Because of enhanced management of high-risk pregnancies and advancements in neonatal medical care, survival rates for infants born very prematurely (VP, <32 weeks) and/or at very low birth weight (VLBW, <1500 g) are improving (Allen, Donohue, & Dusman, Reference Allen, Donohue and Dusman1993; Hoekstra, Ferrara, Couser, Payne, & Connett, Reference Hoekstra, Ferrrara, Couser, Payne and Connett2004; Horbar, Wright, & Onstad, Reference Horbar, Wright and Onstad1993; Schwartz, Luby, Scanlon, & Kellogg, Reference Schwartz, Luby, Scanlon and Kellogg1994). As such, there is an increased need to examine and find ways to improve the long-term outcomes of these survivors.
Research has shown that many of the smallest survivors of preterm birth face significant, ongoing challenges. Some are affected by physical and health-related problems such as cerebral palsy, seizures, sensorineural hearing loss, and blindness (Aylward, Reference Aylward2002; Foreman, Fielder, Minshell, Hurrion, & Sergienko, Reference Foreman, Fielder, Minshell, Hurrion and Sergienko1997; Goldenberg & McClure, Reference Goldenberg, McClure and Berghella2010; Hellgren et al., Reference Hellgren, Hellström, Jacobson, Flodmark, Wadsby and Martin2007; Msall & Tremont, Reference Msall and Tremont2002; Saigal, Stoskopf, Streiner, & Burrows, Reference Saigal, Stoskopf, Streiner and Burrows2001). Many exhibit poor performance on tests of attention, memory, and both expressive and receptive language, and experience academic struggles in the areas of reading, spelling, and mathematics (Anderson & Doyle, Reference Anderson and Doyle2003; Bennett, Reference Bennett, Avery, Fletcher and MacDonald1999; Cherkes-Julkowski, Reference Cherkes-Julkowski1998; Downie, Frisk, & Jakobson, Reference Downie, Frisk and Jakobson2005; Grunau, Whitfield, & Fray, Reference Grunau, Whitfield and Fray2004; Rickards et al., Reference Rickards, Kitchen, Doyle, Ford, Kelly and Callanan1993). This group is also at elevated risk for problems with aspects of cortical visual function (e.g., Jakobson, Fisk, & Downie, Reference Jakobson, Frisk and Downie2006; Taylor, Jakobson, Maurer, & Lewis, Reference Taylor, Jakobson, Maurer and Lewis2009), and for other cognitive and behavioral problems (Bhutta, Cleves, Casey, Cradock, & Anand, Reference Bhutta, Cleves, Casey, Cradock and Anand2002; Caravale, Tozzi, Albino, & Vicari, Reference Caravale, Tozzi, Albino and Vicari2005; Delobel-Ayoub et al., Reference Delobel-Ayoub, Arnaud, White-Koning, Casper, Pierrat and Garel2009; Hack et al., Reference Hack, Taylor, Drotar, Schluchter, Cartar and Andreias2005; Hille et al., Reference Hille, den Ouden, Saigal, Wolke, Lambert and Whitaker2001; Litt, Taylor, Klein, & Hack, Reference Litt, Taylor, Klein and Hack2005; Marlow, Wolke, Bracewell, & Samara, Reference Marlow, Wolke, Bracewell and Samara2005; Moster, Lie, & Markestad, Reference Moster, Lie and Markestad2008; Saigal, Pinelli, Hoult, Kim, & Boyle, Reference Saigal, Pinelli, Hoult, Kim and Boyle2003).
Of particular relevance to the current study is research showing that children born VP/VLBW are at increased risk for social, emotional, and mental health difficulties (Dahl et al., Reference Dahl, Kaaresen, Tunby, Handegård, Kvernmo and Ronning2006; Farooqi, Hägglöf, Sedin, Gothefors, & Serenius, Reference Farooqi, Hägglöf, Sedin, Gothefors and Serenius2007; Gardner et al., Reference Gardner, Johnson, Yudkin, Bowler, Hockley and Mutch2004; Reijneveld et al., Reference Reijneveld, de Klein, van Baar, Kollée, Verhaak and Verhulst2006; Saigal et al., Reference Saigal, Pinelli, Hoult, Kim and Boyle2003). For example, some reports point to elevated risk for internalizing problems (such as anxiety, depression, withdrawal, and somatic complaints), problems with attention and hyperactivity, thought problems, and social difficulties (Farooqi et al., Reference Farooqi, Hägglöf, Sedin, Gothefors and Serenius2007; Gardner et al., Reference Gardner, Johnson, Yudkin, Bowler, Hockley and Mutch2004). Others have shown an increased risk for psychiatric hospitalization in young adulthood, and increased vulnerability to a range of psychiatric diagnoses, such as nonaffective psychosis, depressive disorder, and bipolar affective disorder (Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe and Yin2012).
