Burmese amber (Burmite) from northern Myanmar is now a celebrated source of Cretaceous fossils, throwing light on the evolution of insects, plants, pollination and squamate reptiles (Ross et al. Reference Ross, Mellish, York, Crighton and Penney2010 and references therein; Daza et al. Reference Daza, Stanley, Wagner, Bauer and Grimaldi2016; Grimaldi & Ross Reference Grimaldi, Ross, Fraser and Sues2017).
A commonly observed club-shaped structure, 2–6 mm in width, was described by Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002, p. 9) and interpreted as “sporangia of a fungus or possibly plant.” These were noted as the “most common organismal inclusion” in the material studied. Scanning electron micrographs of the exposed surfaces of these forms (Grimaldi et al. Reference Grimaldi, Engel and Nascimbene2002, fig. 6) revealed a reticulated, in places ‘herringbone’ pattern of fine ridges. A possible explanation involved sporangia growing on the wood surface within cavities into which resin flowed and then hardened, being released as the enclosing wood decayed during burial (Grimaldi et al. Reference Grimaldi, Engel and Nascimbene2002, fig. 4).
Poinar & Brown (Reference Poinar and Brown2003) formally described this club-shaped form as Palaeoclavaria burmitis (Hymenomycetes: Palaeoclavariaceae), referring to it as the “first fossil record of the Aphyllophorales”. Echoing Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002), Poinar & Brown (Reference Poinar and Brown2003, p. 767) argued that, “the new species was probably lignicolous … and may have developed around wounds on the resin producing tree … Resin released from the wound could have engulfed the surrounding sporocarps, resulting in their preservation on both sides of the amber pieces.”
These club-like forms can be shown to be bivalve borings produced after hardening of the amber-forming resin and a brief note in Ross et al. (Reference Ross, Mellish, York, Crighton and Penney2010) recognises this, citing a personal communication from Paul Jeffery (then at Oxford University Museum of Natural History). This fact was independently recognised by the current first author on the basis of truncation relationships and external form, who then contacted D. Grimaldi and the second author with enquiries. Here, we present new observations on the clavate features, clavate features with associated bivalve fossils and bivalve fossils not associated with the clavate features.
1. Geological setting
Zherikhin & Ross (Reference Zherikhin and Ross2000) reviewed the historical localities and concluded that amber of Cretaceous age had been reworked into secondary Eocene deposits. Cruickshank & Ko (Reference Cruikshank and Ko2003) subsequently studied the primary (not reworked) sedimentary succession bearing Burmese amber at the Noije Bum hill site in the Hukawng Basin. They observed disk-shaped clasts of amber, orientated parallel to bedding in siliciclastic sedimentary rocks which contained a rare ammonite (Mortoniceras sp.). They inferred a coastal deltaic or estuarine depositional setting. Shi et al. (Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012) noted the abundance of volcanic lithic fragments in the mud rocks and dated enclosed zircons. The youngest zircons in the primary amber-containing deposit were radiometrically dated to 98.79±0.62 Ma, providing a maximum age for the deposit.
2. Material and methods
Three specimens in the first author's personal collection and two specimens in the National Museums Scotland's collection have been studied in detail. They are:
RS.P1445, a 36×25×7 mm polished piece of amber illustrating the development of the clavate forms on both major sides of the piece of amber (Fig. 1). The clavate features cluster into groups with densities ranging from 15 per square cm to 0 per square cm. Fills of the clavate features are either fine sand, coarse upper sand or calcite cement. Photographs taken by the first author (using an RX100 III camera attached to a Euromex microscope and Zerene stacking software) and CT scans (made with an XT H 225 system) of this specimen are presented here.
