ angle values are corresponding with 27.153° for milling at 460 r/min, 27.193° for milling at 480 r/min, 27.153° for milling at 500 r/min and 27.141° for milling at 520 r/min. According to the indexed results of planar indices, the unit-cell parameters a and c decrease with increasing diffraction angle 2θ, thus, the peak position of (1 1 2) plane is found to shift to the higher angle as reported by Olejnicek et al. (Reference Olejnicek, Kamler, Mirasano, Martinez-Skinner, Ingersoll, Exstrom, Darveau, Huguenin-Love, Diaz, Ianno and Soukup2010). The unit-cell parameters are related to the atomic radius. Since the atomic radius of Ga (0.61 Å) is less than that of In (0.81 Å), thus, it implies that low rotation speeds are beneficial for enhancing Ga content in CIGS and the possibility of Ga substitution for In decreases with increasing milling speed.

Figure 3. The ball milling speed dependence of unit-cell parameters (a and c).
Table I. Milling speed dependence of unit-cell parameters determined by TREOR.
C. Rietveld structure refinement
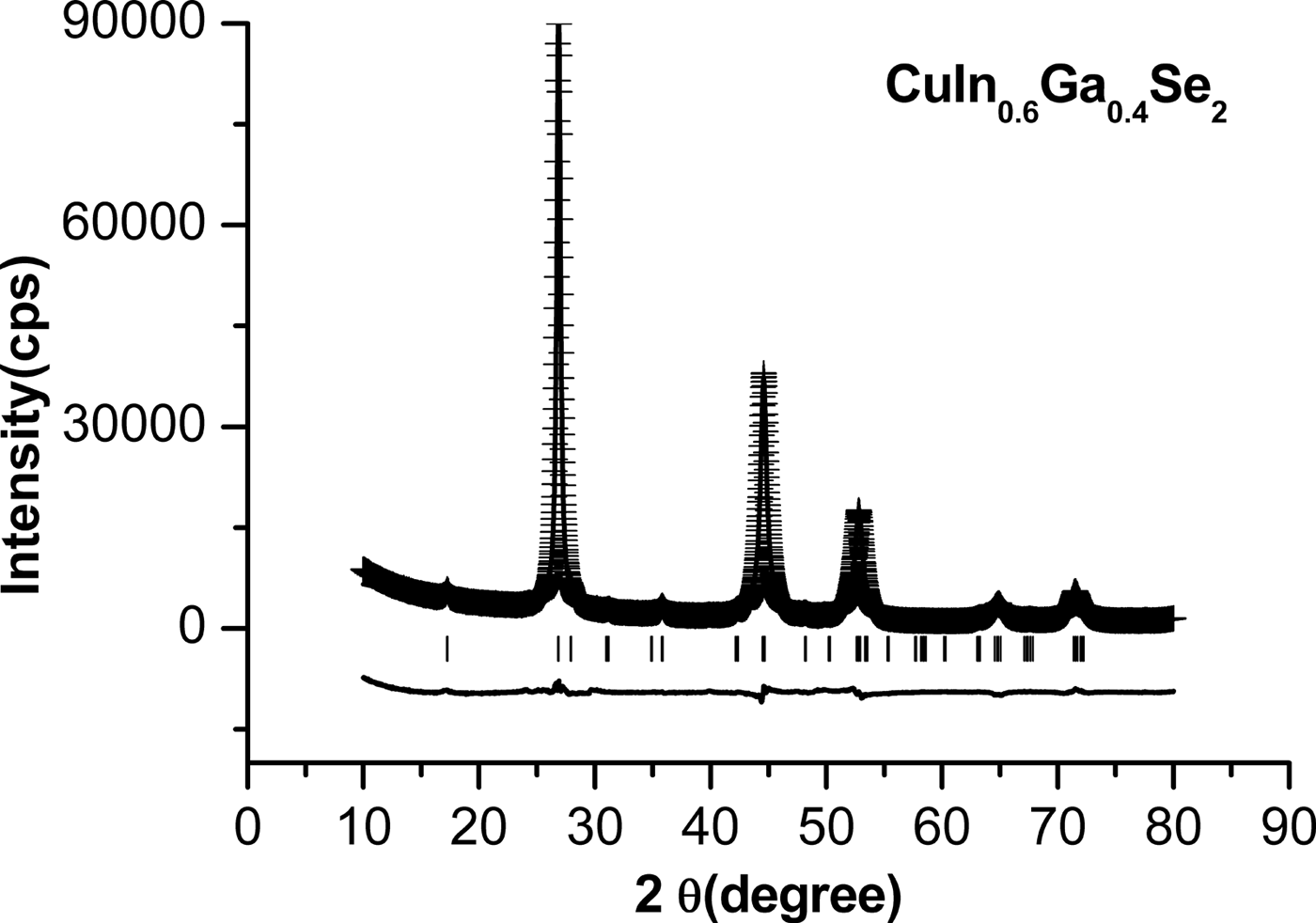
The typical refinement X-ray diffraction patterns of CuIn0.6Ga0.4Se2 nanopowders milled at 500 r/min are shown in Figure 4. The “ + ” points represent the experimental data; the line represents the calculated pattern, and the lowest line indicates the difference between the calculated and experimental pattern. A good overlapping of the experimental and calculated peaks has been observed. The pattern factor R P = 3.42(7)% and the weighted pattern factor R wp = 4.50(5)%.