In addition, although there are some contradictory findings (e.g., Bilder, Pinborough-Zimmerman, Miller, & McMahon, Reference Bilder, Pinborough-Zimmerman, Miller and McMahon2009; Croen, Grether, & Selvin, Reference Croen, Grether and Selvin2002), a number of recent studies suggest that children born VP or at low birth weight (<2500 g) have a higher incidence of “autistic-like” traits when compared to full-term controls (e.g., Eaton, Mortensen, Thomsen, & Frydenberg, Reference Eaton, Mortensen, Thomsen and Frydenberg2001; Hultman, Sparén, & Cnattingius, Reference Hultman, Sparén and Cnattingius2002; Indredavik et al., Reference Indredavik, Vik, Evensen, Skranes, Taraldsen and Brubakk2010; Johnson et al., Reference Johnson, Hollis, Kochhar, Hennessy, Wolke and Marlow2010; Johnson & Marlow, Reference Johnson and Marlow2009; Kuban et al., Reference Kuban, O'Shea, Allred, Tager-Flusberg, Goldstein and Leviton2009; Lampi et al., Reference Lampi, Lehtonen, Tran, Suominen, Lehti and Banerjee2012; Maimburg & Væth, Reference Maimburg and Væth2006; Stephens et al., Reference Stephens, Bann, Watson, Sheinkopf, Peralta-Carcelen and Bodnar2012), and that this relationship is mediated by a number of maternal and perinatal/neonatal risk factors (Buchmayer et al., Reference Buchmayer, Johansson, Johansson, Hultman, Sparén and Cnattingius2009; Gardener, Spiegelman, & Buka, Reference Gardener, Spiegelman and Buka2009; Glasson et al., Reference Glasson, Bower, Petterson, de Klerk, Chaney and Hallmayer2004; Limperopoulos et al., Reference Limperopoulos, Bassan, Sullivan, Soul, Robertson and Moore2008). Unfortunately, much of this research has relied on screening measures administered in retrospective or prospective studies. Some authors caution that use of such tools may lead to high false positive rates, given the increased incidence of other impairments in preterm children (Stephens et al., Reference Stephens, Bann, Watson, Sheinkopf, Peralta-Carcelen and Bodnar2012). It is significant, then, that in a recent study that followed children prospectively and used diagnostic tools, researchers estimated the prevalence of autism spectrum disorder (ASD) in children born weighing <2000 g to be 5%, which is approximately five times higher than the rate seen in the general population (Pinto-Martin et al., Reference Pinto-Martin, Levy, Feldman, Lorenz, Paneth and Whitaker2011).
Despite evidence that preterm children are at increased risk for a behavioral phenotype similar to that seen in autism, Johnson and Marlow (Reference Johnson and Marlow2011) point out that the causal pathway may be different in full-term and preterm children. To date, the literature examining links between prematurity and autism has focused very much on findings obtained from behavioral and symptom checklists. It is important to extend this work by studying specific cognitive and perceptual skills in this population, to determine if/how deficits in particular areas may contribute to the characteristic social and behavioral profile of the preterm child. In this regard, it has been found that 6-month-old infants born prematurely have difficulties engaging in joint play and initiating joint-attention episodes with their mothers, and exhibit more gaze aversion, compared to full-term controls (Landry, Reference Landry1986, Reference Landry1995). This is significant because joint attention skills are thought to be important precursors to higher order skills that are fundamental to social development (Baron-Cohen, Reference Baron-Cohen and Whiten1991; Carpenter, Nagell, & Tomasello, Reference Carpenter, Nagell and Tomasello1998; Mundy & Gomes, Reference Mundy and Gomes1998), including the ability to attribute mental states (such as beliefs, intentions, or desires) to oneself and others, and to appreciate that others' mental states may differ from our own (Premack & Woodruff, Reference Premack and Woodruff1978). These latter abilities have been termed theory of mind (ToM; Premack & Woodruff, Reference Premack and Woodruff1978), mindreading (Baron-Cohen, Reference Baron-Cohen1997), or mentalizing (Abu-Akel, Reference Abu-Akel2003) abilities.
Little is known about the mentalizing skills of children born preterm at VLBW. Much of the literature on mentalizing has centered on children and adults with ASDs, who exhibit a range of impairments in these skills. For example, children with autism produce fewer mental state words in their descriptions of scenarios and stories, and in natural speech patterns, when compared to neurotypical controls (Baron-Cohen, Leslie, & Frith, Reference Baron-Cohen, Leslie and Frith1986; Tager-Flusberg, Reference Tager-Flusberg1992). They also exhibit a lower frequency of symbolic (or pretend) play behaviors in spontaneous play (Baron-Cohen, Reference Baron-Cohen1987; Lewis & Boucher, Reference Lewis and Boucher1988; Ungerer & Sigman, Reference Ungerer and Sigman1981; Wing, Gould, Yeates, & Brierley, Reference Wing, Gould, Yeates and Brierley1977), and even high-functioning children with autism have been shown to have difficulties understanding pretense, persuasion, sarcasm, jokes, double bluffs, irony, and lies (Happé, Reference Happé1994). These latter impairments are evident in a range of tasks requiring an appreciation of first- and second-order false beliefs (Baron-Cohen, Reference Baron-Cohen1989; Baron-Cohen, Leslie, & Frith, Reference Baron-Cohen, Leslie and Frith1985; Leekam & Perner, Reference Leekam and Perner1991; Perner, Frith, Leslie, & Leekam, Reference Perner, Frith, Leslie and Leekam1989; Reed & Peterson, Reference Reed and Peterson1990; Swettenham, Reference Swettenham1996), and in other ToM tasks, including, for example, Happé's Strange Stories task (Happé, Reference Happé1994), the eyes task (Baron-Cohen, Jolliffe, Mortimore, & Robertson, Reference Baron-Cohen, Jolliffe, Mortimore and Robertson1997; Baron-Cohen, Wheelwright, & Jolliffe, Reference Baron-Cohen, Wheelwright and Jolliffe1997), and the Happé–Frith animated triangles task (e.g., Abell, Happé, & Frith, Reference Abell, Happé and Frith2000; Jones et al., Reference Jones, Swettenham, Charman, Marsden, Tregay and Baird2011; Salter, Seigal, Claxton, Lawrence, & Skuse, Reference Salter, Seigal, Claxton, Lawrence and Skuse2008; White, Coniston, Rogers, & Frith, Reference White, Coniston, Rogers and Frith2011; Zwickel, White, Coniston, Senju, & Frith, Reference Zwickel, White, Coniston, Senju and Frith2010).
