INTRODUCTION
The anthropogenic translocation of marine species between disjunct biogeographical regions is one of the most serious and gradually increasing threats to marine ecosystems. This human-mediated process has created significant, unpredictable and irreversible changes to abiotic and biotic environments in a large variety of water bodies worldwide and may cause severe economic damage (Carlton, Reference Carlton1996; Ruiz et al., Reference Ruiz, Carlton, Grosholz and Hines1997). When alien species proliferate populations in an area they are capable of re-structuring the food web, introducing new disease agents or parasites, altering habitat structures and changing gene pools (Holland, Reference Holland2000; Occhipinti Ambrogi, Reference Occhipinti Ambrogi2001; Schwindt et al., Reference Schwindt, Bortolusi and Iribarne2001; Occhipinti Ambrogi et al., Reference Occhipinti Ambrogi, Marchini, Cantone, Castelli, Chimenz, Cormaci, Froglia, Furnari, Gambi, Giaccone, Giangrande, Gravili, Mastrototaro, Mazziotti, Orsi-Relini and Piraino2011). Some invasions may trigger a set of changes in the ecosystem such as energy flow between trophic groups, primary production, relative extent of organic material decomposition and benthic–pelagic coupling (Vitousek et al., Reference Vitousek, D'Antonio, Loope, Rejmanek and Westbrooks1997). Alien species cause a total annual economic loss of $120 billion in the United States (Pimentel et al., Reference Pimentel, Zuniga and Morrison2005) and $15 billion in China (Xu et al., Reference Xu, Ding, Li, Qiang, Guo, Han, Huang, Sun, He, Wu and Wan2006). In the US, almost 42% of the threatened or endangered species are at risk due to impacts of alien species (Pimentel et al., Reference Pimentel, Zuniga and Morrison2005). Impacts of alien species are largely exacerbated by on-going climatic change and pollution in the sea (Stachowicz et al., Reference Stachowicz, Terwin, Whitlatch and Osman2002; Çinar et al., Reference Çinar, Katagan, Öztürk, Egemen, Ergen, Kocatas, Önen, Kırkım, Bakır, Kurt, Dağlı, Kaymakçı, Açik, Doğan and Özcan2006). Walther et al. (Reference Walther, Roques, Hulme, Sykes, Pyšek, Kühn, Zobel, Bacher, Botta-Dukát, Bugmann, Czúcz, Dauber, Hickler, Jarošík, Kenis, Klotz, Minchin, Moora, Nentwig, Ott, Panov, Reineking, Robinet, Semenchenko, Solarz, Thuiller, Vilà, Vohland and Settele2009) emphasized that global warming has enabled alien species to expand into regions where previously they could not survive and reproduce.
Three main vectors for species introductions are recognized: building canals (e.g. Suez and Panama), ship transport (hull fouling or ballast water), and deliberate introductions (i.e. mariculture, including associated species with cultured species) (Elton, Reference Elton1958; Carlton, Reference Carlton1985; Taylor et al., Reference Taylor, Williams and Strong2001). The number of introduced species has increased since trans-oceanic shipping crossings have increased in speed and time which is less than the larval stages of most marine invertebrates (Jensen & Knudsen, Reference Jensen and Knudsen2005). Huge volumes of ballast water (~12 billion t annually) are being discharged into or near ports (Netwig, Reference Netwig and Nentwig2007), leading to the establishment of some alien species. Fouling species that are attached to underwater surfaces of ships can become invasive in some areas. Sabellids and serpulid polychaetes are known to travel around the world on ships' hulls. For example, Ficopomatus enigmaticus, Hydroides elegans and Sabella spallanzanii have become a fouling problem as they have the ability to change the prevailing ecosystem drastically (Vitousek et al., Reference Vitousek, D'Antonio, Loope, Rejmanek and Westbrooks1997; Koçak et al., Reference Koçak, Ergen and Çinar1999; Hayes et al., Reference Hayes, Sliwa, Migus, McEnnulty and Dunstan2005). Except for the areas close to man-made canals, such as the eastern Mediterranean, the main vectors for species introductions were hull fouling (33% of all marine alien species worldwide) and ballast water (27%) (Gollasch, Reference Gollasch and Nentwig2007).