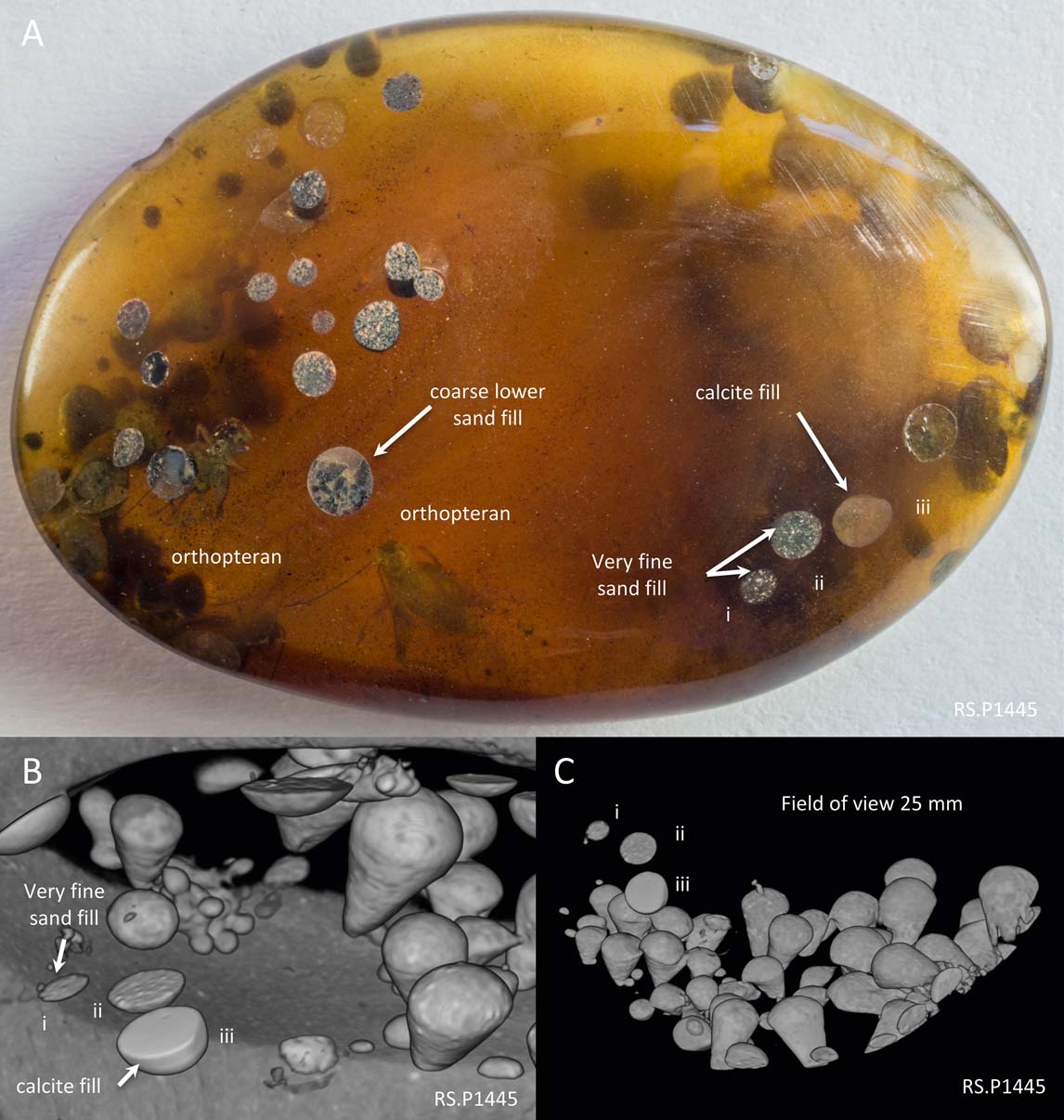
Figure 1 Form, distribution and fill of clavate borings in Cretaceous amber from Myanmar: (A) photograph of RS.P1445, showing patchy distribution of clavate borings, with density ranging from 15/cm2 to 0/cm2. As noted by Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002), the features are directed towards the interior of the amber from both sides. They are filled with either siliciclastic sediment or sparry calcite. Specimen width 37 mm; (B) CT scan image of the right-hand side of the same specimen, showing clavate borings directed from the lower surface of the amber upwards and (now truncated) from the top surface down. Annotated borings i, ii and iii can be used as a spatial cross-reference. Field of view 15 mm; (C) CT scan image showing distribution of a concentration of clavate borings.
RS.P1450 is a 24×14×6 mm piece of amber containing a fragment of Metasequoia and 25 of the clavate objects, ranging in size from 3 mm to 4 mm in length and <1 mm to 3 mm in maximum diameter. Several of the clavate features contain in situ bivalve fossils. Truncation of the plant specimen by the clavate features clearly documents their origin through boring. The same equipment as for RS.P1445 was used.
RS.P1460 is an unusual specimen, 11×9×3 mm, which contains two articulated pholadid individuals (each approximately 2 mm long), not enclosed within one of the common clavate structures seen in other specimens. One of these individuals is associated with an irregularly shaped feature filled with moderately sorted very fine upper to fine lower sand. The same equipment as for RS.P1445 was used.
NMS G.2015.21.1 is an 88×47×31 mm unpolished broken rounded piece of amber with sand-filled clavate borings around its circumference, though with more borings on one side (Fig. 2). Much of the surface of the more populated side is obscured by adhering sand, although where the apertures can be seen there is a density of about 25 crypts to one square cm. The circular apertures at the surface range from 0.5 mm to 0.8 mm in diameter. On the fractured surface of the piece, the largest crypt seen is 3.5 mm long and the shortest is 0.8 mm. The specimen was generously donated to National Museums Scotland by Mr Fan Yong of the Fushun Amber Institute, China.