Figure 4. The refined XRD patterns of CuIn0.6Ga0.4Se2 nanopowders milled at 500 r/min.
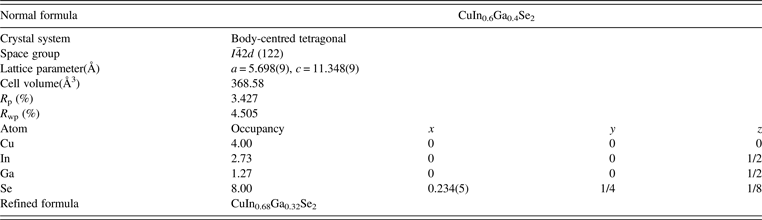
Table II lists the structural parameters, atomic positions and residual R factor of CuIn0.6Ga0.4Se2. The results agree very well with those of TREOR. On the basis of the results, the space group of CuIn0.6Ga0.4Se2 phase is ![]() $I\bar{4}2d$, and the unit-cell parameters are a = 5.698(9) Å and c = 11.348(9) Å.
$I\bar{4}2d$, and the unit-cell parameters are a = 5.698(9) Å and c = 11.348(9) Å.

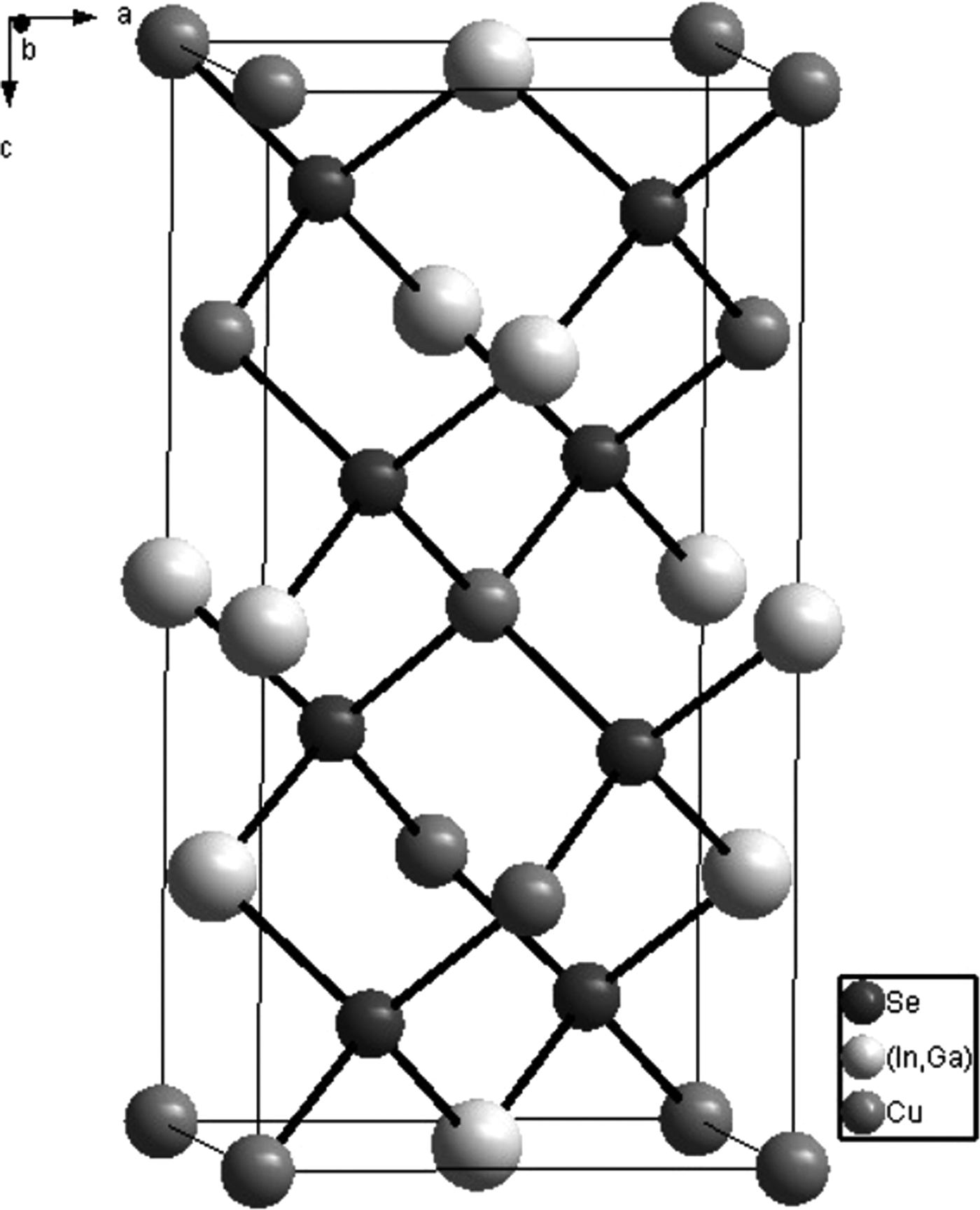
Figure 5. The crystal structural frame of CuIn0.6Ga0.4Se2 with chalcopyrite structure.
Table II. The refined structural parameters of CIGS nanoparticles milled with a speed of 500 r/min.

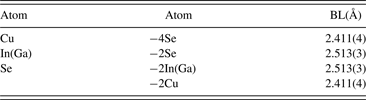
According to the refined results, the crystal structure of CuIn0.6Ga0.4Se2 is drawn by Diamond software, as shown in Figure 5. The nearest bond lengths in CuIn0.6Ga0.4Se2 are listed in Table III.
Table III. Bond length (BL) in CuIn0.6Ga0.4Se2.

IV. CONCLUSION
CIGS nanoparticles have been successfully prepared using mechanical milling technology. Based on XRD analyses, the main phase in CIGS nanoparticles milled at various rotation speeds is CuIn1−xGaxSe2 solid solution with a chalcopyrite structure. The unit-cell parameters are a = 5.693(8)–5.744(9) Å, c = 11.334(9)–11.524(4) Å for Ga content ranging from 0.5 to 0.3. The phase structures are related to the rotation speed. CIGS single phase is obtained with a speed more than 500 r/min. it implies that the low rotation speeds is beneficial for enhancing Ga content in CIGS and the possibility of Ga substitution for In decreases with increasing milling speed. The least grain size observed from SEM is around 200 nm. The grain size of the CIGS powders decreases with increasing milling speed. However, the large agglomerates of CIGS nanoparticles present with speed more than 500 r/min. It might be because of the high surface energy of the nanoparticles and strong van der Waals and Coulomb force between the grains. The milling speed plays an important role in preparation of CIGS nanoparticles. The high purity CIGS nanoparticles could be prepared by further optimization of milling speed.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No. 11274110). The authors appreciate the research group of Prof. Dr. Y.H. Ren in New York City University and their cooperation institute in Sun Harmonics Ltd in Hangzhou, China for providing vacuum nano-material synthesis equipments for preparing CIGS nanoparticles.