Successful settlement of alien species largely depends on the coincidence between delivery of the species to the new location and suitable conditions for establishment, including the absence of predators and the availability of resources (Hayden et al., Reference Hayden, Inglis, Schiel, Rilov and Crooks2009). Species-poor communities such as brackish-water environments and polluted waters are known to be more vulnerable to introductions (Elton, Reference Elton1958; Koçak et al., Reference Koçak, Ergen and Çinar1999; Nehring, Reference Nehring2006). Stachowicz et al. (Reference Stachowicz, Whitlatch and Osman1999) postulated that more diverse communities were less easily invaded as the amount of open space available for invasion decreased. Such spaces (niches) are also available in estuarine and polluted areas. For example, Baltic Sea estuaries are known to serve as stepping stones for the establishment of alien species (Leppäkoski & Olenin, Reference Leppäkoski and Olenin2000b). Crooks et al. (Reference Crooks, Chang and Ruiz2011) also demonstrated that alien species were less affected by pollutants (e.g. copper) than native species. A seasonal analysis of soft-bottom benthic communities in the eastern Mediterranean showed a positive, significant correlation between the density of Streblospio gynobranchia, and ammonium and silicate concentrations (Çinar et al., Reference Çinar, Katagan, Öztürk, Egemen, Ergen, Kocatas, Önen, Kırkım, Bakır, Kurt, Dağlı, Kaymakçı, Açik, Doğan and Özcan2006).
Polychaete species showing a great variety of feeding and reproductive strategies have been introduced from one area to another via ballast water, hull fouling (Carlton, Reference Carlton1985; Godwin, Reference Godwin2003) and canals (Çinar, Reference Çinar2009). Some polychaete species such as Terebrasabella heterouncinata and Polydora uncinata are known to bore into commercially important bivalve species and were transferred to different regions with their host species (Fitzhugh & Rouse, Reference Fitzhugh and Rouse1999; Moreno et al., Reference Moreno, Neill and Rozbaczylo2006). Polychaetes comprise 33% and 12% of the total number of alien species observed off the coasts of California (Foss et al., Reference Foss, Ode, Sowby and Ashe2007) and the Mediterranean Sea (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010), respectively. Alien polychaete species have become a subject of interest regionally: i.e. the Mediterranean Sea (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010); Chile (Moreno et al., Reference Moreno, Neill and Rozbaczylo2006); Brazil (Neves & Rocha, Reference Neves and Rocha2008); the North Sea (Reise et al., Reference Reise, Gollasch and Wolff1999); South Africa (Mead et al., Reference Mead, Carlton, Griffiths and Rius2011); the Black Sea (Kurt Sahin & Çinar, Reference Kurt Sahin and Çinar2012); and Japan (Iwasaki, Reference Iwasaki, Koike, Clout, Kawamichi, De Poorter and Iwatsuki2006). Nishi & Kato (Reference Nishi and Kato2004) analysed alien polychaete species worldwide and reported 74 species belonging to 15 families. This estimate is below the diversity of alien polychaetes. For example, only in the Mediterranean basins, a total of 134 alien polychaetes have been reported so far (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010).
The aims of this review are to present the worldwide status of marine and brackish-water alien polychaete species, and also to emphasize the importance of these species in the ecosystem and economy.
MATERIALS AND METHODS
A list of alien polychaete species was prepared based on species records updated in January 2012. The species data were mainly extracted from the regional reviews on alien species and check-lists of polychaete species. Alien species are investigated within all regions in terms of their establishment success, their area of origin and their mode of introduction.
Alien species were grouped into 4 main categories, namely established, casual, questionable and cryptogenic. The definition of the terms can be found in Zenetos et al. (Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010). Briefly, established species are the alien species with self-maintaining populations; casual species are the species which were reported only once in the region; questionable species are the species which were reported without sufficient information and their taxonomic status is uncertain; cryptogenic species are the species with no definite evidence of their native or introduced status according to the categories proposed by Carlton (Reference Carlton1996). Invasive species are the established aliens that have overcome biotic and abiotic barriers and are able to expand their geographical range through the production of fertile offspring with noticeable impact on the invaded habitats.
RESULTS AND DISCUSSION
How many alien polychaete species?
A compilation of the literature revealed that 292 polychaete species belonging to 164 genera were reported as alien species in at least one locality in the world's oceans (Table 1). According to Beesley et al. (Reference Beesley, Ross and Glasby2000), almost 8348 polychaete species belonging to 1099 genera have been described worldwide. Based on this finding, it was estimated that almost 15% of total number of polychaete genera and 3.4% of total number of species have been reported as alien species throughout the world. The most speciose genera are Hydroides (16 species) and Polydora (16 species), both accounting for 11% of total number of alien species, followed by Boccardia (9 species) and Syllis (8 species) (Figure 1). Many genera are represented by a single species.