The animated triangles task has been widely used as a nonverbal (visual) measure of mentalizing abilities. This task was based on research done by Heider and Simmel (Reference Heider and Simmel1944) in which it was found that viewers often attributed intentionality to geometric shapes that appeared to move in a goal-directed fashion. Happé and Frith extended this task by developing three different types of animations, each featuring two moving triangles (Abell et al., Reference Abell, Happé and Frith2000). In all of the animations, the triangles appeared to move as if they were self-propelled agents, but their specific movements differed in ways that affected how viewers interpreted what they saw. Random displays depicted two triangles that each appeared to move in a nondeliberate (non-goal-directed) manner (e.g., “rotating”). Goal-directed displays depicted one triangle that appeared to respond to the other's “behavior” in a goal-directed (intentional) manner (e.g., one shape “following” the other). Finally, ToM displays depicted one triangle interacting with the other, or in response to environmental cues, in an intentional way that was often described using mental state words (e.g., one shape “coaxing” the other). In short, the three display types were designed to evoke social attributions in typical viewers to differing degrees. By quantifying the number of social attributions a viewer made, one could obtain a measure of his/her mentalizing ability.
Since its introduction, the animated triangles task has been used with neurotypical viewers across a range of ages (Castelli, Happé, Frith, & Frith, Reference Castelli, Happé, Frith and Frith2000; Klein, Zwickel, Prinz, & Frith, Reference Klein, Zwickel, Prinz and Frith2009; Knickermeyer, Baron-Cohen, Raggatt, Taylor, & Hackett, Reference Knickermeyer, Baron-Cohen, Raggatt, Taylor and Hackett2006; Moriguchi, Ohnishi, Mori, Matsuda, & Komaki, Reference Moriguchi, Ohnishi, Mori, Matsuda and Komaki2007) and with a variety of different clinical populations, including individuals with autism, Asperger syndrome, Turner syndrome, schizophrenia, medial frontal lobe damage, right hemisphere damage, and alexithymia (Bird, Castelli, Malik, Frith, & Husain, Reference Bird, Castelli, Malik, Frith and Husain2004; Campbell et al., Reference Campbell, Lawrence, Mandy, Mitra, Jeyakuma and Skuse2006; Castelli, Frith, Happé, & Frith, Reference Castelli, Frith, Happé and Frith2002; Koelkebeck et al., Reference Koelkebeck, Pedersen, Suslow, Kueppers, Arolt and Ohrmann2010; Lawrence et al., Reference Lawrence, Jones, Oreland, Spektor, Mandy and Campbell2007; Moriguchi et al., Reference Moriguchi, Ohnishi, Lane, Maeda, Mori and Nemoto2006; Russel, Reynaud, Herba, Morris, & Corcoran, Reference Russel, Reynaud, Herba, Morris and Corcoran2006; Weed, McGregor, Feldbæk Nielsen, Roepstorff, & Frith, Reference Weed, McGregor, Feldbæk Nielsen, Roepstorff and Frith2010). Research involving individuals with autism has produced a fairly consistent pattern of results. Abell et al. (Reference Abell, Happé and Frith2000) found that children with autism who could pass first- and second-order false belief tasks nonetheless used fewer and less appropriate mental state terms than did typically developing children when describing ToM displays, specifically. Similar results have been described in more recent work involving children (Salter et al., Reference Salter, Seigal, Claxton, Lawrence and Skuse2008), adolescents (Jones et al., Reference Jones, Swettenham, Charman, Marsden, Tregay and Baird2011), and adults (White et al., Reference White, Coniston, Rogers and Frith2011) with autism. In addition to the above, Zwickel et al. (Reference Zwickel, White, Coniston, Senju and Frith2010) found that, in addition to offering less appropriate descriptions of ToM displays than controls, adults with autism overattributed intentionality/mental states to triangles in the random displays and tended to underattribute intentionality/mental states to the triangles in the goal-directed and ToM displays.