Figure 2 (A) Rounded piece of Burmese amber showing pholadid bivalve domichnia all around the edge of a fractured surface. There is a higher concentration of borings on the lower side as viewed in the photograph, which was probably uppermost for longer to enable more colonisation whilst it lay on the sea-bed. Specimen NMS G.2015.21.1. Scale bar = 10 mm. (B) Close-up of the lower surface, showing the sand-filled apertures of the borings. Some sand is still adhering to the surface of the amber. Scale bar = 1mm.
NMS G.2010.20.3 is a 28×16×10 mm polished piece of amber with clavate borings penetrating a mayfly (Ephemeroptera). One boring has gone through the hind margins of the left wings, another has gone through the left cercus and a third has gone through most of the head and through the left foreleg, thus demonstrating that the mayfly was trapped in the resin and the resin hardened before it was bored. There are also small circular pits at the surface of the amber, 0.2–0.4 mm wide, which are likely to be failed attempts at boring into the resin, probably due to premature death. The specimen was purchased from Scott Anderson, USA.
Two additional specimens in the first author's collection (RS.P1449 and RS.P1429) illustrate the truncation relationship between the cross-cutting clavate features and a spider and a feather (Fig. 3).
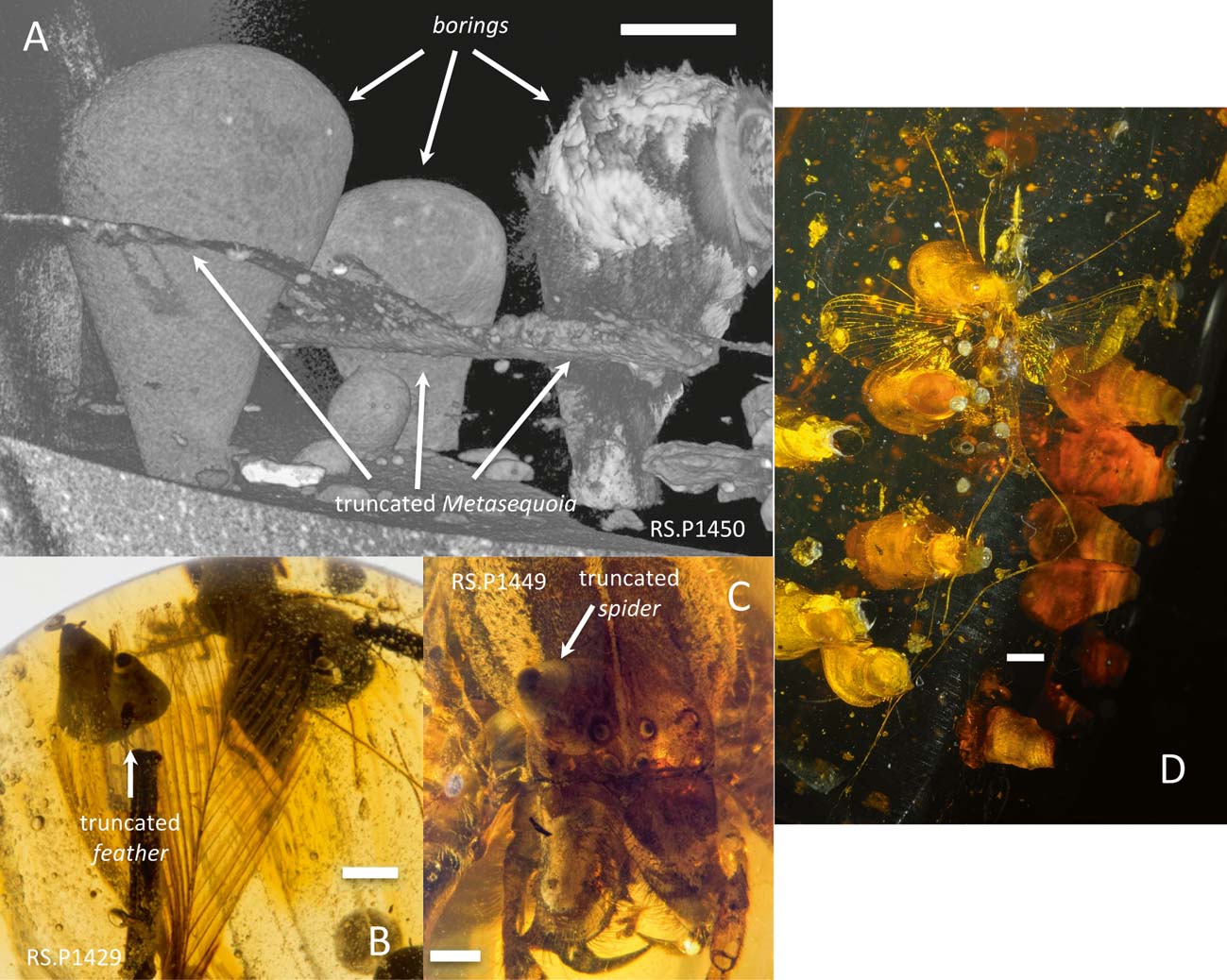
Figure 3 Photographs and CT scan images showing truncation relationships between clavate borings and fossils in Cretaceous amber from Myanmar: (A) CT scan image of RS.P1450, showing domichnia of varying sizes, three of them truncating a Metasequoia fossil; (B) photograph of RS.P1429, showing two domichnia truncating a feather. Borings are directed towards the centre of this piece of amber from both sides; (C) photograph of RS.P1449, showing domichnia truncating an araneid spider exoskeleton. Pyrite microcrystals are present in the central portions of the spider's eyes; (D) mayfly (Ephemeroptera) in Burmese amber, which has been bored by pholadid bivalves. The borings go through the hind margins of the left wings, the left cercus, head and left foreleg. Specimen NMS G.2010.20.3. Scale bars = 1 mm.
3. Description of the borings
Clavate borings in amber with a circular aperture and circular cross-section throughout length. The clavate features show a very narrow neck at the external surface of the amber pieces, broadening towards the centre (Figs 1, 2). Most are evenly conical though some have a slightly elongate neck with a more bulbous chamber, but there is no distinct division between these forms. Terminations are either rounded or in some cases approaching flat. Observed lengths range from 0.8 mm to 6 mm and observed maximum diameters from 0.8 mm to 3 mm.
3.1. Surface ornament
