Introduction
The olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), long a pest in the Mediterranean region (Greathead, Reference Greathead1976; Fimiani, Reference Fimiani, Robinson and Hooper1989), was discovered in southern California in 1998 (Rice et al., Reference Rice, Phillips, Stewart-Leslie and Sibbett2003). Within four years, it had spread nearly throughout the state, posing a serious economic threat to the olive industry. The rapid dispersal and establishment of the olive fruit fly, facilitated by the longevity of the adults and their flight characteristics, allowed little opportunity to conduct a statewide eradication programme (R. Dowell, personal communication). Current research efforts emphasize the development of long-term management practices. Although broad spectrum or baited insecticides, currently the only available options to suppress the fly in California, provide some control, their effectiveness is limited by the abundant roadside and residential olive trees that act as reservoirs for reinvasion into treated orchards (Collier & Van Steenwyk, Reference Collier and Van Steenwyk2003). Furthermore, the biological controls that have been successfully implemented for scale pests in California olives (Daane et al., Reference Daane, Rice, Barnett, Zalom, Johnson, Sibbett and Ferguson2005) may be disrupted by insecticides applied for the flies. As there are no natural enemies in California that can adequately suppress the olive fruit fly, classical biological control has been a major research focus for sustainable management (Hoelmer et al., Reference Hoelmer, Kirk, Wharton, Pickett and Woods2004; Sime et al., Reference Sime, Daane, Andrews, Hoelmer, Pickett, Nadel, Johnson and Messing2006a,Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickettb,Reference Sime, Daane, Messing and Johnsonc).
In Europe, longstanding biological control programmes for the olive fruit fly have relied almost exclusively on Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae), an abundant and widespread African species that parasitizes olive fruit fly and various Ceratitis species in its native range (Narayanan & Chawla, Reference Narayanan and Chawla1962; Wharton et al., Reference Wharton, Trostle, Messing, Copeland, Kimani-Njogu, Lux, Overholt, Mohamed and Sivinski2000; El-Heneidy et al., Reference El-Heneidy, Omar, El-Sherif and El-Khawas2001; Billah et al., Reference Billah, Kimani-Njogu, Overholt, Wharton, Wilson and Cobblah2005). However, there are reasons to consider other parasitoid species for the California programme. Firstly, P. concolor is evidently not a narrow host specialist (Wharton & Gilstrap, Reference Wharton and Gilstrap1983). Field release of exotic parasitoids in California requires demonstration of low risk for non-target species, which in this case include both native tephritid species and beneficials used to control weeds (Sobhian, Reference Sobhian1993; Headrick & Goeden, Reference Headrick and Goeden1996; Turner et al., Reference Turner, Piper and Coombs1996; Lang et al., Reference Lang, Richard, Parker and Wendel2000). Secondly, P. concolor has not proved particularly successful in Europe, failing to establish in most regions and requiring regular inundative releases in others to provide an acceptable level of control (Greathead, Reference Greathead1976; Clausen, Reference Clausen1978; Copeland et al., Reference Copeland, White, Okumu, Machera and Wharton2004). In part, the reliance on P. concolor is due to the ease with which it is mass-reared on the Mediterranean fruit fly (Medfly), Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), in artificial diet, whereas other parasitoids of the olive fruit fly have proved more difficult to rear (Wharton, Reference Wharton, Robinson and Hooper1989; Sime et al., Reference Sime, Daane, Andrews, Hoelmer, Pickett, Nadel, Johnson and Messing2006a). However, use of Medfly as a rearing host is impossible in California, because it is not established and federal regulations forbid its importation to the continental United States (Headrick & Goeden, Reference Headrick and Goeden1996). Moreover, use of Medfly as a factitious host in artificial media may contribute to the poor performance of P. concolor on olive fruit fly in the field by changing mating and oviposition behaviour (Kimani-Njogu et al., Reference Kimani-Njogu, Trostle, Wharton, Woolley and Raspi2001). One goal of the California biological control programme, therefore, is to develop mass-rearing protocols for olive fruit fly parasitoids using olive fruit fly and olive fruit. Parasitoid species that are amenable to this rearing technique and that are specific to the olive fruit fly are particularly desirable.