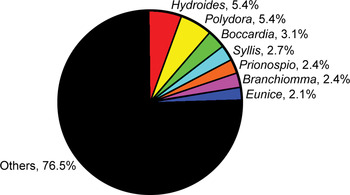
Fig. 1. Relative dominance of polychaete genera by the number of alien species.
Table 1. The list of alien polychaete species reported from the world's ocean. MI, probable means of introduction (Sh, ship (ballast water or fouling); Su, Suez Canal; D, deliberate stocking; A, aquaculture); Succ, establishment success (E, established; C, casual; Cr, cryptogenic; Q, questionable).

W Atlantic, western Atlantic; E Atlantic, eastern Atlantic; NE Atlantic, north-eastern Atlantic; W Pacific, western Pacific; SW Pacific, south-western Pacific; NW Atlantic, north-western Atlantic; NW Tropical Atlantic, north-western Tropical Atlantic; N Pacific, northern Pacific.
Thirty-nine polychaete families (almost half of the known families) include alien species. Spionidae ranked highest in terms of number of alien species (53 species, 18% of the total number of species). Other families represented by a high number of alien species worldwide are Serpulidae (46 species, 15% of the total number of species), Nereididae (26 species, 9% of the total number of species) and Sabellidae (23 species, 7% of the total number of species) (Figure 2). Thirteen families had only a single record of an alien species.

Fig. 2. Relative dominance of polychaete families by the number of alien species.
The number of alien polychaete species greatly varied according to regions. The highest number of alien species (134 species) were reported from the Mediterranean Sea (Figure 3). This region has been greatly influenced by the influx of lessepsian migrants (i.e. species migration from the Red Sea to the Mediterranean via the Suez Canal) after the opening of the Suez Canal in November 1869, and was relatively well studied in this respect (Ben-Eliahu, Reference Ben-Eliahu, Petersen and Kirkegaard1991; Çinar, Reference Çinar2009). The other regions with high number of alien polychaetes are the coasts of the Hawaii Islands (47 species) and the USA Pacific (34 species). The coasts of Australia (20 species), the Red Sea (20 species), New Zealand (17 species), the North Sea (16 species) and Argentina (16 species) were also affected by dense invasions by polychaete species. In some areas, such as the coasts of Pakistan (Hydroides elegans) (Ishaq & Mustaquim, Reference Ishaq and Mustaquim1996), Singapore (Hydroides sanctaecrucis) (Lewis et al., Reference Lewis, Watson and ten Hove2006) and the Philippines (Boccardia berkeleyorum) (Williams, Reference Williams2001), only a single alien polychaete species has been reported so far. The main difference in the number of alien polychaete species among regions is related to a number of factors. This includes sampling area, scientific effort, resistance of native marine populations to invasion, frequency of inoculation by invading populations, and difficulties in resolving the species-level taxonomy of alien organisms.

Fig. 3. The number of alien polychaete species worldwide.
Alien polychaetes are grouped into four broad categories namely established, casual, questionable and cryptogenic. Of the total number of alien species (292 species), 211 species were classified as established or casual alien species in a given area. A total of 180 species have become established in the world's oceans and 31 species (casual species) have a potential to establish viable populations. The number of established and casual polychaete species vary among regions, with the highest score (94 species) in the Mediterranean Sea, followed by the Hawaiin Islands (25 species) and the USA Pacific coast (28 species) (Figure 4). The Red Sea (20 species), Australia (16 species) and New Zealand (13 species) had also high number of established alien species.

Fig. 4. The number of established and casual alien species worldwide.
The Mediterranean (38 species) and Sea of Marmara (9 species) possessed a high number of questionable alien species (Figure 5). They are the species mostly reported from ecological or faunal studies in the region without detailed descriptions (i.e. Rullier, Reference Rullier1963). Species such as Spiophanes bombyx (parentheses in Table 1) cited as aliens require confirmation after the recent generic revisions (e.g. Meißner, Reference Meißner2005).

Fig. 5. The number of questionable alien species worldwide.
The highest number of cryptogenic species (19 species) were reported from the Hawaiin Islands (Carlton & Eldredge, Reference Carlton and Eldredge2009) (Figure 6). The south-west coast of the Atlantic Ocean (Argentina and Brazil) also had a high number of cryptogenic species (Figure 6). Ten cryptogenic species were reported on the coast of Argentina by Orensanz et al. (Reference Orensanz, Schwindt, Pastorino, Bortolus, Casas, Darrigran, Elías, López Gappa, Obenat, Pascual, Penchaszedeh, Piriz, Scarabino, Spivak and Vallarino2002). Regions such as the USA Pacific coast (Boyd et al., Reference Boyd, Mulligan and Shaughnessy2002; Wasson et al., Reference Wasson, Fenn and Pearse2005), the USA Atlantic coast (Ruiz et al., Reference Ruiz, Fofonoff, Carlton, Wonham and Hines2000) and Norway (Hopkins, Reference Hopkins, Leppäkoski, Gollasch and Olenin2002) also had a relatively high number of cryptogenic species. In the Mediterranean Sea, only Paraprionospio coora and Chaetozone corona were known to be cryptogenic species (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010).