Fine pattern of intersecting spiral ridges on external surface of main chamber. The serrated, often herringbone-like, intersecting spiral ornament of micro-ridges on the external surfaces of the clavate objects was well documented by Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002, fig. 6) and is illustrated here in association with a body fossil of the producer in Figure 4B. Such ornament is produced by pholadid bivalves through the repetition of a series of movements known as the ‘boring cycle’ (Ansell & Nair Reference Ansell and Nair1969).

Figure 4 Photographs of RS.P1450, showing domichnia containing the pholadid bivalves that produced them: (A) two domichnia truncating a Metasequoia specimen and containing articulated pholadid bivalves. Field of view 7 mm; (B) detail showing domichnia containing an articulated pholadid bivalve (4 mm long). Anterior is to the left. The sulcus of the bivalve is clearly seen. Rasped ornament is visible on the bored surface at left. Such ornament was well documented in Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002).
3.2. Identification
A difficulty in classifying bivalve borings is the different criteria used for forms produced in wood and forms in lithic substrates, including shell and bone (Bertling Reference Bertling and Miller2007, p. 85; Tapinala et al. Reference Tapanila, Roberts, Bouaré, Sissoko and O'Leary2004). The morphology of the amberground crypts is consistent with woodground Teredolites clavatus Leymerie, 1842 and hardground Gastrochaenolites lapidicus Kelly & Bromley, Reference Kelly and Bromley1984. Given that Teredolites clavatus is specifically associated with pholadoid trace-producers (usually Martesia), this attribution is preferred over Gastrochaenolites lapidicus.
3.3. Cross-cutting relationships
Four specimens are illustrated here that truncate plant and animal fossils with no mechanical deformation of the inclusions at the interface (Fig. 3). This kind of truncating relationship indicates a post-hardening boring (e.g., Bromley Reference Bromley, Maples and West1992).
3.4. Variability of fill
The fills of the clavate forms are variable, including sand of varying grainsize distribution (Fig. 1), from very fine sand to coarse sand in grade, and also examples which received no sediment fill but were filled with sparry calcite cement, which fluoresces yellow under shortwave ultraviolet light. The sand is composed of quartz and lithic fragments.
4. Associated bivalve fossils
4.1. Bivalves within domichnia
One of the specimens illustrated (RS.P1450) shows body fossils within the clavate structures (Fig. 4). These can be seen to be pholadid bivalves with a distinctive anterior shell morphology and a visible sulcus, in addition to the surface ornament on the anterior portion of the shells. They are approximately 4 mm long. The rounded morphology is consistent with the pholadid subfamily Martesiinae, as first suggested by Jeffery in Ross et al. (Reference Ross, Mellish, York, Crighton and Penney2010). This subfamily has been recorded as far back as the Jurassic (Evans Reference Evans1999). The morphology is comparable with Late Cretaceous forms documented from New Zealand (Crampton Reference Crampton1990) and modern Martesia.