Among others, the Pakistani species, Psyttalia ponerophaga (Silvestri) (Hymenoptera: Braconidae), is currently being considered for release in California. Quarantine evaluations of its responses to olive fruit fly and various non-target species indicate that P. ponerophaga is a specialist on olive fruit fly (K. Daane, K. Sime and H. Nadel, unpublished data). This conclusion is supported by evidence from the field: P. ponerophaga has only been obtained from olive fruit fly, despite intensive rearing of this species and similar tephritids in more than a century of searching for natural enemies of various pest fruit flies (Cameron, Reference Cameron1941; Narayanan & Chawla, Reference Narayanan and Chawla1962; Wharton, Reference Wharton, Robinson and Hooper1989). Although P. ponerophaga was identified as a parasitoid of olive fruit fly nearly 100 years ago, no systematic effort has been made to include it in biological control programmes in Europe (Greathead, Reference Greathead1976). Presumably, this is because P. ponerophaga has been more difficult to rear than other Psyttalia species, and indeed one obstacle to its use as a biological control agent in California is the lack of a proven and practical rearing method. To this end, we investigated several basic biological parameters relevant to insectary and field performance, including host-stage preference, adult responses to feeding regimes, and adult and immature responses to temperature.
Another potential obstacle to using P. ponerophaga in California is its geographic origin, which may also explain its failure to establish in the Mediterranean region. The Pakistani population of the olive fruit fly is a well-differentiated subgroup, sufficiently distinct from the Mediterranean and African populations to warrant recognition as a subspecies or variety (Nardi et al., Reference Nardi, Carapelli, Dallai, Roderick and Frati2005). Because genetic data indicate that the California population of olive fruit fly is derived from populations in the Mediterranean basin (Nardi et al., Reference Nardi, Carapelli, Dallai, Roderick and Frati2005), we could not assume that P. ponerophaga would readily parasitize the California population. Possible incompatibility would best be tested by comparing the responses of P. ponerophaga to hosts from each population; unfortunately, though, we are unable to import Pakistani olive fruit fly to California to make such comparisons. Nonetheless, comparison of the performance of P. ponerophaga on California olive fruit fly to the performance of other parasitoid species on this host could provide a useful indication as to whether host population origin ought to be considered in the course of foreign exploration.
Yet another potential barrier to the use of P. ponerophaga as a biological control agent, and a possible explanation for the poor performance of olive fruit fly parasitoids in Europe as well, is suggested by observations of parasitoids reared from wild and commercial olives in Africa and in the insectary. The parasitoid species most commonly reared from olive fruit fly feeding in wild olives (Olea spp.) are the braconids Bracon celer Szépligeti, Psyttalia lounsburyi (Silvestri), and Utetes africanus (Szépligeti) (Neuenschwander, Reference Neuenschwander1982; Copeland et al., Reference Copeland, White, Okumu, Machera and Wharton2004). In cultivated olives, B. celer predominates, and the other species tend to be rare (Annecke & Moran, Reference Annecke and Moran1982; Neuenschwander, Reference Neuenschwander1982). In the insectary, using fly-infested cultivated olives, P. lounsburyi and U. africanus have proved surprisingly difficult to rear (J. Andrews, unpublished data). In contrast, two Diachasmimorpha species, for which olive fruit fly is an entirely novel host, reproduce readily on it under the same conditions (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b). A possible explanation for this variable performance, which has not previously been addressed, concerns the relative size of commercial olive fruit compared to the fruit of wild Olea. Wild fruit are small, usually 1 cm in diameter, whereas cultivated varieties are significantly larger, usually 2–3 cm in diameter (Bartolini & Petruccelli, Reference Bartolini and Petruccelli2002; Tzanakakis, Reference Tzanakakis2003). The parasitoid species in question bore into the fruit with their ovipositors to parasitize their hosts. The length of the ovipositor relative to the depth of the host within the fruit may limit their ability to successfully parasitize certain hosts, a problem that has been well documented for other fruit fly parasitoids (Sivinski et al., Reference Sivinski, Vulinec and Aluja2001; Sivinski & Aluja, Reference Sivinski and Aluja2003). The ovipositors of most Psyttalia species and U. africanus are very short (<2 mm), whereas those of B. celer and the Diachasmimorpha species are longer, approaching 1 cm. The species that parasitize olive fruit fly in natural environments, though evidently well adapted to attacking hosts in small wild olives, may have difficulty reaching hosts in the larger cultivated fruit. Olive fly larvae, particularly in the second instar, tend to feed close to the pit (K. Daane & K. Sime, unpublished data), where they would be beyond the reach of the parasitoid. We investigated this problem by comparing the reproductive success of P. ponerophaga on hosts in large and small olives.