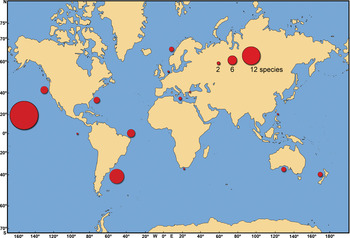
Fig. 6. The number of cryptogenic species worldwide.
After re-evaluating the distribution patterns and taxonomic positions of the species, 16 polychaete species were excluded from the alien list of the Mediterranean Sea (see Zenetos et al., Reference Zenetos, Çinar, Pancucci-Papadopoulou, Harmelin, Furnari, Andaloro, Bellou, Streftaris and Zibrowius2005). Alien species lists of some regions have been prepared by non-polychaete taxonomists so these lists should be used with some reservations. After further examination the species currently identified as aliens could be re-identified as new or different taxa. In Table 1, some species from the alien list have been eliminated as there is more information about their distribution pattern. For example, Ficopomatus enigmaticus was considered as alien for the coasts of Australia (Hewitt, Reference Hewitt2002), but Pillai (Reference Pillai2008) indicated that it could be a species native to Australia and might have been spread to other tropical and subtropical regions by shipping.
Where do they come from?
The geographical origins of marine aliens are largely correlated with the predominant shipping routes in and out of a country (Hayden et al., Reference Hayden, Inglis, Schiel, Rilov and Crooks2009). The majority of alien polychaete species (80% of the total number of species) in the Mediterranean Sea originated from the Red Sea and Indo-Pacific areas (Figure 7). The Suez Canal is the main vector for the species introductions in the region. The Atlantic species accounted for 18% of the total number of species and were introduced to the region mainly via ships. The Pacific coasts of America have been largely colonized by the species of Atlantic origin. For example, the Atlantic-originated species on the Pacific coast of USA, Mexico and Colombia accounted for 49%, 40% and 100% of total number of alien species, respectively. The shallow-water benthic habitats of the areas between 40°N and 40°S have alien polychaete species mostly originating from other tropical and subtropical regions. The Mediterranean species invade the Red Sea via the Suez Canal (anti-lessepsian migrants, 90% of total alien species in the Red Sea) (Por, Reference Por1978). The Mediterranean species were also transferred to the Indo-Pacific areas such as Taiwan (33% of total alien polychaete species) (Paxton & Chou, Reference Paxton and Chou2000; Radashevsky & Hsieh, Reference Radashevsky and Hsieh2000a, Reference Radashevsky and Hsiehb), India (22%) (Subba Rao, Reference Subba Rao2005), Australia (21%) (Polland & Hutchings, Reference Polland and Hutchings1990; Currie et al., Reference Currie, McArthur and Cohen2000; Hewitt et al., Reference Hewitt, Campbell, Thresher, Martin, Boyd, Cohen, Currie, Gomon, Keough, Lewis, Lockett, Mays, McArthur, O'Hara, Poore, Ross, Storey, Watson and Wilson2004; Hayes et al., Reference Hayes, Sliwa, Migus, McEnnulty and Dunstan2005) and New Zealand (18%) (Handley, Reference Handley2000; Glasby et al., Reference Glasby, Read, Lee, Blakemore, Fraser, Pinder, Erséus, Moser, Burreson, Govedich, Davies and Dawson2009) via ships. The coasts of Azores, France, Ireland and Britain were also largely colonized by the Mediterranean species. The alien polychaete species in the Caspian Sea and Aral Lake were mainly introduced from the Black Sea (Leppäkoski & Olenin, Reference Leppäkoski, Olenin and Pederson2000a; Grigorovich et al., Reference Grigorovich, Therriault and MacIsaac2003). The Indo-Malaysian region can be identified as a centre of alien species originating from south-east Asia, China, Japan, the Philippines and Australian regions (Subba Rao, Reference Subba Rao2005).

Fig. 7. The origins of alien polychaete species in regions.
What are the main vectors for species introductions?