4.2. Bivalves not associated with domichnia
Specimen RS.P1460 shows two articulated bivalves (approximately 2 mm long) ‘floating’ within the amber (Fig. 5). One of the bivalves is associated with an irregular void at the amber surface, which later became filled with sand grade sediment. The other has no associated structure produced by boring or burrowing. A leaf inclusion is bent in the vicinity of the bivalve fossil, as opposed to the cases of clear truncation observed in the domichnia specimens. Similar ‘floating’ articulated valves can be seen in Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002, fig. 2a, b).
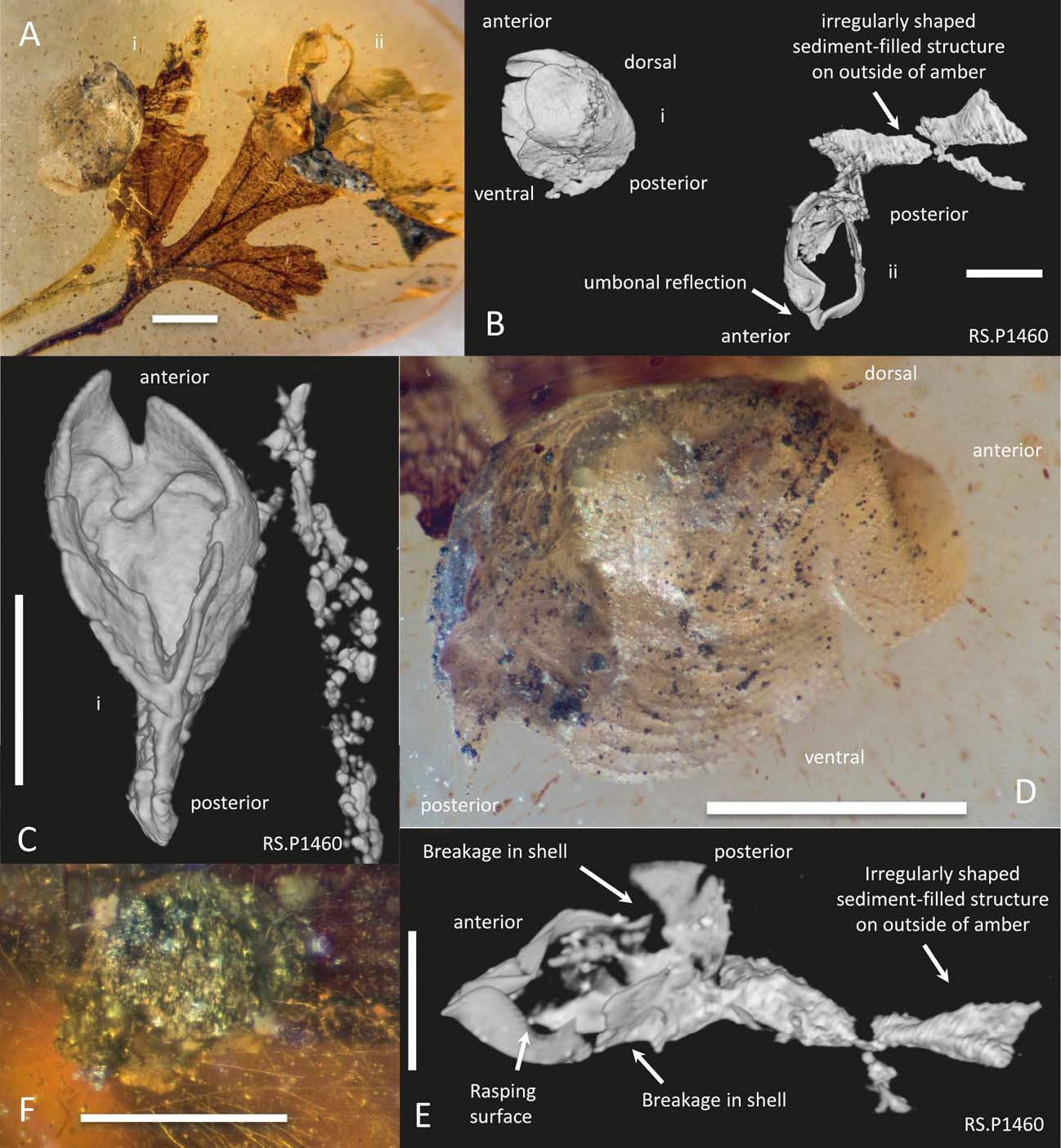
Figure 5 Photographs and CT scan images of RS.P1460, showing pholadid body fossils not associated with domichnia: (A) photograph, showing leaf with two articulated pholadid individuals (i and ii). Individual ii occurs at the end of an elongate irregular body containing a fill of find-sand grade. The leaf was bent adjacent to the bivalves rather than truncated; (B) CT scan image, showing the two bivalves and the sediment-filled structure associated with individual ii; (C) CT scan ventral view of bivalve I, showing anterior gape. (D) photograph of bivalve in lateral view, with anterior to the right; (E) CT scan detail of bivalve ii in ventral view, showing rasping surface at left, breakage in the shell and connected irregular sediment-filled structure at right; (F) cluster of pyrite microcrystals in Burmese amber. Scale bars = 1 mm.
