Introduction
The genus Ahtiella Öpik, Reference Öpik1932 is a distinctive resupinate and variably geniculate plectambonitoid brachiopod described originally from the Baltic region (Öpik, Reference Öpik1932, Reference Öpik1933; Hessland, Reference Hessland1949) but subsequently recognized in Wales (Bates, Reference Bates1968) and central Newfoundland (Neuman, Reference Neuman1976). In South America, Ahtiella is very common in the lower−middle Darriwilian carbonate-ramp deposits of the Precordillera basin of west-central Argentina where it defines the uppermost of the six brachiopod biozones recognized through the San Juan Formation (Herrera and Benedetto, Reference Herrera and Benedetto.1991; Benedetto, Reference Benedetto2002, Reference Benedetto2007). Later, Ahtiella was reported from the Floian-Dapingian volcaniclastic succession of the Famatina Range (Benedetto et al., Reference Benedetto, Dávila and Astini2003; Benedetto Reference Benedetto2003a) but these specimens remain undescribed. Its presence in southern Peru (Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007), together with its record in the central Andean Basin of northwestern Argentina (this paper) and probably Bolivia (described as Valcourea sp. by Havlíček and Branisa, Reference Havlíček and Branisa1980), indicate that this genus not only attained a wide geographic range in South America but also experienced a significant speciation event encompassing at least five species. As Gutiérrez-Marco and Villas (Reference Gutiérrez-Marco and Villas2007) pointed out, the records of Ahtiella in the Floian of Peru and Dapingian of Famatina are the oldest known of the genus, strongly suggesting that it originated on the Andean margin of Gondwana and later migrated to other regions.
One of the objectives of this study is to update the taxonomy of the genus Ahtiella from the three major Ordovician basins of Argentina: Precordillera, Famatina, and Central Andes. This includes: (1) the redescription of the Precordilleran species Ahtiella argentina Benedetto and Herrera, Reference Benedetto and Herrera1986, on the basis of extensive collections made in the past twenty years from the upper part of the San Juan Formation, as well as the proposal of a new species of Ahtiella from the somewhat younger Las Chacritas Formation; (2) the first description of the Ahtiella specimens from volcaniclastic rocks of the Famatina Range; and (3) the reassignment to the genus Ahtiella of Monorthis coloradoensis Benedetto, Reference Benedetto1998b, from northwestern Argentina.
Evidence presented here aims to shed light on the long-standing and not yet resolved issue of the origin of plectambonitoid brachiopods. Although parsimony analysis constitutes an indispensable tool for unravelling the phylogeny of fossil groups, the most difficult task is to corroborate in the fossil record the phyletic lineages predicted in such analyses, and even more problematic is to detect those morphological transitions leading to the origin of new taxa. According to the punctuated equilibrium hypothesis (Eldredge and Gould, Reference Eldredge and Gould1972; Gould and Eldredge, Reference Gould and Eldredge1977; Benton and Pearson, Reference Benton and Pearson2001), this can be explained by the conjunction of the rapidity as cladogenesis events occur and the relatively small size and geographic restriction of populations undergoing phenotypic change. In this respect, the continuous and richly fossiliferous volcanosedimentary succession of the Famatina Range provides an invaluable frame to establish well-resolved phylogenies based on the fossil record. In this paper, evidence is presented suggesting that Ahtiella originated from the hesperonomiid orthoid Monorthis transversa Benedetto, Reference Benedetto2003b, which always occurs in strata below those bearing Ahtiella famatiniana new species (described herein). A general trend of morphological change through time emerges from the comparative morphology of Ahtiella species and its putative ancestor Monorthis. Relevant for our phylogenetic hypothesis is the fact that the earliest species of Ahtiella recorded in Gondwana exhibit transitional characteristics between Monorthis and the typical Ahtiella species from younger strata of Cuyania and Baltica. Finally, a phylogenetic analysis is presented herein to investigate the evolutionary relationships among the Gondwanan species of Ahtiella and those from Cuyania, Baltica, and Avalonia.
Stratigraphic provenance and age
The early Paleozoic geodynamic history of southern South America involved three main sedimentary domains (Fig. 1), which were inhabited at different times by species of Ahtiella. They are: (1) the autochthonous Central Andean Basin developed around the Brazilian craton through Peru, Bolivia, and northwestern Argentina; (2) the volcanosedimentary Famatina Basin, which together with the Puna volcanic arc developed peripheral to the active pre-Andean Gondwana margin; and (3) the Precordillera Basin developed on the Laurentian-derived Cuyania terrane, which accreted to Gondwana during the early Paleozoic (for a comprehensive review of the Ordovician basins of Argentina, see Astini, Reference Astini2003).
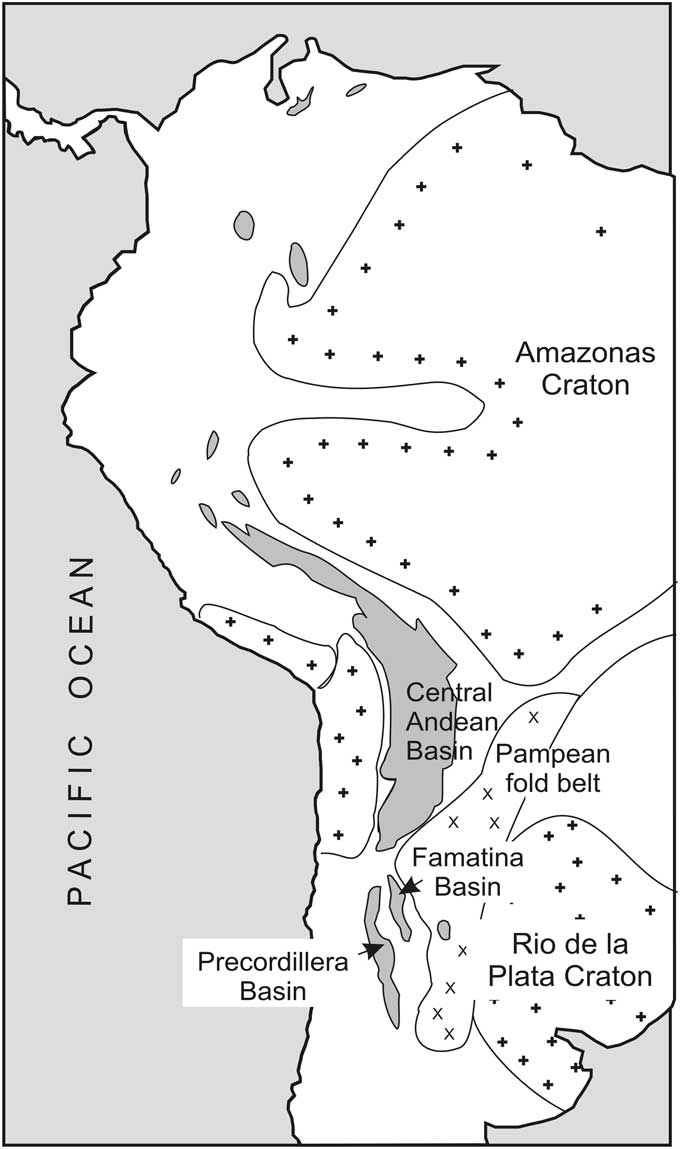
Figure 1 Map of central-western South America showing the main Ordovician sedimentary basins discussed in the text (gray shading).