Materials and methods
Sources of insects and plants and colony maintenance
Laboratory cultures of olive fruit fly originated with infested olives collected near Davis, California (Yolo County), USA, in 2002, and were maintained at the University of California Insectary and Quarantine Facility in Berkeley, California (Berkeley I&Q). The culture was replenished with additional flies from this location two to three times per year. Flies were reared on olive fruit following the procedures of Tzanakakis (Reference Tzanakakis, Robinson and Hooper1989, Reference Tzanakakis2003). Because the flies do not develop on small fruit less than 2 months old, and olives picked when fully ripe tend to rot before the fly larvae (or their parasitoids) complete development, we used a variety of olive cultivars (mostly Manzanillo, Sevillano and Mission) that have varying seasonal periods of ripening. These cultivars could be collected at different times across a long section of the state (south to north: Riverside, Kern, Tulare, Fresno and Yolo Counties), thereby providing fruit of an acceptable quality for 7–9 months of the year. Olives held in cold storage were used for the remaining period.
Olives were exposed to adult flies in an oviposition chamber (45 cm3 wooden cage, with organdy sides and a glass top) that was kept in a temperature-controlled insectary room, with humidity kept relatively low to retard mould growth (22±2°C, 16:8 (L:D) h, 40% RH). Flies had free access to water and a mixture (approximately 2:1 by volume) of honey and a dry yeast extract (FisherBiotech, Fairlawn, New Jersey, USA). Olives were left in the cage for 1–2 days or until they each had 5–10 oviposition marks. Infested olives were transferred to plastic boxes (36×18×10 cm3) with a nylon mesh top. To reduce mould growth, infested olives were placed in the box no more than 2–3 layers deep and were held 2–3 cm off the bottom of the container by a metal screen. Under these conditions, the mature larvae left the fruit and pupated on the bottom of the boxes after 10–14 days. Puparia were collected and transferred to the oviposition chamber to emerge as adults and repeat the process.
Parasitized olive fruit fly pupae, collected from wild Olea europea ssp. cuspidata (Wall. ex. G. Don) in the Northwest Frontier Province, Pakistan, in autumn 2004, were reared at the USDA-ARS European Biological Control Laboratory (EBCL) in Montferrier, France. Adult P. ponerophaga from this collection were sent directly to the Berkeley I&Q. Upon receipt, the parasitoids were placed in a 45×45×45 cm cage that was freely provisioned with fly-infested olives, water, and a honey-water solution (50% by volume). Similar Psyttalia species that attack fruit-infesting tephritids oviposit into second or third instar larvae (Biliotti & Delanoue, Reference Biliotti and Delanoue1959; Mohamed et al., Reference Mohamed, Overholt, Wharton, Lux and Eltoum2003; Billah et al., Reference Billah, Kimani-Njogu, Overholt, Wharton, Wilson and Cobblah2005), with the offspring completing development in the host's puparium. The parasitoids were, therefore, provided with olives infested 6–10 days earlier, containing a mixture of second- and third-instar hosts. After a 1–3 day exposure period, depending on parasitoid density, the inoculated material was transferred to plastic rearing boxes, as described above. The olive fly larvae exited the fruit and dropped to the bottom of these containers to pupate. The puparia were transferred to transparent plastic Petri dishes (9-cm diameter) that were monitored for the emergence of adult flies and parasitoids. The experiments described below began in August 2005, after approximately ten generations of P. ponerophaga had been reared at Berkeley I&Q.
Host-stage preference and reproductive success
Host-stage preference and ovipositional success on different developmental stages were examined in choice tests. To produce an age series of immature flies, fresh olives were exposed to adult flies for 8 h every 2 days and then held at 25±1°C. Immature stages inside the olives were presented to the parasitoids when 2, 4, 6, 8, 10 and 12 days old. A sub-sample of olives from each set was dissected shortly before each test to determine which olive fly stages were present. Under these conditions, 2-day-old olives contained eggs and, rarely, first instars; 4-day-old olives contained first instars; 6-day-old olives contained second instars; 8-day-old olives contained second and young third instars; 10-day-old olives contained third instars; and 12-day-old olives contained mature third instars and were occasionally accompanied by prepupal larvae (emerging from fruit) and pupae.