5. Discussion
Amongst numerous other examples, very similarly shaped (though much larger) clavate borings, have been reported from the Miocene Isidro Formation, Baja California Sur, Mexico (Demetrion & Squires Reference Demetrion and Squires1994), some examples showing the serrated tool marks produced by the pholadid bivalve excavators (Demetrion & Squires Reference Demetrion and Squires1994, fig. 3). Gastrochochaenolites ranges from Ordovician to Recent (Taylor & Wilson Reference Taylor and Wilson2003; Vinn & Wilson Reference Vinn and Wilson2010) and is a common and distinctive feature of the Trypanites ichnofacies (Frey & Pemberton Reference Frey, Pemberton and Walker1984).
Bandel et al. (Reference Bandel, Shinaq and Wetschat1997) recognised similar borings to those illustrated here in Cretaceous amber from Jordan. They note, “Now and then larger amber pebbles are bored. The boreholes have a club-shaped outline quite like those found to be excavated by modern bivalves of the Pholadidae.” They cite the reports by Morton (Reference Morton1971) that Martesia and the related Xylophaga may also attack a variety of plastics in addition to wood. Tunnelling by Martesia striata into polyvinyl chloride tubes in Hong Kong Bay, a substance which cannot be dissolved by weak acids or alkalis, is evidence that the bivalves bore strictly mechanically. The borings observed in Jordanian Cretaceous amber were comparable in size and shape with those found in the modern plastic. Other reports of Martesia boring in non-wood substrates include Turner (Reference Turner1955), Turner & Johnson (Reference Turner, Johnson, Jones and Eltringham1971), Scott (Reference Scott1991) and Dhevendaran et al. (Reference Dhevendaran, Rajashree and Balakrishnan Nair2001).
The rimming, inward-directed character of the assemblages of the club-shaped features in Burmese amber noted by Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002) and Poinar & Brown (Reference Poinar and Brown2003) is readily explained as the resin being rolled and bored from multiple sides by pholadid bivalves during their residence (and overturning) in a brackish to marine environment (as also noted by Ross et al. Reference Ross, Mellish, York, Crighton and Penney2010).
Poinar & Brown (Reference Poinar and Brown2003) described microscopic structures on the outside surface as “oleocystidia.” It is possible that the bivalves secreted a substance that attacked the amber, or perhaps they are microfractures caused by the grinding action of the valves (or both), although the purely mechanical action of pholadids has been emphasised by Bandel et al. (Reference Bandel, Shinaq and Wetschat1997). Poinar et al. (Reference Poinar, Jacobson and Eisenberger2006) reported phlebotomine sand fly (Diptera: Psychodidae) larvae associated with the “fungal fruiting bodies” and hypothesised that they were feeding on them. Given their true identity, the association is coincidental, as the larvae would have become trapped and died before the resin was bored by the bivalves.
A specimen illustrated by Grimaldi et al. (Reference Grimaldi, Engel and Nascimbene2002, fig. 2), shows what were interpreted as “dehisced sporangia”, but which can be seen to be pholadid bivalve shells. Their figure 5, in particular, shows the anterior gape and rasping surfaces (e.g., Rudy & Rudy Reference Rudy and Rudy1979), the strap-like structure (previously interpreted as a possible hinge involved in explosive discharge of spores by Grimaldi et al. Reference Grimaldi, Engel and Nascimbene2002) being the styloid apophysis, the attachment of the pedal retractor muscle (Voight Reference Voight2015).