Functional neuroimaging studies have shown that viewing ToM displays is associated with increased activation in areas involved in mentalizing (i.e., medial prefrontal cortex, superior temporal sulcus [STS], basal temporal regions, and extrastriate cortex; Castelli et al., Reference Castelli, Happé, Frith and Frith2000). Anatomical and/or functional abnormalities in at least one of these areas, the STS, have been found both in children with ASD (Boddaert et al., Reference Boddaert, Chabane, Gervais, Good, Bourgeois and Plumet2004; Pelphery & Carter, Reference Pelphrey and Carter2008; Zilbovicius et al., Reference Zilbovicius, Meresse, Chabane, Brunelle, Samson and Boddaert2006) and in those born prematurely (Dubois et al., Reference Dubois, Benders, Cachia, Lazeyras, Leuchter and Sizonenko2008; Lax et al., Reference Lax, Duerden, Lin, Chakravarty, Donner and Lerch2013; Zubiaurre-Elorza et al., Reference Zubiaurre-Elorza, Soria-Pastor, Junqué, Vendrell, Padilla and Rametti2009). This is significant because some have suggested that, in ASD, such abnormalities could have cascading effects, altering the development of white matter connections between the STS and other key regions of the social brain (Zilbovicius et al., Reference Zilbovicius, Meresse, Chabane, Brunelle, Samson and Boddaert2006). Several reports describing altered connectivity in the ToM network among those with ASD have appeared (e.g., Barnea-Goraly et al., Reference Barnea-Goraly, Kwon, Menon, Eliez, Lotspeich and Reiss2004; Lee et al., Reference Lee, Bigler, Alexander, Lazar, DuBray and Chung2007). In one recent study (Kana, Keller, Cherkassky, Minshew, & Just, Reference Kana, Keller, Cherkassky, Minshew and Just2009) functional activations within and between regions of the ToM network were examined as individuals with high functioning autism and controls completed the animated triangles task. Even though group differences in functional activation were not evident in the posterior STS in this study (but see Castelli et al., Reference Castelli, Frith, Happé and Frith2002), individuals with autism showed functional underconnectivity (a lower degree of synchronization) between this region and frontal ToM areas when making mental state attributions. The idea that problems with mentalizing might arise, in part, from inefficient communication between the STS (a posterior region implicated in ToM cue extraction) and other parts of the social brain gains additional support from the fact that, even in healthy young adults, there is a relationship between the number of autistic-like traits individuals display and the strength of white matter connections between the STS and the amygdala (Iidaka, Miyakoshi, Harada, & Nakai, Reference Iidaka, Miyakoshi, Harada and Nakai2012).
The present study was designed to explore the social attribution skills of children born preterm at VLBW (preterm children). Specifically, we examined whether these children would show impaired ability to attribute intentionality/mental states in the animated triangles task when compared to full-term, age-matched controls whose birth weights were >2500 g (full-term controls). We also asked whether their social attribution skills were related to the number of autistic-like traits they displayed, and whether either of these measures was related to specific neonatal risk factors.
Materials and Methods
Participants
A sample of 34 preterm children and 36 full-term controls took part in this study. All participants were between 8 and 11 years of age. See Table 1 for additional demographic information.
Table 1. Demographic and screening measures for the full-term and preterm samples
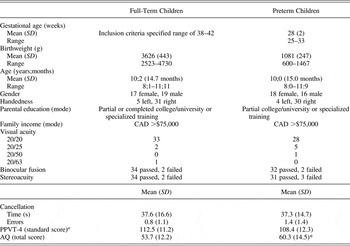
Note: AQ, Autism Spectrum Quotient—Child version.
a Age-corrected standard scores on the Peabody Picture Vocabulary Test, Fourth Edition (PPVT-4), were used to estimate verbal intelligence; see the Intellectual screening Section and Results Section for further details.
*Group difference was significant at p < .05.
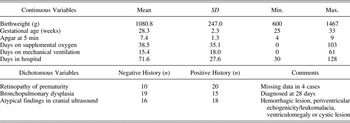
Preterm children were recruited through the High-Risk Newborn Follow-Up Programs at Children's Hospital and at St. Boniface Hospital (both in Winnipeg, MB). Preterm children were excluded if they (a) had a birth weight of ≥1500 g; (b) suffered from major sensory impairment (e.g., blindness or deafness), and/or (c) had undergone ventriculo-peritoneal shunting for posthemorrhagic hydrocephalus. With parental consent, information about the following medical variables was obtained from neonatal medical records of study participants: birthweight, gestational age, APGAR score at 5 min, duration of mechanical ventilation (days), days on supplemental oxygen, history of bronchopulmonary dysplasia, length of hospital stay (days), information regarding the presence/severity of retinopathy of prematurity, and results of neonatal cranial ultrasound scans and any other available brain imaging studies (see Table 2).
Table 2. Medical variables describing the preterm sample
Full-term controls were recruited through elementary schools and the community via recruitment letters and word of mouth. All were born at term (within 2 weeks of their due date) without medical complications and were an appropriate size for their gestational age. None had a history of developmental problems.
General procedure
Upon arrival, a parent provided written consent and then completed a general information questionnaire and a questionnaire designed to measure autistic-like traits in children, as described below. The participating child gave verbal assent. Participating children were tested individually in a quiet room. Each child completed a letter cancellation task and a test of receptive vocabulary. This was followed by completion of the animated triangles task and a number of other tasks administered for a separate investigation. A brief description of each measure used in the present study is provided below.
Demographic and screening measures
General information questionnaire
For each participating child, a parent (or legal guardian) completed a questionnaire, providing information about the child's early development and demographic information relating to parental education and family income (variables that have been linked to cognitive development in preterm children; Braid, Donohue, & Strobino, Reference Braid, Donohue and Strobino2012; Sommerfelt, Ellertsen, & Markestad, Reference Sommerfelt, Ellertsen and Markestad1995; Voss, Jungmann, Wachtendorf, & Neubauer, Reference Voss, Jungmann, Wachtendorf and Neubauer2012).
Visual screening
The Lighthouse Distance Acuity Chart was used to measure linear acuity (Lighthouse International, New York). The Worth 4 Dot Test (Richmond Products, Albuquerque, NM) was used to measure binocular fusion. The Titmus Test of Stereoacuity (Stereo Optical Company, Chicago) was used to measure stereoacuity. The administration and scoring of these tasks followed standardized procedures (see Kniestedt & Stamper, Reference Kniestedt and Stamper2003; Taylor et al., Reference Taylor, Jakobson, Maurer and Lewis2009).