Central Andean Basin
The southern part of the large Central Andean Basin is widely exposed in the Cordillera Oriental of northwestern Argentina, where the uppermost Cambrian to Lower Ordovician siliciclastic succession of the Santa Victoria Group attains 3,800 m in thickness. In the study area of Los Colorados (Fig. 2), it is overlain by a succession of reddish purple sandstones named the Alto del Cóndor Formation, which is succeeded by fossiliferous greenish mudstones and marls. This interval, which was referred to as the ‘Green Member of the Sepulturas Formation’ by Astini (Reference Astini1994) and as the Sepulturas Formation by Astini et al. (Reference Astini, Toro, Waisfeld and Benedetto2004a), is well exposed at Quebrada Chamarra and Quebrada del Cardonal (Fig. 2). Brachiopods consist of Monorthis coloradoensis (reassigned herein to Ahtiella) and rare specimens of Paralenorthis sp., Dinorthis? sp., and small dalmanellids. Trilobites are represented by Neseuretus sp., a trinucleid of the Anebolithus-Incaia group (personal communication, B.G. Waisfeld, 2017), and a new species of Hoekaspis, the latter recorded elsewhere in the Subandean Ranges of northwestern Argentina from beds not older than the upper Darriwilian (Waisfeld and Vaccari, Reference Waisfeld and Vaccari2003). Albanesi and Astini (Reference Albanesi and Astini2002) reported from interbedded carbonate-rich layers a conodont assemblage consisting of Erraticodon, Erismodus, and Plectodina, as well as microremains of the agnathan Sacabambaspis considered of late Darriwilian age.

Figure 2 Geological map of the Los Colorados area of the Central Andean Basin (modified from Astini et al., Reference Astini, Toro, Waisfeld and Benedetto2004a) showing fossil locations (stars) and integrated stratigraphic column of the study area showing levels yielding Ahtiella coloradoensis. 1=Quebrada Chamarra; 2=Quebrada Cardonal.
Famatina Basin
The Famatina Range is characterized by a thick succession of sedimentary and volcanosedimentary rocks deposited in a retroarc basin almost synchronously with the emplacement of crust-derived magmatism along the proto-Andean margin (Pankhurst et al., Reference Pankhurst, Rapela and Fanning2001; Dahlquist et al., Reference Dahlquist, Pankhurst, Rapela, Galindo, Casquet, Fanning, Saavedra, Baldo and González-Casado2005). Marine intervals are well exposed to the north of the basin in the Chaschuil area (Catamarca Province), and to the south in the Cachiyuyo-Saladillo rivers area (La Rioja Province) (Fig. 3). In the Chaschuil area, a regressive volcanosedimentary sequence acummulated on a high-gradient narrow platform flanking the volcanic chain (Mángano and Buatois, Reference Mángano and Buatois1996, Reference Mángano and Buatois1997). Its lower part, ~150 m thick, was referred to the Loma del Kilómetro Member of the Suri Formation; it has been interpreted as deposited in a storm- and mass flow-dominated shelf, whereas the upper Punta Pétrea Member is a coarse-grained volcaniclastic wedge that records the progradation of a fan delta system onto the shelf sediments. The Dapingian age of the Loma del Kilómetro Member, containing Monorthis transversa (discussed below) and other brachiopods (Benedetto, Reference Benedetto1994), is based on conodonts of the Baltoniodus navis Biozone (Albanesi and Vaccari, Reference Albanesi and Vaccari1994) and the Baltoniodus triangularis Biozone (Carlorosi et al., Reference Carlorosi, Heredia and Aceñolaza2017) recovered from the upper shell beds of this member. That age is consistent with the Whitlandian British regional stage suggested by the underlying trilobite fauna (Vaccari and Waisfeld, Reference Vaccari and Waisfeld1994). The Punta Pétrea Member, which crops out between Agua del Médano and Puesto Chaschuil, yielded an undescribed species of Famatinorthis and Ahtiella famatiniana n. sp. (this paper). This succession is partially interbedded with (Cisterna, Reference Cisterna2001), or is unconformably overlain by (Astini and Dávila, Reference Astini and Dávila2002), the Sierra de Las Planchadas rhyolite, which provided a 206Pb/238U SHRIMP age of 468.3±3.4 Ma (Baldo et al., Reference Baldo, Fanning, Rapela, Pankhurst, Casquet and Galindo2003), i.e., close to the Dapingian-Darriwilian boundary.

Figure 3 Location of the Famatina Range showing sampled areas (circled numbers), stratigraphic columns, and fossiliferous horizons. 1=Chaschuil River area; 2=Central Famatina Range (Cachiyuyo-Saladillo rivers area).
The thicker and more continuous volcanosedimentary succession exposed in the central Famatina Range has been referred to the Famatina Group, encompassing the Suri and Molles formations (Harrington and Leanza, Reference Harrington and Leanza1957). Both units are profusely fossiliferous, containing rhynchonelliform (Benedetto, Reference Benedetto2003b, Reference Benedetto2008, Reference Benedetto2013) and lingulate brachiopods (undescribed), bivalves (Sánchez and Babin, Reference Sánchez and Babin1993; Sánchez, Reference Sánchez1997), trilobites (Harrington and Leanza, Reference Harrington and Leanza1957; Vaccari and Waisfeld, Reference Vaccari and Waisfeld1994), and graptolites (Toro and Brussa, Reference Toro and Brussa1997). The up to 2,000 m thick succession reflects deposition on a high-gradient, mixed siliciclastic-volcaniclastic platform (Astini and Benedetto, Reference Astini and Benedetto1996; Astini, Reference Astini1999, Reference Astini2003; Mángano et al., Reference Mángano, Astini, Buatois and Dávila2002). The Suri Formation displays a shallowing-upward trend ranging from dark shales deposited in relatively deep oxygen-deficient waters to shallow platform facies. The Molles Formation consists of fossiliferous grey mudstones alternating with silicified tuffs, volcanogenic sandstones, and reddish sandstones with evidence of tidal action. Concerning the age, the lower part of the Suri Formation yielded graptolites that indicate the Floian Baltograptus deflexus and Didymograptellus bifidus biozones (Toro and Brussa, Reference Toro and Brussa1997), whereas the shell beds from the top of the Suri Formation and the base of the overlying Molles Formation contain conodonts referable to the upper part of the Oepikodus evae Biozone of Floian age (late Fl2 stage slice of Bergström et al., Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009) (Albanesi and Astini, Reference Albanesi and Astini2000). Lehnert et al. (Reference Lehnert, Bergström and Vaccari1997) reported from the Molles Formation conodonts of the Paroistodus originalis Biozone of middle Dapingian age.
The Molles Formation is unconformably overlain by a 720 m thick volcanosedimentary succession named the Cerro Morado Group, which starts with acidic volcanics and ignimbrites (Portillo Formation) and culminates with silicified tuffs, pyroclastic flows, and bioturbated fossiliferous green shales interbedded with coquina layers (La Escondida Formation) (Astini and Dávila, Reference Astini and Dávila2002) (Fig. 3). The fauna is dominated by Famatinorthis turneri (Benedetto, Reference Benedetto2013), a distinctive taxon of the underlying Molles Formation. A volcanosedimentary unit probably equivalent to the La Escondida Formation crops out to the north of the Cachiyuyo River at Las Pircas anticline. These levels yielded an undescribed Skenidioides? sp. , Paralenorthis sp., and Ahtiella sp. (Benedetto et al., Reference Benedetto, Dávila and Astini2003), the last described herein as A. famatiniana n. sp. The age of this interval, in absence of conodont data, is still poorly constrained, but given that in the Chaschuil area the levels of A. famatiniana n. sp. are interbedded with isotopically dated volcanic rocks (see above), a middle-late Dapingian age seems most likely.