The existence of body fossils of the pholadid bivalves in amber with no associated boring can be explained by the scenario of juvenile pholadids boring into resin pieces that were still internally liquid. Schmidt & Dilcher (Reference Schmidt and Dilcher2007) discussed the incorporation of aquatic organisms in tree resin with reference to observations in a Florida swamp forest, where resinous trees are found standing in shallow water. They observed that pieces of resin at the water–air interface were nearly solidified after one week; whereas subaqueous bodies of resin formed a thin hardened skin after one to two days, while remaining liquid within. They noted that “large arthropods that were able to break through this skin could become entrapped over a couple of weeks.”
The presence of the bivalve body fossils within the amber suggests a close proximity between the resin-producing trees and brackish or marine water, since it indicates that they were, at times, in contact with resin that had not yet fully solidified. Intriguingly, Cruickshank & Ko (Reference Cruikshank and Ko2003) briefly noted that, “a bivalve shell found embedded in amber suggests that the latter was soft when deposited”, but provided no further description or illustration.
Poinar (Reference Poinar2001) described a new genus and species, Lycoperdites teriarius, as puffballs (Gasteromycetes: Lycoperdales) in Mexican amber; however, these also appear to be pholadid bivalve borings (Paul Jeffery, pers. comm. 2010).
6. Implications for palaeoenvironments
Adult pholadids have one to three additional shell plates attached over the hinge and over the margins of the two primary shells (Rice et al. Reference Rice, Johnson and Estevez1990), although juveniles do not, as seen with the body fossils illustrated here. They excavate burrows/borings and feed on suspended material in the water as a primary food source. Pholadids are usually subtidal to mudline in their habitat and salinity tolerance varies among species, with Martesia striata being known to tolerate low salinities associated with mangrove forests (e.g., Singh & Sasekumar Reference Singh and Sasekumar1994; Sivakumar & Kathiresan Reference Sivakumar and Kathiresan1996; Çevik et al. Reference Çevik, Ozcan and Gündoğdu2015). Laboratory experiments reported in Cheriyan & Cheriyan (Reference Cheriyan and Cheriyan1980) show lethal salinity levels below 4–6 parts per thousand, which is in the brackish range (0.5–30 parts per thousand). Cheriyan & Cheriyan (Reference Cheriyan and Cheriyan1980) note the possibility of some Martesia striata surviving near freshwater conditions, perhaps “due to the fact that the area attains fresh water conditions only within the course of a few days of heavy rains, which may be sufficient for the animals to slowly acclimatise.”
Growth rates for the genus Martesia of 0.383 mm/day over the first 30 days have been recorded (Turner Reference Turner1954). Mann & Gallager (Reference Mann and Gallager1984) noted minimum estimates of post-settlement growth rates of Martesia cuneiformis as 0.19 mm/day during the summer months in a North Carolina estuary. These rates, if broadly applicable to the Cretaceous forms under discussion, suggest that the fossil pholadids seen in specimen RS.P1460 became trapped and died within approximately 1–2 weeks of having settled on the resin. The implication is that the resin-producing tree was very close to an environment in which the pholadid bivalves lived.
A brackish water bay or estuary is a plausible palaeoenvironment in which larval pholadid bivalves could have encountered only partially solidified resin. The Zuari estuary (Goa, India) could be a suitable modern analogue (Yennewar et al. Reference Yennewar, Thakur, Anil, Venkat and Wagh1999). This is consistent with the inferences on depositional environment of Cruickshank & Ko (Reference Cruikshank and Ko2003), “a nearshore marine environment, such as a bay or estuary”. They elaborated that, “the depositional area must have been nearshore, because of the abundance of amber, coalified plant fragments, and common coal laminations in the fine clastic facies. According to Davies (Reference Davies2001), the dinoflagellates he identified are typical of inner neritic to littoral environments. A marine environment is also indicated by his recognition of organic-walled foraminiferal liners and zynemataceous algae.” Cruickshank & Ko (Reference Cruikshank and Ko2003) noted that palynomorphs of genera from both Araucariaceae and the Taxodiaceae families were identified in samples collected from the amber horizon at Noije Bum, and that both of these families have been identified as sources of Cretaceous amber elsewhere (e.g., Grimaldi et al. 2000; Poinar & Milki Reference Poinar and Milki2001). The presence of pyrite in Burmese amber pieces is additionally consistent with the presence of sulphate-bearing water.
Langenheim (Reference Langenheim, Anderson and Crelling1995, p. 21) and Solórzano Kraemer (Reference Solórzano Kraemer and Penney2010) inferred such a coastal setting for at least a portion of the Mexican and Dominican Republic resin-producing trees. It was noted that Hymenaea courbaril trees line mangrove-dominated estuaries and swamps along the modern Mexican Pacific coast.