Letter cancellation task
This task (Geldmacher, Reference Geldmacher1996) provided a measure of visual attention and processing speed. The child was presented with an array of 100 letters printed on a 8.5 × 11 in. sheet of white paper and was asked to cross out all 20 instances of the target letter “X.” Errors and time (in seconds) to complete the task were recorded.
Intellectual screening
The fourth edition of the Peabody Picture Vocabulary Test (PPVT-4; Dunn & Dunn, Reference Dunn and Dunn2007) was used to estimate verbal intelligence. Scores on this test are highly correlated with scores on the third edition (Dunn & Dunn, Reference Dunn and Dunn1997) that are in turn highly correlated with both full-scale IQ (r = .90) and verbal IQ (r = .91) measured with the third edition of the Wechsler Intelligence Scale for Children (Wechsler, Reference Wechsler1991). On each trial of the PPVT-4, the examiner says a word, and the participant is then required to point to one of four pictures that correspond to that word. The items are grouped into sets of 12, with more difficult items at the end. The test continues until “basal” and “ceiling” sets are found for each child. The basal set refers to an item set in which the child makes one or no errors. The ceiling set refers to an item set in which the child makes eight or more errors. The test results are expressed in standard scores (M = 100, SD = 15). This measure was included to ensure that any difficulties observed in the animated triangles task could not be attributed to the presence of a general cognitive delay (that would also affect verbal functioning) or to difficulty in comprehending experimental instructions.
Autism Spectrum Quotient—Child Version (AQ)
A parent/guardian of each participating child completed the child version of the AQ, a 50-item parent-report questionnaire that is designed to measure autistic-like traits in children ages 4–11 years (Auyeung, Baron-Cohen, Wheelwright, & Allison, Reference Auyeung, Baron-Cohen, Wheelwright and Allison2008). The AQ has good test–retest reliability (r = 0.85, p < .001), a high Cronbach α (0.97), and has been shown to discriminate between individuals with Asperger syndrome/high functioning autism and those who are typically developing with a high degree of accuracy (Auyeung et al., Reference Auyeung, Baron-Cohen, Wheelwright and Allison2008; see also Woodbury-Smith, Robinson, Wheelwright, & Baron-Cohen, Reference Woodbury-Smith, Robinson, Wheelwright and Baron-Cohen2005). While it does not include items relating to the full range of autistic symptoms, it does allow one to assess functioning in five broad areas associated with autism and the broader autism phenotype, including social skills, attention switching, attention to detail, communication, and imagination. Each area is assessed using 10 items. The respondent indicates the extent to which each trait accurately describes the child using a 4-point Likert scale (0 = definitely agree, 3 = definitely disagree). The maximum total score on this measure is 150, representing full endorsement of all autistic-like traits.
Experimental measure
Animated triangles task
In this task, participants were presented with a series of animations, each depicting one large red triangle and one small blue triangle, moving about in a framed white background (Abell et al., Reference Abell, Happé and Frith2000; Castelli et al., Reference Castelli, Happé, Frith and Frith2000). Three different types of animations were presented: random, goal directed, and ToM. As noted earlier, random displays do not typically evoke descriptions of interactions or mental states. In contrast, goal-directed displays (in which one triangle moves as though intentionally “responding” to the other's behavior) typically evoke descriptions of specific interactions (e.g., following, fighting, chasing, or dancing), while ToM displays (in which one triangle appears to intentionally act in a way designed to affect the other's “mental state”) typically evoke descriptions that include mental state terms describing the action (e.g., persuading, mocking, coaxing, or surprising) and/or the thoughts or feelings of one or both triangles (e.g., happy or afraid).
Children completed familiarization and experimental trials, which were presented in a fixed, random order, with the stipulation that no more than two trials in a row were from the same condition. For the familiarization task, one practice animation of each type was presented. Participants described each scenario, and corrective feedback was given. A second administration was allowed if necessary. For the experimental task, four animations were presented in each condition (12 trials in total), each lasting from 34 to 45 s.
After viewing each animation, the child was asked, “What happened in the cartoon?” The child's verbal description of the scenario was recorded verbatim and then scored using Castelli et al.'s (Reference Castelli, Happé, Frith and Frith2000) protocol. Responses were initially scored on three different dimensions: certainty (0–3 Likert scale; 0 = long hesitations or silence, 3 = no hesitations), appropriateness (0–3 Likert scale; 0 = no answer or an answer of “I don't know,” 3 = an appropriate and clear answer), and intentionality (0–5 Likert scale; 0 = a nondeliberate [i.e., non-goal-oriented] action, 3 = a deliberate action in response to another's actions, and 5 = a deliberate action taken with the explicit goal of affecting another's mental state). It is important to note that, even though the movements of the triangles in both goal-directed and ToM displays appear to be interactive and intentional, it is only in the latter case that one triangle appears to act in a way intended to affect the mental state of the other. For this reason, descriptions of ToM displays would normally be expected to include mental state terms, and to receive higher “intentionality” scores than would goal-directed displays (as defined in the scoring protocol).
In addition to the three measures mentioned above, we also recorded a total word count for each participant. The primary researcher and two raters who were blind to group membership carried out all scoring independently. Any discrepancies in scoring were resolved through discussion between all three raters; thus, 100% agreement was obtained for every score.