For each of 14 replicates, five female parasitoids were held for 24 h in an ovipositional chamber (a plastic cylinder 13 cm deep×20 cm diameter, with a fine mesh top) provided with 24 olives of which four each represented one of the six olive fly age categories. The olives were placed in the bottom of the container, grouped by age category in plastic Petri dishes (5-cm diameter) marked with the age of the olives. The position of the grouped age categories on the chamber floor was randomly assigned. During the first 8 h of the exposure period, the activity at each dish within each oviposition chamber was observed 10 times for 6 s each (c. one observation every 50 min, for a total of 70 min of observation). The age category of the olives contacted by adult P. ponerophaga was recorded. After the parasitoids were removed from the oviposition chamber, the olives were held at 25±1°C to rear either adult parasitoids or flies.
Pre-imaginal development at constant temperatures
The developmental rate (egg to adult eclosion) of P. ponerophaga was assessed at moderate to high constant temperatures (22, 25, and 30°C, T±0.5°C, RH 25±4%). High temperatures were of particular interest because parasitoid success in California is most likely to be limited by summer heat, rather than by low temperatures. At lower temperatures (<20°C), the olives tended to become mouldy before parasitoid development was completed, and thus this method could not be used to estimate the developmental threshold. Olives infested with 10-day-old olive fly (third instars) were exposed to the parasitoids in the colony for 20–24 h. Each replicate (of ten) consisted of six infested olives in a paper cup (9-cm diameter×4.5 cm deep). After exposure to the parasitoids, the cup was covered with a clear, ventilated plastic lid and randomly assigned to one of the temperature treatments. The cups were then checked daily for fly or parasitoid emergence. The conditions in each incubator were monitored using a data logger (Onset Computer Corp., Bourne, Massachusetts).
Adult longevity and reproduction
Adult longevity at different temperatures
Adult male and female P. ponerophaga longevity were measured at six constant temperatures (15.2±0.3, 22.0±0.2, 25.2±0.5, 30.0±0.3 and 34.0±0.3°C; RH 25±2%). Newly emerged parasitoids were placed in glass vials (1 cm diameter×5 cm long, with an organdy-mesh lid), which were provisioned with a streak of honey-water (50% solution by volume), and then randomly assigned to a temperature cabinet. To maintain a higher humidity (60±5%), the vials were kept inside an airtight plastic container. The parasitoids were checked daily for mortality. The honey-water was refreshed every 2–3 days (as needed). At each temperature, ten male and ten female parasitoids were tested (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b,Reference Sime, Daane, Messing and Johnsonc).
Adult longevity given different provisions
Female P. ponerophaga longevity was compared among five treatments with access to: (i) olives containing hosts, honey-water (50% by volume) and water; (ii) uninfested olives, honey-water and water; (iii) honey-water and water only; (iv) water only; and (v) no provisions (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b,Reference Sime, Daane, Messing and Johnsonc). Newly emerged adult females were collected daily, transferred to a small container with males, supplied with water and honey-water, and held for one day to mate. The females were then randomly assigned to one of the five treatments, with each parasitoid isolated in a small plastic container (15 cm diameter×6 cm deep) with a hole (7 cm diameter) cut in the lid and covered with nylon mesh for ventilation. The olives (four per container) were replaced every other day. Where olives with hosts were offered, the fly larvae were at a suitable stage for parasitoid oviposition (second and third instars). Each of the four olives had 5–10 olive fly oviposition marks, and, therefore, 20–40 larvae were available for each 2-day interval (an estimate confirmed by the subsequent rearing). Honey-water, streaked along the sides of the container, and distilled water, in a soaked cotton wick, were freely available. Parasitoids were checked daily for mortality. All treatments were kept in a temperature-controlled room (22±2°C, 40±5% RH, 16:8 (L:D) h supplemented by natural daylight). There were ten replicates for each treatment.
Lifetime reproductive potential
To determine lifetime reproductive potential, the infested olives that were collected every other day in the experiment described above were held in plastic cups for emergence of adult flies or parasitoids. An additional ten replicates were completed, bringing the total to 20 replicates. The number and sex of the emerging adult offspring were recorded.