Figure 8 indicates that shipping is a major vector for the introduction of polychaete species worldwide. Polychaetes can be transferred via ballast waters of ships or hull fouling. Almost 82% of serpulid species were introduced in a new region by ship fouling. Spionids, except for boring species, are translocated in ballast water (Carlton, Reference Carlton1985). The Suez Canal seems to have played an important role for the species exchange between the Mediterranean and Red Seas. Almost 48% of the alien polychaetes in the Mediterranean Sea are lessepsian migrants (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010). However, the importance of lessepsian migrants changes according to the basins of the Mediterranean. For example, lessepsian migrants comprised almost 51% of the alien polychaete species on the Levantine and Aegean coasts of Turkey, but only 6% on the Italian coast. The majority of alien polychaete species reported on the coasts of England (Eno et al., Reference Eno, Clark and Sanderson1997), Spain (Atlantic) (Martinez et al., Reference Martinez, Adarraga and Lopez2006), the Philippines (Williams, Reference Williams2001) and Chile (Moreno et al., Reference Moreno, Neill and Rozbaczylo2006) were introduced to the areas in association with the aquaculture of some mollusc species such as Crassostrea virginica and Haliotis rufescens. Almost 30% of total alien species reported on the coasts of Australia (Hayes et al., Reference Hayes, Sliwa, Migus, McEnnulty and Dunstan2005; Sato-Okoshi et al., Reference Sato-Okoshi, Okoshi and Shaw2008), New Zealand (Handley, Reference Handley2000; Glasby et al., Reference Glasby, Read, Lee, Blakemore, Fraser, Pinder, Erséus, Moser, Burreson, Govedich, Davies and Dawson2009; Read, Reference Read2010) and Brazil (Radashevsky et al., Reference Radashevsky, Lana and Nalesso2006) were introduced to the regions via aquaculture activities. Some large polychaete species are widely used as fish bait (Brown, Reference Brown1993; Dagli et al., Reference Dagli, Ergen and Çinar2005) and the bait trade has led to the introductions of some species. Two nereidid species are known to have been transferred by the worm bait trade. Namalycastis abiuma was exported from an Indo-Pacific area to the Pacific coast of the USA (Cohen & Carlton, Reference Cohen and Carlton1995), and Nereis aibuhitensis from Korea to Japan (Nishi & Kato, Reference Nishi and Kato2004) and Portugal (Fidalgo e Costa et al., Reference Fidalgo, Costa, Gil, Passos, Pereira, Melo, Batista, Cancela da Fonseca, Sarda, San Martín, Lopez, Martín and George2006). About 620 million live animals were brought into Japan in 2003 and more than 90% of these are classified as worms for fishing bait (Mito & Uesugi, Reference Mito and Uesugi2003).

Fig. 8. The main vectors of species introductions worldwide.
Three alien polychaete species, namely Hediste diversicolor, Streblospio gynobranchiata and Ficopomatus enigmaticus, were reported from the Caspian Sea (Grigorovich et al., Reference Grigorovich, Therriault and MacIsaac2003; Taheri et al., Reference Taheri, Seyfabadi, Abtahi and Foshtomi2009). Hediste diversicolor was intentionally transferred from the Black Sea to the Caspian Sea for stocking, whereas the other species were introduced to the area via shipping. Only one alien species (H. diversicolor) was reported from Aral Lake (Leppäkoski & Olenin, Reference Leppäkoski, Olenin and Pederson2000a) and was intentionally introduced to the area from the Black Sea.
Invasive species and their impacts
According to Williamson & Fitter (Reference Williamson and Fitter1996), 10% of the alien species could become established in the recipient area and 1% of them will eventually become invasive. This rule cannot be applied everywhere. In the Mediterranean, for example, 45% of the total number of alien species have become established and 18% of the total number have invasive characters (Zenetos et al., Reference Zenetos, Verlaque, Gofas, Çinar, Garcia Raso, Azzurro, Bilecenoglu, Froglia, Siokou, Bianchi, Morri, Sfriso, San Martin, Giangrande, Katağan, Ballesteros, Ramos Espla, Mastrototaro, Ocana, Zingone, Cantone, Gambi and Streftaris2010). These ratios also change according to the sub-areas of the Mediterranean. Established and invasive species accounted for up to 53% and 23% of the total number in the eastern Mediterranean; 50% and 8% of the total number in the western Mediterranean; and 43% and 29% of the total number in the Sea of Marmara, respectively.