Results
A series of statistical tests were conducted to assess the comparability of the preterm and full-term samples on the demographic and screening measures (see Table 1 for group means). An independent samples t test confirmed that the two groups were comparable in terms of age (months). They also had similar handedness and gender distributions, parental education levels, and family incomes (chi-squared tests). Visual acuity was comparable in both groups (chi-squared test), being 20/25 or better in 68 of the 70 participants. The remaining participants had measured acuities that are considered adequate for neuropsychological testing (Capruso, Hamsher, & Benton, Reference Capruso, Hamsher, Benton, Mapou and Spector1995; Norman, Payton, Long, & Hawkes, Reference Norman, Payton, Long and Hawkes2004). In addition, low and comparable rates of problems with binocular fusion and stereoacuity were seen in both groups (chi-squared tests).
Independent samples t tests were used to compare the two groups on measures of visual attention/processing speed (speed and accuracy on the cancellation task), verbal intelligence (standard scores on the PPVT-4), and autistic-like traits (total scores on the AQ). As can be seen in Table 1, children in the preterm and full-term samples performed similarly on the cancellation task and the PPVT-4, but they differed in the number of autistic-like traits they displayed, with parents of preterm children reporting significantly more symptoms of autism in their children than parents of full-term peers, t (68) = 2.06, p = .043. Despite this, the mean score of the preterm group fell below the clinical cut-scores specified by the test developers, and even those who were in the clinical range scored below the mean reported for individuals with Asperger syndrome/high functioning autism (see Auyeung et al., Reference Auyeung, Baron-Cohen, Wheelwright and Allison2008).
Animated triangles task
The descriptions provided by children in the preterm sample were shorter than those provided by their peers, overall (total word count: M preterm = 488, SD = 210.6 vs. M full-term = 633, SD = 286.5); t (68) = 2.39, p = .019. For this reason, certainty, appropriateness, and intentionality scores were analyzed using separate, mixed analysis of covariance (ANCOVA) tests that controlled for total word count. Each analysis had one between-groups factor (group: preterm vs. full-term) and one within-groups factor (display type: random, goal-directed, or ToM).
The analysis of certainty ratings produced no significant main effects or interactions. Thus, across conditions, mean certainty ratings were very similar in the two groups (M preterm = 2.84, SD = 0.24 vs. M full-term = 2.94, SD = 0.25), with descriptions provided by children in the two groups being equally fluent (i.e., one group was not more hesitant, or prone to long pauses, than the other).
The ANCOVA on appropriateness ratings produced significant main effects of group, F (1, 67) = 8.79, p = .004, η2 = 0.116, and display type, F (2, 134) = 5.93, p = .005, η2 = 0.08. Full-term children provided more appropriate descriptions of the animations overall (M preterm = 1.36, SD = 0.23 vs. M full-term = 1.52, SD = 0.23), but both groups of children found the ToM displays the hardest to describe appropriately (see Figure 1). The Group × Display Type interaction was not significant.
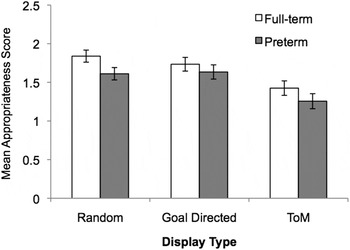
Figure 1. Mean appropriateness scores (SE) displayed as a function of display type (random, goal directed, and theory of mind [ToM]) and group membership (full-term and preterm samples). Although descriptions provided by preterm children were less appropriate than those of their peers, overall, both groups found the ToM displays the most difficult to describe.
The ANCOVA on intentionality ratings produced a significant main effect of display type, F (2, 134) = 50.71, p < .001, η2 = 0.43, and a significant interaction between display type and group, F (2, 134) = 7.04, p = .001, η2 = 0.095 (see Figure 2). Follow-up tests of simple main effects showed that intentionality scores increased from random to goal directed, and from goal directed to ToM (display type: F > 18.05, p < .001 in both cases) but that, compared to full-term peers, preterm children overattributed intentionality/mental states to triangles in random displays, F (1, 67) = 4.83, p = .03, η2 = 0.067, and underattributed intentionality/mental states to triangles in ToM displays, F (1, 67) = 3.97, p = .05, η2 = 0.056. Sample descriptions illustrating these findings are provided in Table 3. The two groups performed similarly on the goal-directed displays, F (1, 67) = 0.63, p = .80, η2 = 0.001.
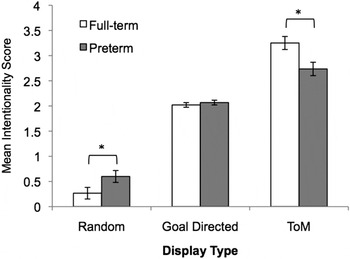
Figure 2. Mean intentionality scores (SE) displayed as a function of display type (random, goal directed and theory of mind [ToM]) and group membership (full-term and preterm samples). Preterm children overattributed intentionality to the random displays and unattributed intentionality to the ToM displays (group difference, *p < .05).
Table 3. Sample descriptions of the drifting random animation and the coaxing ToM animation provide by one PT and one FT child

Note: ToM, theory of mind; PT, preterm; FT, full term. The descriptions show overattribution and underattribution of intentionality/mental states to triangles in the random and ToM displays, respectively, by PT children.
Relationships between variables
Generally speaking, we expected social attribution skills to improve with age, and to be worse in children showing more autistic-like symptoms. Because we were interested in possible group differences in these relationships, we tested correlations between variables in each group separately; if these correlations were of similar magnitude (Fisher z test), the analysis was rerun on the combined data set to increase power. We also expected to find that, among preterm children, those with more complicated medical histories would show weaker social attribution skills and more autistic-like traits than those with less complicated medical histories. Given the directional nature of all the predictions noted above, we assessed relationships between variables using one-tailed tests. In all analyses involving the data from the animated triangles task, we partialled out total word count.