Comparing reproductive success on large and small olives
Olives were collected in a single Fresno County orchard containing a mixture of cultivars in late August and early September 2005. At this point in the season, a variety of sizes of fruit were present, but all fruit were green and firm, 2–3 months away from ripening. The olives were divided into two groups by size. Most of the ‘large’ olives were Ascolano and Sevillano cultivars, typical commercial table olives, about 3 cm diameter; and most of the ‘small’ olives were Mission and Rubra cultivars, used for oil, with fruit sizes within the range (6–19 mm) reported for a typical Pakistani wild olive, Olea europea ssp. cuspidata (Bartolini & Petruccelli, Reference Bartolini and Petruccelli2002). All varieties are susceptible to the olive fruit fly. The lengths of a subsample of 25 small and 25 large olives were measured to confirm the visual categorization. Olives were grouped in paper cups (five small and five large olives each), which were then placed in a fly ovipositional cage for 2 days. Following exposure to flies and allowing 10 days for fly larval development, the infested olives were placed in a parasitoid ovipositional chamber for 2 days, then transferred to an incubator (25±0.5°C) for rearing. There were 25 replicates, with each paper cup of ten olives serving as a replicate.
Statistical analysis
Results are presented as means per treatment (±SEM). To determine treatment effect, we used analysis of variance (ANOVA), with treatment means separated using Tukey's HSD test (three or more treatments) or t tests (two-way comparisons) and linear regression. For observations of parasitoid–host encounters, we summed the number of encounters for each treatment and replicate over the 1 min observation period (ten 6–s observations), and then regressed age category against the mean per treatment. For measuring parasitism rates and comparing the number of offspring produced, only those replicates where one or more adult flies or P. ponerophaga emerged were included.
Results
Host-stage preference and reproductive success
Olives containing all age categories of immature flies were examined by P. ponerophaga. Although there were no differences among individual age categories (F=1.197, df=5, 72, P=0.319), the number of parasitoid–host encounters was a positive function of olive fly age (fig. 1a). A relatively low level of parasitoid–host encounters was observed (<1 per five adults per 1 min observation period), with most of the adults resting on the oviposition chamber walls or screen top during the observation intervals. All but the 2-day age category (eggs and neonate larvae) contained acceptable host stages from which parasitoids were reared. The number of parasitoids reared was also a positive function of olive fly age category (fig. 1b), and there were differences among age categories in the number of parasitoids reared (F=8.744, df=5, 44, P<0.001). Significantly more (P<0.01) parasitoids were reared from flies in the oldest age category (12 days, late third instar) than from the 2, 4, 6 or 10 day old age categories. Similarly, significantly more (P<0.05) parasitoids were reared from flies in the 8-day-old age (second and young third instars) category than from the 2- or 4-day-old categories.

Fig. 1. Mean (±SEM) number of (a) parasitoid–host encounters observed during a 1 min period and (b) parasitoids reared per four-olive replicate were positive functions of fly age category (encounters: y=0.005+0.072x, r2=0.783, df=1, 4, F=18.99, P=0.012; offspring: y=−0.944+0.354x, r2=0.642, df=1, 4, F=9.966, P=0.034).
Pre-imaginal development at constant temperatures
The developmental times for parasitoids reared at 22, 25 and 30°C were a negative function of temperature for both males and females, with developmental times ranging from 12 to 26 days (fig. 2). The developmental times shown for olive fruit fly are for individuals that escaped parasitism and represent the periods following exposure to parasitoids (i.e. duration of the pupal stage plus the last 1–3 days of the third instar). Under these conditions, fly development was a negative function of temperature, and flies emerged earlier than parasitoids at all temperatures tested (fig. 2).

Fig. 2. Mean (±SEM) immature (egg to adult eclosion) development time was a negative function of three tested temperatures for Psyttalia ponerophaga females (y=32.40–6.51x, r2=0.94, F=236.1, df=1, 14, P<0.001), and males (y=30.67–5.61x, r2=0.92, F=155.3, df=1, 11, P<0.001). Development time for fly larvae that escaped parasitism (the periods following exposure to parasitoids) was also a negative function of temperature (y=18.24–2.48x, r2=0.92, F=154.0, df=1, 27, P<0.001). –●–, female P. ponerophaga; - -○- -, male P. ponerophaga; –▾–, olive fly.
Temperature also affected fly and parasitoid survival. Across all temperature treatments, there were 33.1±3.0 insects per replicate that reached the pupal or adult stages (fly or parasitoid). Of these, there was greater mortality in the 30°C temperature than in either of the lower temperatures tested (table 1). Overall, less than 7.1% of the exposed insects that reached the adult or pupal stage were reared to adult P. ponerophaga, and most of these were in the lowest temperature treatment (table 1).