Polluted or physically degraded environments are known to be more prone to invasion than pristine habitats (Çinar et al., Reference Çinar, Katagan, Öztürk, Egemen, Ergen, Kocatas, Önen, Kırkım, Bakır, Kurt, Dağlı, Kaymakçı, Açik, Doğan and Özcan2006; Galil, Reference Galil2000). Native species might not be adapted to the changed environmental conditions, reducing their ability to exploit resources which provides opportunities for invaders (Shea & Chesson, Reference Shea and Chesson2002). Increased pollution exposure affects hard bottom assemblages dominated by natives to become either dominated by alien species or equally occupied by native and alien species (Piola & Johnston, Reference Piola and Johnston2008). The polluted soft-bottom benthic environments in Izmir Bay where a large international harbour is located have been densely invaded by the species originated from the north-west Atlantic Ocean (Polydora cornuta and Streblospio gynobranchiata) and the coast of Japan (Pseudopolydora paucibranchiata) (Çinar et al., Reference Çinar, Ergen, Dagli and Petersen2005; Dagli & Çinar, Reference Dagli and Çinar2008). They constituted more than 95% of the total individuals and 70% of the total biomass at some stations. Çinar et al. (Reference Çinar, Ergen, Dagli and Petersen2005) indicated that these species had a great impact on the prevailing ecosystem and seemed to have replaced some opportunistic species previously known from the polluted Izmir Bay such as Capitella capitata and Malacoceros fuliginosus. Ranasinghe et al. (Reference Ranasinghe, Mikel, Velarde, Weisberg, Montagne, Cadien and Dalkey2005) found that P. paucibranchiata accounted for 52% of total zoobenthic populations in southern California embayments.
In the shallow-water benthic habitats of the eastern Mediterranean, some lessepsian migrants such as Pseudonereis anomala and Eunice antennata became dominant components of benthic communities and competed with some native species. Ben-Eliahu (Reference Ben-Eliahu, Spanier, Steinberger and Luria1989) postulated that Perinereis cultrifera, a native nereidid species of the Mediterranean, was excluded from algal habitats along the Israeli coast, probably due to an efficient dispersal. Perinereis cultrifera has direct, non-pelagic development and its dispersal is consequently more restricted than that of the migrant species (Pseudonereis anomala), which has the pelagic Heteronereis stage. The changes that have occurred in benthic communities of the Levant coast of Turkey due to the dense populations of E. antennata (Kurt Sahin & Çinar, Reference Kurt Sahin and Çinar2009) are unknown at this stage and require further investigation.
Some serpulid species such as Hydroides elegans, H. ezoensis, H. sanctaecrucis and Ficopomatus enigmaticus invade artificial substrates in polluted or brackish water environments in tropical and subtropical areas (i.e. Schwindt et al., Reference Schwindt, Bortolusi and Iribarne2001; Hewitt, Reference Hewitt2002; Zvyagintsev, Reference Zvyagintsev2002; Lewis et al., Reference Lewis, Watson and ten Hove2006). The reef builder species, F. enigmaticus, was considered an important part of the ecosystem by providing a suitable habitat for other species and also by changing physical factors of the invaded environment (Schwindt et al., Reference Schwindt, Bortolusi and Iribarne2001). This species may attain an annual production in dry weight of almost 21 kg.m−2.yr−1 (Fornós et al., Reference Fornós, Forteza and Martínez-Taberner1997) and a density of 150,000 ind.m−2 (Bianchi & Morri, Reference Bianchi and Morri2001) in the Mediterranean Sea. In harbours and estuarine areas it causes economic problems due to fouling (Read & Gordon, Reference Read and Gordon1991). However, dense populations of F. enigmaticus in enclosed waters including harbours have very beneficial effects on water quality, reducing suspended particulate loads and improving both the oxygen and nutrient status (Davies et al., Reference Davies, Stuart and Villiers1989). This species also hosts other alien species such as Polydora cornuta and Conopeum seurati (Read & Gordon, Reference Read and Gordon1991).