Precordillera Basin (Cuyania terrane)
There is a general agreement that the up to 2,500 m thick, passive-margin carbonate succession started to accummulate during the Cambrian Series 2 on the Laurentian continental margin, a segment of which subsequently rifted from the Ouachita Embayment originating the Cuyania terrane (Astini et al., Reference Astini, Benedetto and Vaccari1995; Thomas and Astini, Reference Thomas and Astini1996, Reference Thomas and Astini2003; Astini, Reference Astini1998; Benedetto, Reference Benedetto1998a, Reference Benedetto2004; Ramos, Reference Ramos2004; but see Finney, Reference Finney2007, for a para-autochthonous Gondwanan hypothesis). Cuyania became part of the Gondwana continent since its accretion to the proto-Andean margin by Middle Ordovician or more probably Late Ordovician times.
Depositional environments evolved from tidal flats, shoals, shallow subtidal settings, and restricted subtidal settings during the Cambrian−early Tremadocian, to open shelf settings by late Tremadocian-Darriwilian times (Cañas, Reference Cañas1999; Keller, Reference Keller1999, Reference Keller2012; Gómez and Astini, Reference Gómez and Astini2015). The carbonate succession referred to the San Juan Formation consists of ~350 m of burrowed skeletal wackestones and packstones capped by a 25−30 m thick interval of mid-outer ramp nodular limestones bearing a rich benthic fauna dominated by rhynchonelliform brachiopods and sponges, with trilobites, bryozoans, gastropods, crinoids, and linguliforms as secondary components (Carrera, Reference Carrera2003; Waisfeld et al., Reference Waisfeld, Sánchez, Benedetto and Carrera2003; Sorrentino et al., Reference Sorrentino, Benedetto and Carrera2009; Carrera and Ernst, Reference Carrera and Ernst2010; Lavié and Benedetto, Reference Lavié and Benedetto2016) (Fig. 4). The brachiopod association from these beds encompasses the Ahtiella argentina Biozone, which is the uppermost of the six biozones recognized through the San Juan Formation (Herrera and Benedetto, Reference Herrera and Benedetto.1991; Benedetto, Reference Benedetto2002, Reference Benedetto2007). This interval is particularly well exposed along the western slope of Cerro Viejo, ~20 km northeast of the city of San José de Jáchal, where the San Juan Formation forms a westward-dipping homoclinal succession. The A. argentina specimens described herein were collected mainly at Quebrada Los Gatos and the adjacent Quebrada Honda stratigraphic sections from a 10−12 m thick package of nodular limestones lying immediately below the contact with the Los Azules Formation black shales (Fig. 4). The age of this interval is well constrained by conodonts of the Paroistodus horridus Subzone within the Lenodus variabilis Biozone (Albanesi and Ortega, Reference Albanesi and Ortega2002; Ortega et al., Reference Ortega, Albanesi and Frigerio2007) and the lower part of the succeeding Yangtzeplacognathus crassus Biozone (Mestre and Heredia, Reference Mestre and Heredia2013; Serra et al., Reference Serra, Albanesi, Ortega and Bergström2015). According to the time-slices schema proposed by Bergström et al. (Reference Bergström, Chen, Gutiérrez-Marco and Dronov2009), the A. argentina beds fall mostly within Dw1 reaching the lower part of Dw2.

Figure 4 Outcrops of Ordovician rocks in the Precordillera mountain belt, sampled areas (circled numbers), and stratigraphic columns showing fossiliferous horizons. 1=Cerro Viejo (Qebrada Los Gatos and Qebrada Honda); 2=Sierra de la Trampa (Quebrada Las Chacritas and Quebrada La Tuna).
At Sierra de la Trampa, near 40 km south of the city of San José de Jáchal, a ~60 m thick succession of nodular limestones crops out at Quebrada Las Chacritas and Quebrada La Tuna (Fig. 4). This package was originally referred by Espisúa (Reference Espisúa1968) to the ‘upper member’ of the San Juan Formation, then to the ‘Las Tunas calcareous unit’ by Carrera (Reference Carrera1997), and finally to the Las Chacritas Formation by Astini (Reference Astini1998), which was formally defined by Peralta et al. (Reference Peralta, Heredia and Beresi1999). The thin bedded wackestones, bioclastic grainstones, and mudstones of the Las Chacritas Formation have yielded rich poriferan assemblages (Carrera, Reference Carrera1997), excellently silicified trilobite larval stages (Waisfeld et al., Reference Waisfeld, Vaccari, Chatterton and Edgecombe2001, and references therein), and numerous brachiopods not yet described, including Skenidioides? sp. and Ahtiella tunaensis new species (this paper). Several conodont studies led to the recognition the Y. crassus Zone in the lower part of the Las Chacritas Formation, the Eoplacognathus pseudoplanus Biozone from 36 m above the base, and the Eoplacognathus suecicus Biozone near the top (Albanesi and Astini, Reference Albanesi and Astini2000; Heredia et al., Reference Heredia, Beresi and Peralta2011; Mestre and Heredia, Reference Mestre and Heredia2012, Reference Mestre and Heredia2013; Serra et al., Reference Serra, Albanesi, Ortega and Bergström2015). Accordingly, A. tunaensis n. sp. can be confidently dated as middle Darriwilian (Dw2).
Remarks on the biogeography of Ahtiella and related ahtiellins
The earliest known representative of the genus is Ahtiella zarelae Villas in Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007 from the upper Floian San José Formation of southern Peru (Gutiérrez-Marco et al., Reference Gutiérrez-Marco, Albanesi, Sarmiento and Carlotto2008). The slightly younger A. famatiniana n. sp. occurs in volcaniclastic rocks of the Famatina Range of middle-late Dapingian age. In Anglesey (northwestern Wales), Ahtiella is represented by A. quadrata Bates, Reference Bates1969, from the Expansograptus hirundo Biozone, which in the Atlantic Province encompasses the Dapingian and the base of Darriwilian (Zalasiewicz et al., Reference Zalasiewicz, Taylor, Rushton, Loydell, Rickards and Williams2009), and A. concava Bates, Reference Bates1969, from the slightly younger Bob Deiniol Formation. Ahtiella paucirugosa Neuman, Reference Neuman1976 has been reported from the lower Darriwilian Summerford Group of New World Island, central Newfoundland. These volcaniclastic rocks were interpreted as recording intra-Iapetus volcanic islands related to the Avalonian paleocontinent (Neuman, Reference Neuman1976, Reference Neuman1984; Neuman and Harper, Reference Neuman and Harper1992; Harper et al., Reference Harper, MacNiocaill and Williams1996). Monorthis coloradoensis occurs in the Cordillera Oriental of northwestern Argentina from beds probably not older than mid-Darriwilian. Ahtiella is common in the carbonate or mixed carbonate-clastic rocks of Baltica and Cuyania. In Sweden and Estonia, Ahtiella encompasses the Kunda and Asseri regional stages, the latter reaching the middle Darriwilian Pterograptus elegans Biozone and E. suecicus Zone (Tolmacheva et al., Reference Tolmacheva, Egerquist, Meidla, Vinn and Holmer2003; Suyarkova and Koren, Reference Suyarkova and Koren2009). As stated above, in the Precordillera basin, Ahtiella ranges from the lower Darriwilian (A. argentina) to the middle Darriwilian (A. tunaensis n. sp.) but does not reach the E. suecicus Zone. Such a distribution led Gutiérrez-Marco and Villas (Reference Gutiérrez-Marco