Table 1. Rearing record for olive fruit fly larvae exposed to adult Psyttalia ponerophaga and then reared at three different temperatures (percentage±SEM).
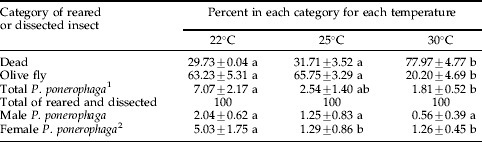
Statistical output for each insect category are as follows, dead: F=40.16, df=2, 26, P<0.001; olive fly: F=31.10, df=2, 26, P<0.050; female P. ponerophaga: F=3.360, df=2, 26, P<0.050; male P. ponerophaga F=1.264, df=2, 26, P=0.299; total parasitoids (female and male): F=3.320, df=2, 26, P<0.052.
1 Significant at P<0.06.
2 Significant at P<0.09.
Adult longevity and reproduction
Adult longevity at different temperatures
Adult longevity was a negative function of temperature for both females (y=57.55–1.55x, r2=0.432, F=38.30, df=1, 48, P<0.001) and males (y=34.15–0.90x, r2=0.347, F=27.01, df=1, 48, P<0.001). Paired comparisons at each temperature indicated no significant differences in male and female longevity except at 22°C, where females lived longer (table 2).
Table 2. Longevity (±SEM) of adult female and male Psyttalia ponerophaga when held at constant temperatures and provisioned with honey and water.

Means within column followed by the same letter are not significantly different at P<0.05 (Tukey's pairwise comparisons, female: F=13.41, df=4, 45, P<0.001, male: F=10.58, df=4, 45, P<0.001).
Adult longevity given different provisions
Adult female parasitoids lived longest when provided with olives (with or without hosts), honey and water; or just honey and water (fig. 3). Provision with water alone, or with nothing, significantly decreased longevity compared to treatments that included honey.

Fig. 3. Adult female longevity (mean±SEM) of Psyttalia ponerophaga was significantly affected by provisioning (F=17.20, df=4, 45, P<0.001). Different letters above each bar represent significant differences (Tukey's multiple comparison test, P<0.01).
Lifetime reproductive potential
Average lifetime production of progeny was 18.7±2.8 adult offspring obtained per female. Progeny production rates were highest during the first 10–12 days of adult life and declined thereafter (fig. 4), dropping to near zero after 20 days, although the parasitoids used in the experiment continued to live to an average of 29.5±2.5 days. The mean proportion of female offspring obtained was 0.41±0.11, which does not differ significantly from a female:male ratio of 1:1.

Fig. 4. Mean (±SEM) lifetime production of offspring produced by Psyttalia ponerophaga provisioned with hosts, honey and water and held at 22°C.
Comparing reproductive success on large and small olives
The small olives measured 1.75±0.03 cm and the large olives measured 3.25±0.05 cm in length. The two olive size categories differed significantly (t-statistic=23.94, df=42, P<0.001). More olive flies were reared from the large olives (fig. 5a), but more P. ponerophaga were reared from the small olives (fig. 5b), with higher percentage parasitism in the small olives (fig. 5c). Because there were significantly fewer host larvae available for oviposition in small (n=109) compared with large olives (n=367), the percentage parasitism may not be an appropriate treatment comparison. For that reason, the data were also submitted to a 2×2 contingency table (SYSTAT, 2000), which showed a significant effect of olive size on the expected numbers of adult fly and parasitoids reared (χ2=20.16, P<0.001).

Fig. 5. Mean (±SEM) number of adult (a) olive fruit flies, (b) Psyttalia ponerophaga reared from olives categorized as either small or large and (c) the resulting levels of percentage parasitism. In each graph, different letters above each mean bar indicate a significant difference for (a) flies (t=5.612, df=44, P<0.001), (b) parasitoids (t=−1.843, df=38, P=0.073) and (c) percentage parasitism (−3.338, df=38, P=0.002).