Hydroides elegans and H. dianthus can form dense populations in harbour environments. In the Mediterranean Sea, the population densities of H. elegans and H. dianthus may attain 110700 ind.m−2 and 33050 ind.m−2, respectively (Çinar et al., Reference Çinar, Katagan, Koçak, Öztürk, Ergen, Kocatas, Önen, Kirkim, Bakir, Kurt, Dagli, Açik, Dogan and Özcan2008). In the lagoon of Orbetello (Italy), H. dianthus built a small (less than 1 m) reef (Bianchi & Morri, Reference Bianchi and Morri2001). These species, with calcareous tubes, can become a nuisance when they attach to hard structures such as quays, mariculture equipment and ships' hulls (Relini, Reference Relini1993). In Hong Kong waters, mariculture cage nets were completely colonized by H. elegans after immersion for one month, the thickness and wet weight were reported to be 38 mm and 12.5 kg.m−2, respectively (Jianjun & Zongguo, Reference Jianjun and Zongguo1993). This species together with other fouling organisms can block the net mesh and greatly reduce water circulation (Zongguo et al., Reference Zongguo, Zhengyan, Morton and Yan1999). They also add considerable weight to the nets which can cause the cages to sink, enabling the stock to escape. In the same area, the density and biomass of H. elegans on ferry docks and boats were estimated as 3.3×105 ind.m−2 and 20.5 kg.m−2 (Jianjun & Zongguo, Reference Jianjun and Zongguo1993). Hydroides elegans is a mariculture pest in Japan, where it competes with oysters for food and oxygen. An outbreak of this serpulid polychaete in Hiroshima Bay has caused heavy economic loss of cultured oyster crops through fouling on their shells (Hirata & Akashige, Reference Hirata and Akashige2004). In a study by Çinar (Reference Çinar2006), alien serpulid species comprised more than 95% of the total serpulid specimens found in hard substrates such as rocks, molluscs and artifical substrates (i.e. docks' pilings, ropes and tires) in the eastern Mediterranean. Spirobranchus kraussii (previously known as Pomatoleios kraussii) formed a densely populated belt in shallow-water areas in Mersin Bay, providing a suitable habitat for small vagile fauna (Çinar, Reference Çinar2006). In the area, some man-made structures and also natural substrates such as stones were completely covered by Hydroides operculatus. The population density and biomass reached up to 384,000 ind.m−2 and 246 g.m−2, respectively (Çinar, Reference Çinar2006). In the United Kingdom, fouling of H. ezoensis (up to 30 cm thick) has caused navigation problems by reducing the flotation of navigation buoys (Eno et al., Reference Eno, Clark and Sanderson1997). Dense settlements of serpulid polychaetes may kill other native species such as young oysters and mussels by overgrowing (Eno et al., Reference Eno, Clark and Sanderson1997). The dense settlement of Neodexiospira brasiliensis on Zostera leaves resulted in decreasing the eel grass' photosynthetic efficiency (Critchley & Thorp, Reference Critchley and Thorp1985).
Some sabellid species have become invasive in some regions. Sabella spallanzani, a native species of the Mediterranean Sea, forms dense populations on artificial substrates in Australian waters and competes with native suspension feeders (i.e. mussels) and interferes with their recruitment (Hayes et al., Reference Hayes, Sliwa, Migus, McEnnulty and Dunstan2005). The same impacts were also noted for Branchiomma luctuosum (Licciano et al., Reference Licciano, Giangrande and Gambi2002) and B. bairdi (Çinar, Reference Çinar2009) in the Mediterranean Sea. Euchone limnicola forms dense populations in soft substratum of Portland Harbour, Dorset, UK (mean density of 2127 ind.m−2) and competes with native species for food and space (Wilson, Reference Wilson, Hewitt, Campbell, Thresher and Martin1999). The tubes retain sediment, thereby altering the habitat for other organisms (Wilson, Reference Wilson, Hewitt, Campbell, Thresher and Martin1999).
Marenzelleria viridis, a North American spionid, was first recorded in brackish waters of the Wadden Sea and Baltic Sea in the early 1980s (Kube et al., Reference Kube, Zettler, Gosselck, Ossig and Powilleit1996). It has spread rapidly and is now a dominant species of zoobenthos in estuaries and coastal lagoons. Leppäkoski & Olenin (Reference Leppäkoski and Olenin2000b) calculated the mean rate of spread of M. viridis within the Baltic Sea as 480 km/year. Marenzelleria viridis managed to seasonally dominate soft bottom benthic habitats in the Baltic Sea, where the maximum density was estimated as 28,000 ind·m−2 (Kube et al., Reference Kube, Zettler, Gosselck, Ossig and Powilleit1996; Gruszka, Reference Gruszka1999). This species re-circulates organic matter deposited in deeper sediment, links benthic and pelagic subsystems, and provides new microhabitats for its associated fauna (Kube et al., Reference Kube, Zettler, Gosselck, Ossig and Powilleit1996; Gruszka, Reference Gruszka1999). In the Ems estuary (North Sea), increasing densities of M. viridis in a sandy habitat (maximum juvenile density 19300 ind.m−2) coincided with a reduced abundance of the polychaete Hediste diversicolor (Essink & Kleef, Reference Essink and Kleef1988).