Age-related changes in social attribution skills
Partial correlations between age (in months) and both appropriateness and intentionality scores were significant in the full-term sample, appropriateness: r (33) = .34, p = .02; intentionality: r (33) = .58, p < .001, with performance improving as a function of age. In contrast, neither correlation was significant in the preterm sample, appropriateness: r (31) = .10, p = .30; intentionality: r (31) = –.09, p = .32. Fisher z tests confirmed that the difference in the strength of the correlations measured in the two groups was significant for the intentionality scores (z = 2.98, p = .001).
Relationship between social attribution skills and AQ scores
The strength of the partial correlation between ToM intentionality and AQ total scores was comparable in the two groups. In the combined sample, these scores were negatively correlated with one another, r (67) = –.24, p = .025. Thus, as expected, children who displayed more autistic-like traits tended to underattribute intentionality/mental states to triangles in ToM displays, relative to those who displayed few autistic-like traits.
Relationship between social attribution skills and medical risk factors in preterm children
To investigate relationships between medical variables and social attribution skills in preterm children, we computed correlations between ToM intentionality scores and each of the continuous medical variables (birthweight, gestational age, Apgar score at 5 min, days on ventilation, days on supplemental oxygen, and length of hospital stay). For the remaining dichotomous medical variables (presence/absence of bronchopulmonary dysplasia at 28 days, abnormality in brain imaging studies, and retinopathy of prematurity), we compared ToM intentionality scores of children with and without complications using independent samples t tests. The same sets of analyses were undertaken to explore the relationship between medical risk and AQ total scores. Given the small sample size and the large number of analyses that were run, these results should be interpreted with caution. Having said that, even within this sample of VLBW children, birthweight was positively correlated with ToM intentionality scores, r (34) = .30, p = .04. In addition, VLBW children who showed evidence of bronchopulmonary dysplasia at 28 days displayed more autistic-like traits on the AQ than those who did not, t (32) = –2.26, p = .03.
Discussion
The results of the present study lend support to earlier reports indicating that preterm children born at VLBW are at increased risk for displaying autistic-like traits (Eaton et al., Reference Eaton, Mortensen, Thomsen and Frydenberg2001; Hultman et al., Reference Hultman, Sparén and Cnattingius2002; Indredavik et al., Reference Indredavik, Vik, Evensen, Skranes, Taraldsen and Brubakk2010; Johnson et al., Reference Johnson, Hollis, Kochhar, Hennessy, Wolke and Marlow2010; Johnson & Marlow, Reference Johnson and Marlow2009; Kuban et al., Reference Kuban, O'Shea, Allred, Tager-Flusberg, Goldstein and Leviton2009; Lampi et al., Reference Lampi, Lehtonen, Tran, Suominen, Lehti and Banerjee2012; Maimburg & Væth, Reference Maimburg and Væth2006; Pinto-Martin et al., Reference Pinto-Martin, Levy, Feldman, Lorenz, Paneth and Whitaker2011; Stephens et al., Reference Stephens, Bann, Watson, Sheinkopf, Peralta-Carcelen and Bodnar2012). They also extend this work by showing an association between the number of autistic-like traits identified through parental report measures, and impaired ability to attribute intentionality/mental states in a performance-based test of these skills (the animated triangles task). These findings are, perhaps, particularly notable given that our preterm sample comprised children who appeared to have escaped major language/intellectual impairments (as indexed by their performance on the PPVT-4).
The specific problems with social attribution that children in our preterm sample experienced were similar to those that have been described in individuals with ASD (Abell et al., Reference Abell, Happé and Frith2000; Jones et al., Reference Jones, Swettenham, Charman, Marsden, Tregay and Baird2011; Zwickel et al., Reference Zwickel, White, Coniston, Senju and Frith2010). Specifically, compared to full-term controls, preterm children used fewer interactional and mental state words when describing socially relevant ToM displays, and more interactional and mental state words when describing random displays. Despite this, they were not completely insensitive to the manipulation; thus, their mean intentionality scores did increase as a function of display type, suggesting that they had some underlying understanding that the scenes varied in interactional content. However, the fact that their descriptions were less appropriate overall suggests that, like children with ASD (see Zwickel et al., Reference Zwickel, White, Coniston, Senju and Frith2010), they use mentalizing words in a somewhat indiscriminate manner, perhaps in an effort to fulfill the assumed task requirements.
Although it seems likely that problems with social attribution underlie the difficulties that the preterm children experienced, it is important to rule out alternative explanations for our findings. The literature suggests that processing speed, verbal intelligence, and demographic variables (such as gender, family income, and maternal education) can affect performance on ToM tasks (Buitelaar, van der Wees, Swaab-Barneveld, & van der Gaag, Reference Buitelaar, van der Wees, Swaab-Barneveld and van der Gaag1999; Cutting & Dunn, Reference Cutting and Dunn1999; De Sonneville et al., Reference De Sonneville, Verschoor, Njiokiktjien, Op het Veld, Toorenaar and Vranken2002; Happé, Reference Happé1995; Milligan, Astington, & Dack, Reference Milligan, Astington and Dack2007; Pears & Moses, Reference Pears and Moses2003). As such, it is important to highlight that the groups in the present study were matched on these variables, making it unlikely that the group differences we observed were attributable to these factors. Similarly, by controlling for total word count in the analyses of the data from the animated triangles task, we reduced the likelihood that group differences emerged simply because preterm children offered more succinct descriptions of the animations.