Discussion
This study establishes basic guidelines for rearing P. ponerophaga on olive fly in olive fruit. The parasitoids should be provided with hosts in the second and third instar, and, given honey-water, can be expected to lay eggs for approximately two weeks at standard room temperature. Reproductive success will be maximized when smaller olives are used. Larval development on picked olives proceeds better at slightly lower temperatures, around 22°C. Using these methods, we have to date been able to continuously maintain a small culture of P. ponerophaga for over 20 months. The main disadvantage of using fruit is that good quality olives are not available throughout the year, and reproduction may decline during the off-season (Sime et al., Reference Sime, Daane, Andrews, Hoelmer, Pickett, Nadel, Johnson and Messing2006a). We are currently investigating ways to improve storage methods for olive fruit. Alternatively, efforts are also under way to develop mass-rearing techniques for the olive fruit fly and associated parasitoids using artificial diet (C. Pickett, personal communication). The lifetime reproductive potential determined here for P. ponerophaga is similar to that reported for P. concolor reared using the standard method of Medfly in artificial diet as host material (Stavraki-Paulopoulou, Reference Stavraki-Paulopoulou1966), which suggests that artificial diets could yield comparable results. Artificial diet rearing may, however, present an unacceptable tradeoff by selecting for behaviours that impede field performance (Kimani-Njogu et al., Reference Kimani-Njogu, Trostle, Wharton, Woolley and Raspi2001). This possibility has yet to be investigated for P. ponerophaga or in olive fruit fly (as opposed to Medfly) rearing systems.
The biological traits we measured in the present study can be used to compare the performance of P. ponerophaga to other olive fruit fly parasitoids. Climate tolerance, for one, must be considered in selecting natural enemy species for release (Hoelmer & Kirk, Reference Hoelmer and Kirk2005) and is a special concern in California. Olives are grown both in coastal counties, which are characterized by mild temperatures year-round, and in the Central Valley, which has very hot summers and somewhat colder winters. The fly does well in both regions, but we cannot assume that any single parasitoid species will. The European experience suggests that olive fruit fly thrives where many parasitoid species do not (Greathead, Reference Greathead1976; Clausen, Reference Clausen1978). Other biological control programmes in California have documented the ability of parasitoids to provide control inland but not on the coast, or vice versa, with the differences attributed at least in part to climate (Yu et al., Reference Yu, Luck and Murdoch1990; Dahlsten et al., Reference Dahlsten, Daane, Paine, Sime, Lawson, Rowney, Roltsch, Andrews, Kabashima, Shaw, Robb, Geisel, Chaney, Ingels, Varela and Bianchi2005). Based on our adult longevity data, P. ponerophaga appears to be very promising for widespread establishment in California. Its survivorship at both high and low extremes compares favourably, for example, to the survivorship of two Diachasmimorpha species (D. kraussii (Fullaway) and D. longicaudata (Ashmead) (Hymenoptera: Braconidae)) tested under identical experimental conditions (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b), exceeding their performance at constant temperatures greater than 30°C. That P. ponerophaga males and females can live for several days at a constant 34°C indicates that they will perform well even in the Central Valley, where summer highs normally reach (and sometimes exceed) this temperature but are sustained for only a few hours each day.
The responses of immature P. ponerophaga to temperature are less clear because only a limited range of temperatures (22–30°C) was tested. Moreover, because these data were collected on excised fruit, they may not reflect field performance. The olives tended to dry out and shrivel quickly at 25 and 30°C, which may have impaired parasitoid development. Because fruit still on a tree would remain turgid even at high temperatures, the results cannot be used to infer an upper temperature limit for development.
The results of this study also provide specific guidelines for field manipulations of P. ponerophaga and similar parasitoids. Releases should be timed to coincide with the availability of second- and third-instar hosts. Fruit size must be taken into account as well. When fruit are relatively large, second-instar hosts will be less accessible because these tend to feed deeper within the fruit, and releases should correspond to the third instar. In general, the field effectiveness of P. ponerophaga, and other olive fruit fly parasitoids with short ovipositors, is expected to correlate inversely with fruit size. These species are less likely to be successful in large table-olive varieties, particularly those that are heavily irrigated to maximize fruit size. On the other hand, smaller varieties and oil olives (which are not watered to the same extent) should prove more amenable to biological control using these species. Unfortunately, no specialist parasitoids of olive fly are known that have longer ovipositors. Bracon celer, Diachasmimorpha kraussii and D. longicaudata, three species with long ovipositors, are readily reared on olive fly in the laboratory, but all three have relatively broad host ranges and thus may not be suitable for release in California (Sime et al., Reference Sime, Daane, Andrews, Hoelmer, Pickett, Nadel, Johnson and Messing2006a,Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickettb).