and Villas2007) to infer that Ahtiella migrated eastward from the mid-latitude (~30–40°) Andean region into Avalonia and Baltica, and simultaneously moved into the low-latitude Cuyania taerrane. It should be noted that Gutiérrez-Marco and Villas (Reference Gutiérrez-Marco and Villas2007, fig. 8) adopted the paleogeographic reconstruction of Aceñolaza et al. (Reference Aceñolaza, Miller and Toselli2002) and Finney (Reference Finney2007) and placed Cuyania into the gap delimited by southern South America, South Africa, and Antarctica. Because neither tectonostratigraphic (Astini and Rapalini, Reference Astini and Rapalini2003; Ramos, Reference Ramos2004; Thomas et al., Reference Thomas, Astini, Mueller, Gehrels and Wooden2004) nor paleontological evidence (Benedetto, Reference Benedetto2004) supports such a para-autochthonous Gondwanan origin, Cuyania is located here fairly closer to, and at approximately the same paleolongitude as the Famatina-Puna volcanic arc (Fig. 5). Perhaps the major weakness—but not the only one—of the Finney (Reference Finney2007) reconstruction is the complete absence in the Cambrian carbonate rocks of the Precordillera of Redlichiid-realm trilobites, which, as it is known, are distinctive of Australasia and Antarctica. Instead, the Cambrian-Tremadocian trilobites from Cuyania (Astini et al., Reference Astini, Benedetto and Vaccari1995; Vaccari, Reference Vaccari1995; Benedetto, Reference Benedetto2004; Benedetto et al., Reference Benedetto, Vaccari, Waisfeld, Sánchez and Foglia2009), as well as brachiopods (Benedetto and Foglia, Reference Benedetto and Foglia2012) and other fossil groups (Carrera and Rigby, Reference Carrera and Rigby1999; Carrera, Reference Carrera2003; Astini et al., Reference Astini, Thomas and Yochelson2004b) display indisputable Laurentian affinities. On the other hand, the Early Ordovician brachiopod faunas of Famatina—with a strong Celtic province signature (Benedetto, Reference Benedetto2004; Harper et al., Reference Harper, Owen and Bruton2009)—share several genera (though not the same species) with Cuyania, e.g., Skenidiodes Schuchert and Cooper, Reference Schuchert and Cooper1931; Paralenorthis Havlíček and Branisa, Reference Havlíček and Branisa1980; Productorthis Kozlowski, Reference Kozlowski1927; Monorthis Bates, Reference Bates1968; Ffynnonia Neuman and Bates, Reference Neuman and Bates1978; Hesperonomia Ulrich and Cooper, Reference Ulrich and Cooper1936; Hesperonomiella Ulrich and Cooper, Reference Ulrich and Cooper1936; Camerella Billings, Reference Billings1859; and Rugostrophia Neuman, Reference Neuman1971. This indicates that by the Darriwilian, Cuyania was separated from Gondwana by a remnant ocean not large enough to prevent faunal dispersion (Benedetto et al., Reference Benedetto, Dávila and Astini2003; Benedetto, Reference Benedetto2004). It seems likely that brachiopod dispersion from Famatina to Cuyania was facilitated by the gradual approximation of the Cuyania terrane to the Gondwana margin combined with a generalized sea-level rise (Carrera and Astini, Reference Carrera and Astini1998; Cañas, Reference Cañas1999; Astini, Reference Astini2003).

Figure 5 Early−Middle Ordovician paleogeography map (modified from Cocks and Torsvik, Reference Cocks and Torsvik2002 and Popov et al., Reference Popov, Ghobadi Pour, Bassett and Kebriaee-Zadeh2009), showing global distribution of the genus Ahtiella (stars). Paleogeographic map. ATA=Armorican Terrane Assemblage; CAB=Central Andean Basin; CNF=Central Newfoundland (placement based on Neuman, Reference Neuman1984).
An interesting feature is that diversification of the subfamily Ahtiellinae was centered mainly in Avalonia, Cuyania, and Baltica (Fig. 5). The Welsh Treiorwerth Formation yielded Inversella (Reinversella) monensis Bates, Reference Bates1969 (Neuman and Bates, Reference Neuman and Bates1978), whereas the Central Newfoundland Summerford Group contains the ahtiellins Schedophyla potteri Neuman, Reference Neuman1971, Inversella sp., and the endemic Guttasella gutta Neuman, Reference Neuman1976. In the Cuyanian Precordillera Basin, Ahtiella argentina co-occurs with I. (R.) arancibiai Herrera and Benedetto, Reference Herrera and Benedetto1987 (Benedetto et al., Reference Benedetto, Sorrentino, Cech and Sánchez2008) and the endemic ahtiellin Sanjuanella plicata Benedetto and Herrera, Reference Benedetto and Herrera1987. In Estonia, Ahtiella lirata Öpik, Reference Öpik1932 is approximately coeval with I. (Inversella) borealis Öpik, Reference Öpik1933. Outside the Baltic and Celtic faunal provinces, the only ahtiellins reported are Borua Williams and Curry, Reference Williams and Curry1985 from Ireland, and two species of Schedophyla Neuman, Reference Neuman1971 from southern China (Xu and Liu, Reference Xu and liu1984; Zhan et al., Reference Zhan, Jin and Rong2006). However, as noted below, the placement of Schedophyla among the ahtiellinis requires further confirmation. The Norwegian Rutrumella Harper in Bruton and Harper, Reference Bruton and Harper1981 is a poorly known genus that has been referred questionably to the subfamily (Cocks and Rong, Reference Cocks and Rong2000).
The Andean region as a center of origin
As Gutiérrez-Marco and Villas (Reference Gutiérrez-Marco and Villas2007) previously noted, and regardless of the chosen paleogeographic scenario, it is apparent that Ahtiella originated along the proto-Andean Gondwana margin. Several recent paleontological discoveries provided evidence supporting that both the Central Andean Basin and the arc-related Puna-Famatina Basin operated simultaneously as centers of evolutionary radiation (‘centers of origin’) and species pump regions (sensu Harper et al., Reference Harper, Rasmussen, Liljeroth, Blodgett, Candela, Jin, Percival, Rong, Villas and Zhan2013) from which new taxa spread to neighboring areas (Benedetto and Sánchez, Reference Benedetto and Sánchez2003; Muñoz and Benedetto, Reference Muñoz and Benedetto2016; Benedetto and Muñoz, Reference Benedetto and Muñoz2017). Such temperate Gondwana basins acted as sites of origination, as did the equatorial shallow-water shelves of Gondwana and peri-Gondwanan terranes, which have been identified by Bassett et al. (Reference Bassett, Popov and Holmer2002) as the main source of the precursors to the Ordovician radiation. For instance, the earliest known punctate orthide Lipanorthis Benedetto in Benedetto and Carrasco, Reference Benedetto and Carrasco2002 from the upper Tremadocian of northwestern Argentina was not an immigrant from the tropical belt, as Harper et al. (Reference Harper, Cocks, Popov, Sheehan, Bassett, Copper, Holmer, Jin and Rong2004) suggested, but probably originated from a Protorthisina-like plectorthoid ancestor inhabiting the Central Andean Basin in the latest Cambrian (Benedetto, Reference Benedetto2013). Furthermore, based on cladistic analysis, Benedetto and Muñoz (Reference Benedetto and Muñoz2017) showed that plectorthoids not only underwent an important diversification in the Central Andean Basin during the Tremadocian and Floian but also could have been a source for the heterorthids, which through the Ordovician spread along the western Gondwanan shelves (Peru, northern Africa) and peri-Gondwanan terranes (Avalonia, Armorica).