Another possibility that should be considered is that the group differences we observed reflect underlying problems with memory in preterm children. Difficulties in working, spatial, and episodic memory have been described in this population (e.g., Anderson & Doyle, Reference Anderson and Doyle2004; Isaacs et al., Reference Isaacs, Lucas, Chong, Wood, Johnson and Marshall2000; Luciana, Lindeke, Georgieff, Mills, & Nelson, Reference Luciana, Lindeke, Georgieff, Mills and Nelson1999; Woodward, Edgin, Thompson, & Inder, Reference Woodward, Edgin, Thompson and Inder2005). In this regard, we should note that, as part of a separate investigation, we assessed how well participants in the present study recalled details about specific objects that appeared in short video clips (19–40 s in length) depicting social interactions (Williamson & Jakobson, Reference Williamson and Jakobson2014). Not only did preterm children recall just as many details about objects in these scenes as their full-term peers, but we confirmed that the recall scores they achieved in that study were not correlated with the ToM intentionality scores they obtained in the present study, r (34) = .19, p = .28. Given this, we think it unlikely that the group differences we observed in the animated triangles task arose from group differences in the ability recall details about the animations.
It will be interesting in future work to determine if/how problems with executive function contribute to difficulties in social attribution in preterm children. Deficits in both executive function and ToM are often seen in individuals with ASD (e.g., Ozonoff, Pennington, & Rogers, Reference Ozonoff, Pennington and Rogers1991), but whether these deficits are linked or simply co-occur is not clear. Hu, Chan, and McAlonan (Reference Hu, Chan and McAlonan2010) have shown that typical children's performance on social attribution tasks is not related to their performance on tests of executive function, after controlling for age and verbal IQ.
In their study, Hu et al. (Reference Hu, Chan and McAlonan2010) tested social attribution skills in typically developing 6- to 13-year-olds using a task similar to the animated triangles task used here, and a modified version of the task in which the moving figures were animals rather than geometric shapes. While the modified task was sensitive to developmental changes across the full age range of participants, performance on the conventional task only showed age-related improvement in children over the age of 9. The authors concluded that social attribution tasks involving animated shapes may be too abstract for young children, or those with developmental problems, and that difficulties appreciating the symbolic nature of the stimuli may compromise the performance of these groups. In the present study, which involved children between the ages of 8 and 11 years, we found evidence of age-related improvement in the ability to interpret ToM displays in full-term but not preterm children. Given this, it is possible that problems with symbolic representation may have contributed to the difficulties preterm children experienced in attributing social meaning to the animations. It is also possible, however, that in preterm children, social attribution skills simply follow a different developmental trajectory than that seen in typical development. Longitudinal research is needed to address this question. Investigators undertaking such research should consider using less abstract tasks, such as the modified social attribution task described by Hu et al. (Reference Hu, Chan and McAlonan2010).
A variety of other factors could contribute to difficulties with social attribution in preterm children. For example, recent work shows that VLBW children are at risk for experiencing difficulties with low-level and more complex forms of motion perception (Jakobson et al., Reference Jakobson, Frisk and Downie2006; MacKay et al., Reference MacKay, Jakobson, Ellemberg, Lewis, Maurer and Casiro2005; Taylor et al., Reference Taylor, Jakobson, Maurer and Lewis2009). An important focus of future research should be to determine the extent to which such problems contribute to difficulties with social attribution in this population. One approach to this question might be to study the performance of preterm children on a visual ToM task that uses static stimuli, such as the Eyes Test (Baron-Cohen, Joliffe, et al., Reference Baron-Cohen, Jolliffe, Mortimore and Robertson1997). Another might be to study ToM skills in verbal tasks, such as Happé's strange stories task (Happé, Reference Happé1994).
Even within this small sample composed of VLBW children functioning in the normal range intellectually, birthweight was related to social attribution skills, and children with a positive history of bronchopulmonary dysplasia showed more autistic-like traits (by parent report) than did children without such a history. Additional research involving larger samples of preterm children may help to identify important risk markers for poor social outcomes. Research incorporating sophisticated brain-imaging techniques that can detect subtle damage or dysfunction in the preterm brain may be particularly useful in this regard. For instance, future research could map similarities and differences in brain activation patterns as preterm and full-term children watch random, goal-directed, and ToM animations. Here, we might expect to see that difficulties attributing intentionality/mental states are associated with reduced activation in areas of the brain involved in mentalizing (see Castelli et al., Reference Castelli, Frith, Happé and Frith2002, who reported a similar finding in adults with Asperger syndrome). Future researchers might also apply diffusion tensor imaging techniques to determine if impairments in social attribution are related to disruptions of white matter pathways connecting different parts of the social brain.
The results of this study make an important contribution to a growing literature showing that preterm children are at risk for social and behavioral problems similar to, though perhaps milder than, those seen in children with ASD (e.g., Indredavik, Vik, Skranes, & Brubakk, Reference Indredavik, Vik, Skranes and Brubakk2008). More research is needed to identify the causal pathway behind these functional impairments and to determine if they are the same as those operating in full-term children with ASD (see also Johnson & Marlow, Reference Johnson and Marlow2011). The long-term goal of the present research is to improve our understanding of the development and functioning of the social brain and to improve screening tools and clinical interventions used with populations at risk for impaired social functioning.