The overall performance of P. ponerophaga on the California olive fly population, as measured by lifetime fecundity and offspring sex ratio, suggests that there exists no particular barrier to its use of this population as a host. Although it may reproduce more efficiently on the Pakistani strain, a possibility that could not be tested in this study, its performance on California olive flies compares favourably to that of other parasitoids. The lifetime production of offspring (18.7±2.9) by P. ponerophaga is slightly lower but not significantly different from that observed for two laboratory strains of P. concolor (22.5±5.1 and 28.7±4.1 offspring per female) (Sime et al., Reference Sime, Daane, Messing and Johnson2006c) and two Diachasmimorpha species (23.6±5.3 and 22.7±5.5) (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b) tested under identical experimental conditions.
These last results are, however, contrary to the expectation that P. ponerophaga, as a specialist coevolved with olive fly, would perform better than the more generalist P. concolor or the Diachasmimorpha species for which olive fly represents an entirely novel host. There are two likely explanations. Firstly, P. ponerophaga may be better adapted to the Pakistani olive fly population. Secondly, the undistinguished performance of P. ponerophaga compared to the Diachasmimorpha species may be an artefact of using larger olives. Tested under the same experimental conditions, D. kraussii and D. longicaudata also preferentially attack second and third instars (Sime et al., Reference Sime, Daane, Nadel, Funk, Messing, Andrews, Johnson and Pickett2006b). Whatever disadvantages the Diachasmimorpha species may have in coping with a novel host may be outweighed by the possession of longer ovipositors, which allow them to reach almost all available fly larvae and to readily parasitize their preferred stages of host. It would be instructive to compare the performance of Diachasmimorpha species on hosts reared in large and small olives, to confirm whether or not olive size has any effect on their parasitism rates as well.
Finally, olive size and ovipositor length may also help to explain the mixed performance of P. concolor as a biological control agent for olive fly in Europe. Its ovipositor is approximately the same length as that of P. ponerophaga. Field releases of P. concolor are often made when the hosts are in the first and second instar, a recommendation based on performance in diet-reared cultures (Raspi & Canale, Reference Raspi and Canale2000). Our results indicate, however, that these stages are difficult for Psyttalia species to parasitize when the host is feeding in cultivated fruit varieties. More generally, the problem of ovipositor length may help explain the puzzling absence of olive fly parasitoids in Europe. Although olives have been grown in Europe for thousands of years, and there are no obvious geographic barriers to insect dispersal (as evidenced by the spread of the olive fruit fly itself), P. concolor and other parasitoids that are abundant in adjacent regions have failed to spread into and establish in Europe. The problem may be that there only exist domestic varieties of olive in Europe, with their larger fruit. A telling contrast exists in South Africa, where commercial olives grow in the same habitats as wild olives, and olive fly is seldom an economic pest because it is heavily parasitized by various braconids (Annecke & Moran, Reference Annecke and Moran1982). There, parasitoids of olive fruit fly in wild olives serve as a population reservoir for spread into commercial olive orchards. Of the various species attacking the fly in wild olives, only B. celer is consistently reported as abundant in cultivated fruit (Annecke & Moran, Reference Annecke and Moran1982; Neuenschwander, Reference Neuenschwander1982). The ovipositor of this species is much longer than those of the other olive fly parasitoids in the region, and it preferentially parasitizes mature third instars, which vary in feeding depth and are sometimes found close to the surface (Sime et al., Reference Sime, Daane, Andrews, Hoelmer, Pickett, Nadel, Johnson and Messing2006a). The inherent incompatibility of the various short-ovipositor parasitoids of olive fruit fly with cultivated olives may represent a serious challenge for biological control, but it can potentially be moderated by proper seasonal timing of parasitoid release, modifying irrigation regimes to minimize fruit size and taking fruit size and host feeding location into account in field release strategies.
Acknowledgements
The authors thank Mohammed Riaz, CABI Bioscience, Rawalpindi, Pakistan, for collecting fly infested olives, shipping parasitized pupae to EBCL and making field observations; K. Hoelmer, A. Blanchet, M. Roche and D. Coutinot at EBCL France for rearing and processing parasitoids; and R. Wharton (Texas A&M University) for identifying P. ponerophaga. In California, H. Beeson, J. Brown, H. Nadel, C. Pickett, M. Gerik and M. Yamamoto provided additional assistance. Funds provided by the California Specialty Crop Block Grant (administered by the California Department of Food and Agriculture, with funding from the United States Department of Agriculture) and the California Olive Committee are gratefully acknowledged.