The Puna-Famatina volcanic arc (Fig. 5) was another significant center of origin during the Early to Middle Ordovician. As it has been already noted, its shelly faunas exhibit a high level of endemicity, in particular bivalves (Sánchez and Babin, Reference Sánchez and Babin1993; Sánchez, Reference Sánchez1997) and brachiopods (Benedetto, Reference Benedetto2003b; Benedetto and Sánchez, Reference Benedetto and Sánchez2003). Volcanic islands and archipelagos have long been recognized as important evolutionary centers of modern biota (e.g., MacArthur and Wilson, Reference MacArthur and Wilson1967), but their role in promoting faunal diversification in the past was not fully acknowledged until Neuman (Reference Neuman1984) proposed that the distinctive Celtic faunas from the Ordovician volcaniclastic rocks of the Caledonian-Appalachian folded belt inhabited intra-Iapetus volcanic islands. Also relevant was the subsequent study by Webby (Reference Webby1992) on the low-latitude Ordovician faunas from the volcaniclastic rocks of New South Wales. Harper et al. (Reference Harper, Owen and Bruton2009) emphasized the role of such volcanic chains as cradles and centers of origin contributing to the increase of γ-diversity during the Great Ordovician Biodiversification Event.
Current ideas about the origin of Plectambonitoidea
The general statement that the order Strophomenida evolved from the early to middle Cambrian Nisusiidae of the class Kutorginata (Williams and Hurst, Reference Williams and Hurst1977) or, alternatively, from an ancestor similar to Billingsella Hall and Clarke, Reference Hall and Clarke1892 at the Cambrian-Ordovician transition, has been based essentially on the presence in all these groups of an apically perforated pseudodeltidium (Cocks and Rong, Reference Cocks and Rong1989; Williams et al., Reference Williams, Carlson, Brunton, Holmer and Popov1996). However, no further compelling evidence has been presented to support such ancestor-descendant relationships for all members of the order. According to Bassett et al. (Reference Bassett, Popov and Holmer2001), bilingsellides and kutorginates share the well-developed perforate pseudodeltidium and the lack of dental plates, but differ in that sockets and socket plates have a different origin in billingsellides and strophomenides, concluding that their phylogenetic links still remain unclear. Subsequently, Bassett et al. (Reference Bassett, Popov and Egerquist2008) and Bassett and Popov (Reference Bassett and Popov2017), based on a study of the ontogeny of the orthotetide Coolinia Bancroft, Reference Bancroft1949, inferred an early divergence of strophomenate and rhynchonellate brachiopods.
At the superfamily level, it has been assumed that Strophomenoidea was derived from the Plectambonitoidea during the Early Ordovician. Spjeldnaes (Reference Spjeldnaes1957) did not identify the group of plectambonitoideans that gave rise to the strophomenoideans and left open the possibility that the latter group is polyphyletic. According to Cocks and Rong (Reference Cocks and Rong1989, Reference Cocks and Rong2000), strophomenoideans originated from plectambonitoideans by a transformation of the cardinal process from simple to bifid, suggesting as potential ancestor a leptellinid like Apatomorpha Cooper, Reference Cooper1956 or Toquimia Ulrich and Cooper, Reference Ulrich and Cooper1936. Recent discoveries demonstrated that the three basic types of strophomenoid cardinalia were already differentiated in the oldest known members of the clade recorded in the Dapingian of southern China (Zhan et al., Reference Zhan, Jin, Rong and Liang2015), supporting that the Strophomenoidea originated in the Floian from an unknown ‘strophomenide stem group’ shortly after the first appearance of plectambonitoids. Dewing (Reference Dewing2004) challenged the hypothesis of the plectambonitoid derivation of strophomenoids based on their different shell structure (laminar in the former and fibrous in the latter). Unlike the a priori assumption that shell structure is homoplastic (Cocks and Rong, Reference Cocks and Rong2000), i.e., evolved independently in different clades, Dewing (Reference Dewing2004, fig. 3) proposed a phylogenetic scenario in which the Strophomenoidea arose from a Cambrian laminar-shelled billingselloid, whereas the common ancestor of both the fibrous-shelled Plectambonitoidea and Clitambonitoidea was left with interrogation. On the contrary, the parsimony analysis performed by Congreve et al. (Reference Congreve, Krug and Patzkowsky2015) indicated that plectambonitoideans and strophomenoideans are phylogenetically related but, in contrast to previous inferences, plectambonitoideans do not constitute a monophyletic group but a paraphyletic grade of the strophomenoidean clade. Significantly, in the phylogeny presented by Congreve et al. (Reference Congreve, Krug and Patzkowsky2015, fig. 4), Taffia Butts, Reference Butts1926, Railtonella Laurie, Reference Laurie1991, and Ahtiella, all currently included in the family Taffiidae, cluster as basal forms to all other Strophomenida, as Spjeldnaes (Reference Spjeldnaes1957) intuitively depicted in his phylogenetic tree sixty years earlier.
The absence or extreme paucity of undisputed plectambonitoids in the Tremadocian, along with their sudden diversification around the Floian-Dapingian transition, suggests that this superfamily originated in the Early Ordovician rather than deep in the Cambrian. In my opinion, billingselloids are too derived morphologically to be considered direct ancestors of plectambonitoids (excepting the family Plectambonitidae, as discussed below). Since their first appearance in the middle Cambrian, billingsellides developed a proportionally high planar ventral interarea leading in the late Tremadocian to the hemipyramidal shells that characterize most polytoechioids, e.g., Protambonites Havlíček in Havlíček and Josopait, Reference Havliček and Josopait1972 and Tritoechia Ulrich and Cooper, Reference Ulrich and Cooper1936, which form a consistent monophyletic clade (Benedetto, Reference Benedetto2009; Topper et al., Reference Topper, Harper and Brock2013). The apically perforated pseudodeltidium—the main feature linking billingsdelloids and plectambonitoids—could be a plesiomorphic condition of basal rhynchonelliforms already present in some of the earliest members of the clade (e.g., Nisusioidea) or, alternatively, could be an homoplastic feature that appeared and became lost at different times in different clades. In fact, in certain basal plectambonitoids, e.g., Plectella Lamansky, Reference Lamansky1905, Ingria Öpik, Reference Öpik1930, Aporthophyla Ulrich and Cooper, Reference Ulrich and Cooper1936, Tourmakeadia Williams and Curry, Reference Williams and Curry1985, and Pelonomia Cooper, Reference Cooper1956, the pseudodeltidium is rudimentary or lacking. In any cases, this structure is not as phylogenetically informative as previously supposed.
Noteworthily, the widely splayed, rodlike socket ridges running almost parallel to the hinge line of billingselloids are closely comparable to those of the family Plectambonitidae (e.g., Plectella, Plectambonites Pander, Reference Pander1830, and Ingria). Such an arrangement is quite different from the typically orthoid cardinalia seen in taffiids. Therefore, it is not surprising that in the parsimony analysis carried out by Congreve et al. (Reference Congreve, Krug and Patzkowsky2015), Plectambonites appears as monophyletic only if it is excluded from all other ‘plectambonitoids’ and placed in a separate superfamily. In this context, it is worth noting the close resemblance between the billingsellide (?) Kozhuchinella Severgina, Reference Severgina1967 and the oldest known probable plectambonitoid Akelina Severgina, Reference Severgina1967, both from the upper Tremadocian Algan Formation of Kuznetz-Altai, Altai Mountains, Siberia (Severgina, Reference Severgina1967). Despite the poor preservation of the latter (reillustrated by Cocks and Rong, Reference Cocks and Rong1989, figs. 13–17), both genera share a concavoconvex profile; parvicostellate ornamentation (typical billingsellides are multicostellate or ramicostellate); the absence of dental plates; a prominent dorsal median ridge; a simple knob-like cardinal process; long, widely divergent socket ridges; and a well-developed dorsal subperipheral rim. Accordingly, Akelina and Kozhuchinella are likely related forms, which could be considered either as early members of the ‘plectambonitoid’ clade or, alternatively, as derived billingselloids (the presence of pseudopunctae has not yet been demonstrated in these genera). This raises the possibility that only the plectambonitoid clade sensu stricto (the subfamily Plectambonitinae in the current classification) evolved from a billingselloid ancestor, and that ahtiellins (and probably other taffids) had a different ancestor, which should be sought among the Orthoidea, as discussed below.