Although some Burmese amber was reworked into younger Eocene deposits (Zherikhin & Ross Reference Zherikhin and Ross2000), the re-worked specimens (where not polished) have impact pits on their surface, which are not present on the specimen in Figure 2, suggesting that this specimen and its borings came from a primary deposit and are broadly contemporaneous (i.e., the borings did not penetrate a re-worked specimen later on).
7. Implications for the age of Burmese amber
There has been much debate over the age of Burmese amber, summarised by Ross et al. (Reference Ross, Mellish, York, Crighton and Penney2010), who supported a late Albian age based on the presence of a Mortoniceras ammonite and palynomorphs recorded by Cruickshank & Ko (Reference Cruikshank and Ko2003). Shi et al. (Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012) dated zircons from the amber-bearing bed which gave an age of 98.79±0.62 Ma; thus early Cenomanian. Ross (Reference Ross2015; in Grimaldi & Ross Reference Grimaldi, Ross, Fraser and Sues2017) argued that the amber was older, as the presence of the pholadid bivalve borings indicated that the amber was hard and rolling around in the sea before it was buried. Ross (Reference Ross2015) went on to argue that the amber was more likely to be Albian in age, as 15 % of the insect families in Burmese amber are extinct; which is close to the percentage for known extinct Albian insect families, as opposed to the 10 % of extinct insect families in the Cenomanian.
This new study has demonstrated that the outside layer of the resin was certainly hard enough to be bored by pholadid bivalves, but the cores of at least some pieces were liquid, or at least soft enough for the bivalves to end up as internal inclusions. This suggests that the age of the amber and the amber-bearing bed may not be very different, with some of the pieces being bored within weeks of production from the resin-producing tree. The current International Chronostratigraphic Chart (v2016.04) by the International Commission on Stratigraphy (www.stratigraphy.org) has the Albian\Cenomanian boundary at 100.5 Ma, at least one million years older than the age of the amber-bearing bed from the zircon dates. Therefore, the amber is likely to be early Cenomanian in age.
In recent years, many new amber mines have been opened in northern Myanmar to feed the strong desire for amber in China. A large collection has been built up by the Nanjing Institute of Paleontology, and many new taxa are in the process of being described. An urgent study of the geology of the region is required, to confirm whether the amber is collected from only one primary horizon or from several horizons of different ages.
8. Conclusions
As briefly noted in Ross et al. (Reference Ross, Mellish, York, Crighton and Penney2010), clavate features in Cretaceous amber from Myanmar, previously described as fungal sporocarps (named Palaeoclavaria burmitis in the Hymenomycetes) were in fact produced by boring pholadid bivalves. The clavate borings are here recognised as Teredolites clavatus Leymerie, Reference Leymerie1842 domichnia (comparable to Gastrochaenolites lapidicus Kelly & Bromley, Reference Kelly and Bromley1984 in a lithic or shell boring context). The crypts may contain the articulated valves of the martesiine bivalve producers, be filled with sand-grade sediment, or with sparry calcite cement.
The presence of martesiine bivalve fossils in amber, yet not associated with borings, has implications for the proximity of amber-forming trees to a brackish or marine environment. Observations on the hardening history of aquatically deposited resin (Schmidt & Dilcher Reference Schmidt and Dilcher2007) support a model whereby juvenile pholadids settled on the hardened outer rim of the resin, commenced boring and reached the still liquid interior of the resin piece, where they became trapped. The resin-producing trees could have lived adjacent to coastal rivers, or to the landward margins of brackish-marine bays.
Constraining the age of the resin/amber pieces when they were bored has an important bearing on the age of the enclosed biota, since if some considerable time has elapsed, then the amber inclusions could be substantially older than the age of the enclosing sediment; whereas a smaller elapsed time reduces the age difference between the amber-bearing bed and the biota trapped in the amber. Evidence of martesiine pholadid bivalves ‘floating’ in Burmese amber indicates that at least some resin pieces had soft or liquid centres and were being colonised within weeks of production at the source tree. Thus, Burmese amber is similar in age to the amber-bearing bed and therefore early Cenomanian in age.
9. Acknowledgements
Constructive reviews by Paul Jeffery and J Rust are gratefully acknowledged. RDAS thanks D. Grimaldi for discussion on the observed truncation relationships, Alexander Schmidt for a reprint of his paper on the behaviour of modern aquatic resin and Dmitry Shaporov for his assistance in preparing the micro CT scans. The photographs of the NMS specimens were taken by Bill Crighton. Many thanks go to Mr Fan Yong of the Fushun Amber Institute, China for the generous donation of specimen NMS G.2015.21.1 to National Museums Scotland.