Boring polychaetes belonging to the families Spionidae, Cirratulidae and Sabellidae commonly infest shells of cultured mollusc species (Blake, Reference Blake1969; Evans, Reference Evans1969; Moreno et al., Reference Moreno, Neill and Rozbaczylo2006). The intensive shellfish trade has been implicated in the introduction of several boring polychaetes to different parts of the world together with their hosts. Boring spionid polychaetes belonging to the genera Boccardia, Dipolydora and Polydora, and the sabellid Terebrasabella heterouncinata can cause severe damage to the mollusc shells, affecting the fitness of their hosts and often causing enormous financial loss to owners of aquacultures worldwide (Evans, Reference Evans1969; Martín & Britayev, Reference Martín and Britayev1998; Fitzhugh & Rouse, Reference Fitzhugh and Rouse1999; Moreno et al., Reference Moreno, Neill and Rozbaczylo2006; Radashevsky et al., Reference Radashevsky, Lana and Nalesso2006; Simon et al., Reference Simon, Worsfold, Lange and Sterley2010).
Terebrasabella heterouncinata, a native species of the South African coast, densely infests several gastropods (Simon et al., Reference Simon, Kaiser and Britz2005) and has become a pest on cultured abalone in South Africa and California in the early 1990s (Fitzhugh & Rouse, Reference Fitzhugh and Rouse1999; Moore et al., Reference Moore, Juhasz, Robbins and Grosholz2007). This species inhabits burrows that form when the larvae settle on the growing edge of shells of the abalone (Haliotis midae), and the host covers it with shell (Simon et al., Reference Simon, Kaiser and Britz2004). Farmed abalone with heavy infestations reduce or cease production of the prismatic layer of shell and develop brittle shells that are domed in shape with deformed or absent respiratory pores (Moore et al., Reference Moore, Juhasz, Robbins and Grosholz2007). Consequently, dense settlement of the species results in deformation and weakening of the shell, a reduction in growth, or the death of the abalone (Simon et al., Reference Simon, Kaiser and Britz2004). The poor price obtained for abalone with deformed shells was directly responsible for the failure of several farms (Moore et al., Reference Moore, Juhasz, Robbins and Grosholz2007).
Reports of the introductions of boring spionid polychaetes increased with the growth of the shellfish trade worldwide (Radashevsky & Olivares, Reference Radashevsky and Olivares2005; Sato-Okoshi et al., Reference Sato-Okoshi, Okoshi and Shaw2008). Polydora websteri is commonly associated with the shells of commercial oysters and other bivalves of estuaries and nearshore environments (Blake, Reference Blake, Blake, Hilbig and Scott1996). The spread of P. websteri along the east coast of Australia (associated mortalities first recorded in 1880) forced Sydney oyster producers into an intertidal stick and tray culture system (Bower, Reference Bower2001). Dense infestations of this species in cultured oysters caused the collapse of a highly intensive aquaculture industry in Hawaii (Bailey-Brock & Ringwood, Reference Bailey-Brock and Ringwood1982). In Chile, Polydora rickettsi was reported to have infested 24% of mature abalones Haliotis rufescens (Moreno et al., Reference Moreno, Neill and Rozbaczylo2006) while P. uncinata infested 99% of specimens of Haliotis discus hannai (up to 42 worms on one shell) (Radashevsky & Olivares, Reference Radashevsky and Olivares2005). Polydora uncinata and Boccardia knoxi bore into the shells of cultured abalones as well as shells of native bivalve and gastropods (Sato-Okoshi et al., Reference Sato-Okoshi, Okoshi and Shaw2008). Polydorid infestation can reduce the growth rates of shell and meat yield. Moreover, due to the stress caused by the boring spionids, the molluscs are more susceptible to adverse environmental changes leading to increased mortality (Bower, Reference Bower2001).
CONCLUSIONS
The alien polychaete diversity greatly varies according to regions. Although there are many reasons for the difference in diversity the principal factor seems to be the magnitude of scientific efforts focusing on this aspect among regions. The Mediterranean Sea accounted for 47% of the total number of alien species up to date recorded. As lessepsian migrants intensively invade benthic habitats of the eastern Mediterranean and have special attention in scientific and public media, the Mediterranean Sea has relatively been well studied. Although there are only a few data sets regarding alien polychaete species in western and eastern parts of Africa if intensive sampling is applied numbers will greatly increase. Expanding commercial trade between distinct regions via large ships accelerates the introduction of alien species. Large commercial harbours in various countries should be studied and monitored to record alien species distribution.
The alien polychaete species that have been reported in the literature from different parts of the world's oceans are amalgamated as a comprehensive list (Table 1). A list of alien polychaete species should be prepared and updated regularly to monitor their distribution and the adverse effects on the local ecosystem. These data will help to improve the management and regulation of invasive species.