Searching for the Ahtiella ancestors
In his outstanding morphological study of Middle Ordovician strophomenides from Norway, Spjeldnaes (Reference Spjeldnaes1957, fig. 42) presented a diagrammatic evolutionary tree of Strophomenida starting with two main branches, one of them lacking descendants including Plectambonites and allied forms, and the other including the ‘ahtiellinids’ (Ahtiella, Inversella Öpik, Reference Öpik1933, and Ukoa Öpik, Reference Öpik1932), which albeit with a question mark, were placed at the origin of the strophomenoid stock. A third, short-lived basal branch was represented by Taffia. In their comprehensive revision of plectambonitoid classification, Cocks and Rong (Reference Cocks and Rong1989, p. 83, fig. 5) also placed the Taffiidae at the base of the plectambonitoid tree “because we regard the absence of side septa as representing a more primitive state than their presence (as in the Plectambonitidae),” and also that the “oldest plectambonitacean[s] ... are essentially indistinguishable from their orthide (probably billingselloid) ancestors except by their pseudopunctate shell.” In fact, basal plectambonitoids share with orthoids a simple, not undercut cardinal process, the absence of side septa, and the absence of a bema, a structure interpreted as a lophophore support and/or for providing muscle attachment. A more elaborated trifid, often undercut cardinal process, a variably elevated and bilobed bema, paired dorsal valve septa, hinge-line denticles, and more or less prominent internal papillae and/or septules are all features that gradually appear in different combinations in younger, more derived plectambonitoids.
Among taffiids, the ahtiellins—exemplified by the type genus Ahtiella—display such a combination of internal features that, in absence of pseudopunctae, they are virtually indistinguishable from certain basal orthoids (e.g., hesperonomiids). For instance, the inclusion among the ahtiellins of Schedophyla potteri, whose shell structure remains unknown, was cast in doubt by Cocks and Rong (Reference Cocks and Rong1989, p. 97) who stated that “it is possible that the genus is an orthid.” Laurie (Reference Laurie1991) also noted the orthoid dorsal cardinalia and musculature of Schedophyla. Recently Harper et al. (Reference Harper, Popov and Holmer2017, p. 624) noted that “…questions remain regarding the placement of a number of groups such as the toquimiids, that apparently possess orthoid characters.” Perhaps the best example of such a difficulty is the conflicting taxonomic position of ‘Monorthis’ coloradoensis, from the Central Andean Basin, which is hardly differentiable from unquestionable species of Ahtiella such as A. zarelae Villas in Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007 and A. famatiniana n. sp. (described herein). Originally, the species ‘coloradoensis’ was ascribed to the hesperonomiid Monorthis, on the basis of its convexoplanar, slightly resupinate profile, carinate ventral fold, multicostellate ornamentation, and orthoid cardinalia and muscle scars (Benedetto, Reference Benedetto1998b). Such an assignment was further supported by the lack of evidence of pseudopunctae and pseudodeltidium (Benedetto, Reference Benedetto2003b), both considered apomorphic features of plectambonitoids. The shell structure, however, could not be verified because available shells are entirely decalcified, so that its nonpunctate condition was inferred from the lack of evidence of pseudopunctae on internal molds, even though many undisputable strophomenoids do not show internal traces of them.
Comparative morphology of Monorthis and Ahtiella
The orthoid ancestor hypothesis of Ahtiella finds empirical support from the comparison of the species Monorthis transversa (Fig. 6.1–6.12) and Ahtiella famatiniana n. sp. (Fig. 10.9–10.24), which occur in successive strata of the Famatina Basin.

Figure 6 (1−9) Monorthis transversa Benedetto, Reference Benedetto2003b; Loma del Kilometro Member of the Suri Formation (Chaschuil) and Molles Formation, Famatina Range: (1) latex cast of ventral valve exterior, CEGH-UNC 19628a; (2) latex cast of dorsal valve exterior, CEGH-UNC 19628b; (3) latex cast of ventral valve, CEGH-UNC 19628a, showing incipient delthyrium cover; (4, 5) internal mold (4) and latex cast (5) of dorsal valve, CEGH-UNC 19635; (6) internal mold of dorsal valve, CEGH-UNC 19632; (7) internal mold of ventral valve, CEGH-UNC 19625; (8, 9) internal mold of ventral valve (8) and latex cast (9), CEGH-UNC 10962. (10−18) Monorthis cumillangoensis Benedetto, Reference Benedetto2001; San Juan Formation, silicified specimens from Cerro Cumillango and Cerro La Chilca, Precordillera: (10) ventral valve exterior, CEGH-UNC 17915; (11) ventral valve exterior, CEGH-UNC 17917; (12) dorsal valve interior, CEGH-UNC 17920; (13) ventral valve interior, CEGH-UNC 17933; (14, 15) ventral valve interior (14) and detail of incipient delthyrium cover (15), CEGH-UNC 17948; (16) ventral valve interior, CEGH-UNC 21152; (17) dorsal valve exterior, CEGH-UNC 21153; (18) dorsal valve interior, CEGH-UNC 17942. All specimens dusted with ammonium chloride. Scale bars=5 mm.
Shell shape and ornament
Overall, the slightly resupinate, carinate, transversely elongate, alate shells of Monorthis transversa and Ahtiella famatiniana n. sp. are closely comparable. Shells of M. transversa are rather smaller and more transverse than A. famatiniana, resembling the juvenile to medium-sized specimens of the latter. Although ornamentation in M. transversa is slightly coarser than in A. famatiniana n. sp., it is subequally multicostellate in both species.
Pseudodeltidium
Specimens of Monorthis transversa from Chaschuil—originally referred to as Monorthis aff. M. menapiae (Davidson, Reference Davidson1868) by Benedetto, Reference Benedetto1994—and from Central Famatina (Benedetto, Reference Benedetto2003b) were described as having a widely open delthyrium. However, a slightly arched pseudodeltidium can be observed in a few conjoined shells (Fig. 6.1). Its absence in most specimens is likely due to postmortem breakage by hydrodynamic action, but could also reflect ecophenotypical variation within a population, ranging from absent to nearly complete according to specific (but unknown) environmental constraints. A careful revision of the Precordilleran specimens of M. cumillangoensis Benedetto, Reference Benedetto2001, perhaps the better-known species of Monorthis worldwide (Benedetto, Reference Benedetto2001), revealed the presence of an apparently imperforated pseudodeltidium covering the apical region of the delthyrium (Fig. 6.15). A closely comparable structure is also present in Ahtiella coloradoensis (Fig. 11.9). Ahtiella zarelae possesses a small apical pseudodeltidium that is hardly visible in the illustrated specimens (Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007, fig. 4E, F), but in Ahtiella sp. from slightly older strata of the same formation of Peru, it is better developed, almost attaining the delthyrium midlength. Ahtiella famatiniana n. sp. always possesses a well-developed pseudodeltidium covering one-half to two-thirds of the delthyrium (Fig. 10.9, 10.14). In the younger species A. argentina (Fig. 9.3) and A. tunaensis n. sp. (Fig. 10.5), the pseudodeltidium covers the pedicle opening almost entirely at all growth stages, suggesting a general trend from nearly absent to fully developed.
Muscle scars
The ventral muscle field of Monorthis transversa is subtriangular and confined to the delthyrial cavity (Fig. 6.7) as in younger individuals of Ahtiella famatiniana n. sp. (Fig. 10.15). In mature specimens of the latter species (Fig. 10.16, 10.18), as well as in A. coloradoensis (Fig. 11.3, 11.8) and the Peruvian species A. zarelae (Fig. 11.18), the ventral muscle field becomes larger and more or less subpentagonal in outline. The Precordilleran A. tunaensis n. sp. is unique in having a large bilobed muscle field (Fig. 10.4). The dorsal muscle field of M. transversa is quadripartite, with anterior and posterior scars nearly equal in size. A persistent feature in most specimens of M. transversa is the presence of a pair of slightly divergent ridges bounding laterally or bisecting longitudinally the adductor field (Fig. 6.4, 6.5). Remarkably, these ridges are also present in A. famatiniana n. sp., A. coloradoensis, and A. zarelae (Figs. 10.23, 11.14). In the Welsh species A. concava, such ridges extend along the entire length of muscle scars (Bates, Reference Bates1968, pl. 7, figs. 16, 19).
Cardinalia
There are only minor differences between the cardinalia of Monorthis (Fig. 6.5, 6.12) and Ahtiella (Figs. 9.17, 10.20, 10.24, 11.10, 11.15). Monorthis transversa shows some degree of intraspecific variation in the cardinal process, ranging from bladelike to a ridge moderately enlarged anteriorly, occupying the entire length of a raised subtriangular notothyrial platform. The cardinalia of M. transversa are nearly identical to those of A. famatiniana n. sp. and A. coloradoensis, whereas in the Peruvian A. zarelae and Ahtiella sp., the cardinal process tends to be more robust and ovoid in outline. Ahtiella argentina is characterized by a bladelike to slightly enlarged cardinal process erected on a gently convex notothyrial platform (Fig. 9.20, 9.22). The subtriangular to suboval, anterolaterally open sockets excavated on the valve floor and partially under the dorsal interarea of Monorthis and Ahtiella, as well as the slender, distally enlarged socket ridges, are also closely comparable.
Subperipheral rims and platforms
Despite the redefinition of these terms by Cocks and Rong (Reference Cocks and Rong1989), some imprecision persists in the literature, with the term ‘platform’ having been applied to both dorsal and ventral valves (not only to the dorsal one, as these authors proposed), and the two structures are often not easy to differentiate on the basis of their morphology. To avoid confusion, ‘platform’ (= ‘diaphragm’) is used here to designate a low to high, somewhat undercut elevation of the ventral and/or dorsal valve floor originating at or near the cardinal angles and not related to external geniculation, the internal disc, or any kind of valve thickening. On the other hand, following Cocks and Rong (Reference Cocks and Rong1989), the term ‘peripheral rim’ (or ‘subperipheral rim’) is applied to a raised rim running at or near the variably thickened valve margin. When a more or less prominent peripheral rim has developed in the ventral valve, it can be mirrored in the dorsal valve by a similar structure that is often related to an internal deflection of the valve.
According to the original diagnosis of Monorthis (see Bates, Reference Bates1968), platforms are absent in both valves. Valve margins of the Famatinan M. transversa are crenulated but not thickened (Fig. 6.9), whereas the large ventral valves of the Precordilleran M. cumillangoensis show variably thickened margins and an internal geniculation, which is nearly identical to that seen in Ahtiella zarelae (cf. Figs. 6.14, 11.18). The ventral valves of A. famatiniana n. sp. and A. coloradoensis exhibit a conspicuous thickening along the geniculation; in both species, however, it is absent in juvenile individuals (Fig. 10.15), suggesting that this structure developed progressively by peramorphy. In A. argentina, the whole ventral valve margin is geniculated, forming a prominent internal disc deeply incised by the vascula terminalia (Fig. 9.9), like in the Baltic A. lirata. A corresponding discontinuous platform-like structure is usually present in the dorsal valve of A. famatiniana n. sp. (Fig. 10.20). On the contrary, in A. coloradoensis, it is poorly developed or even absent (Fig. 11.10). Unlike other Gondwanan species, the large dorsal valves of A. argentina display a series of curved, roughly radial ridges that can be interpreted as a platform-like structure (Fig. 9.20, 9.22). Ahtiella baltica Öpik, Reference Öpik1932, as can be seen in the specimen figured by Öpik (Reference Öpik1933, pl. 4, fig. 6), possesses a continuous undercut platform, but it is faint or absent in A. lirata.
Vascular system
Vascular trunks are not discernible in Monorthis cumillangoensis in part due to the strong internal impression of the external ornamentation, whereas in M. transversa, a series of short anastomosing canals can be present along valve margins (Fig. 6.4, 6.8). In Ahtiella famatiniana n. sp., the distal portion of mantle canals is well marked on the anterior third of the ventral valve (Fig. 10.22). Adult stages of A. argentina and A. paucirugosa always display a deeply impressed mantle canal system of saccate type, with posteriorly directed branches of vascula media enclosing large gonadal pouches (Fig. 9.10, 9.18).
Trends of morphological change
From the above comparisons, the following trends can be recognized through the inferred Monorthis transversa (and its putative ancestor Hesperonomiella arcuata Benedetto, Reference Benedetto2003b)–Ahtiella argentina lineage (Fig. 7): (1) the nongeniculated ventral valve margin of M. transversa progressively thickens, originating in Ahtiella an internal geniculation, which is low in the species from Wales (A. quadrata, A. concava) and northwestern Argentina (A. coloradoensis), intermediate in the Famatinan (A. famatiniana n. sp.) and Peruvian (A. zarelae) forms, and more prominent in the Darriwilian species from Cuyania and Baltica. In the latter (e.g., A. lirata, A. jaanussoni Hessland, Reference Hessland1949), the main trend is toward a strongly convex gibbous dorsal valve; (2) the pseudodeltidium is absent or incipient in Monorthis, is restricted to the apical region of delthyrium in the oldest known species (A. zarelae) as well as in the Welsh species, and reaches almost two-thirds in the later species from Precordillera and Baltica; (3) external ornamentation evolved from equally multicostellate in Monorthis (retained in the younger A. coloradoensis) to ramicostellate in the Floian A. zarelae, becoming unequally multicostellate to incipiently parvicostellate in the Dapingian A. famatiniana, and definitely parvicostellate in the Darriwilian species; (4) the dorsal platform is absent in Monorthis, is variably developed in the early species A. zaleae and A. famatiniana n. sp., and becomes more prominent in the younger Cuyanian and Baltic species; (5) the mantle canal system is indistinct or confined to the valve margin in Monorthis, has well-impressed distal branches on the margin of disc and trail in the Floian-Dapingian species of Ahtiella, and culminates in A. argentina and A. paucirugosa with a deeply impressed mantle canal system on the entire surface of adult specimens.

Figure 7 Generalized trend of morphological change through selected taxa from the Floian-Dapingian volcanosedimentary succession of Famatina. Ventral internal molds at left, dorsal internal molds at right. Hesperonomiella arcuata Benedetto, Reference Benedetto2003b): left, CEGH-UNC 15740; right, CEGH-UNC 19078. Monorthis transversa Benedetto, Reference Benedetto2003b: left, CEGH-UNC 19095; right, CEGH-UNC 19623. Ahtiella famatiniana n. sp.: left, CEGH-UNC 27140; right, CEGH-UNC 27135b. At the top of the succession, A. argentina Benedetto and Herrera, Reference Benedetto and Herrera1986 illustrates a more derived species of Ahtiella from the Darriwilian of Precordillera (left, CEGH-UNC 27111; right, CEGH-UNC 21118b).
Phylogenetic analysis
Cladistic analysis of Ahtiella species
Comparative morphology makes evident that differences between Monorthis and basal species of Ahtiella are subtle, which makes them difficult to distinguish from each other. The question is whether such similarities reflect homologies and therefore reveal phylogenetic affinities or, on the contrary, they can be viewed as cases of extreme morphological convergence along two independent lineages. Given the striking resemblance in multiple external and internal details along with gradation in some features, convergence seems highly improbable. To investigate whether the trends deduced from comparative morphology are phylogenetically significant, a cladistic analysis of Ahtiella species was performed using TNT (Tree Analysis Using New Technology) version 1.5 (Goloboff and Catalano, Reference Goloboff and Catalano2016), selecting the heuristic search option with multiple random addition sequences and the tree bisection reconnection (TBR) branch-swapping algorithm holding 10 trees in each addition sequence. A total of 23 characters comprising internal and external features were included within the Ahtiella analysis (Table 1). The 23-character matrix was analyzed for 10 taxa (Table 2). The Welsh species A. concava was not considered because it possesses some features atypical for the genus (e.g., apparently smooth exterior, elongate oval anterior adductor scars each flanked by prominent septa), resulting in destabilization of the relationships among other taxa. Hesperonomiella was chosen as the outgroup for rooting the phylogenetic tree because it exhibits an ensemble of nonderived internal features, and lacks the majority of apomorphies present in the group analyzed here. This, along with its first appearance in the middle Cambrian, suggests it as a potential ancestor of the taxa considered in this study. A heuristic search of the data matrix in which all characters were unordered and equally weighted produced four minimal length trees 52 steps long, with consistency index of 0.692 and retention index of 0.754. The strict consensus tree (Fig. 8) is presented here with branch length calibrated to the age of the first appearance datum of each taxon. As the phylogenetic tree shows, the basal member of the Ahtiella clade is Monorthis transversa, which possesses ancestral features such as multicostellate ornamentation, absence of a dorsal platform, an unthickened periphery of the ventral valve, an open delthyrium, and an unmodified notothyrium. Monorthis transversa has in common with the Ahtiella species a resupinate and alate shell (node 1), and cardinalia and muscle scars of the orthoid type. The Welsh species A. quadrata is expressed as the most basal member of the Ahtiella clade displaying vestigial or incipient pseudodeltidium and chilidium and a slightly thickened ventral valve margin (node 2). The apomorphy that defines the remaining Ahtiella species (node 3) is the presence of a dorsal platform, which varies from low and discontinuous in the older species to variably developed in the younger species. The Gondwanan species A. coloradoensis, A. zarelae, and A. famatiniana n. sp., which each retain ancestral uniform or nearly uniform radial ornamentation and an incipient apical pseudodeltidium, appear as basal to the more derived species from Baltica, Cuyania, and Newfoundland. In addition, it should be noted that the presence of pseudopunctae has not been demonstrated in any of these Gondwanan species. Among them, A. famatiniana n. sp. is slightly more advanced by having a comparatively larger pseudodeltidium and chilidium, a more prominent dorsal platform, a deeply impressed vascular system on the disc/trail deflection, and ornamentation tending to be incipiently parvicostellate. The Famatinan species clusters with the group that includes A. tunaensis n. sp., A. lirata, A. jaanussoni, A. argentina, and A. paucirugosa, characterized by a well-defined disc and a dorsally directed trail in the interior of ventral valve (node 4). This group shares unequally parvicostellate ribbing, a well-developed pseudodeltidium and chilidium, and relatively large pseudopunctae (node 5). Ahtiella jaanussoni and A. lirata form a cluster based on a gibbous shell profile and prominent rugae or corrugations covering most of the valve surface, and likely reflect local radiation of the genus in the Baltica paleocontinent (node 6). This group of species served originally to define the genus Ahtiella. On the other hand, A. argentina and A. paucirugosa form a sister group of Baltic species by sharing deeply impressed mantle canals on the entire valve interior and curved ridges in the floor of dorsal valve (node 7).

Figure 8 Phylogenetic relationships of taxa analyzed calibrated to chronostratigraphic scale. Apomorphies defining numbered nodes are discussed in the text.
Table 1 Characters utilized in phylogenetic analysis.

Table 2 Character state distribution for taxa included in phylogenetic analysis.

Our species-level phylogenetic tree shows that since its origin in the Floian, the genus Ahtiella underwent successive speciation events along the Andean margin of Gondwana where the common ancestor would likely have inhabited, and subsequently dispersed and continued speciating as new geographic areas were colonized. The diversification of the Ahtiella clade appears to have occurred by cladogenesis because A. coloradoensis, a conservative species closely related morphologically to Monorthis, persisted in the Central Andean Basin until the late Darriwilian. Perhaps the main conflict posed by the phylogenetic hypothesis of Figure 7 is that A. quadrata is shown as a basal member of the clade because the Welsh species retains some traits ancestral to A. zarelae and A. famatiniana n. sp. A possible explanation is that A. quadrata originated from a Gondwanan ancestor and then migrated along Gondwanan shelves to reach the colder Avalonian waters. However, to date no records of morphologically related forms are known from Gondwana, which could be due either to a lack of extensive sampling in the still poorly known Bolivian and Peruvian sectors of the Central Andean Basin or to the absence of this species in the Andean region. An alternative interpretation is that A. quadrata evolved independently in the Avalonian paleocontinent from a local species of Monorthis. The possibility of parallel evolution at the generic level as well as its possible causes were discussed in a previous paper (Benedetto, Reference Benedetto2008) to account for the nearly simultaneous record of the genus Productorthis in Baltica and the Famatinan volcanic arc, with underlying strata in both regions of the ancestral genus Panderina Schuchert and Cooper, Reference Schuchert and Cooper1931. In our case, the Welsh species M. menapiae of ‘lower Arenigian’ (Floian) age (Bates, Reference Bates1969) closely resembles the Famatinan species M. transversa and could be a potential ancestor of A. quadrata and, eventually, of the ‘anomalous’ species A. concava. It seems likely that cases of parallel evolution in brachiopods, and thus the existence of paraphyletic genera, might be more frequent than previously thought.
In summary, available evidence from the Gondwanan material supports, contrary to previous assumptions, that the basal plectambonitoid Ahtiella could have evolved from the hesperonomiid orthoid Monorthis transversa, and that A. famatiniana n. sp. and A. zarelae are not only the earliest species of the genus, but also are morphologically intermediate between M. transversa and the more derived species of Ahtiella from the Darriwilian of Cuyania and Baltica (Fig. 8).
Some macroevolutionary implications
There is a general consensus that the peculiar strophomenide shell architecture and concomitant anatomical and physiological changes were adaptations (key innovations) allowing invasion of a new ecological niche or adaptive zone, which was essentially the acquisition of an ambitopic or permanent liberosessile life strategy linked to the colonization of low-energy, offshore marine environments (Bassett, Reference Bassett1984). Over time, such morphological changes became so marked that they led to the recognition of a separate higher taxon, the order Strophomenida, which together with other groups was lumped into the large and quite heterogeneous class Strophomenata. Although higher taxa are often viewed as artificial, nonmonophyletic, subjective entities, a number of quantitative studies have confirmed the taxic metrics as an adequate proxy for assessing morphological disparity (Erwin, Reference Erwin2007, p. 59). If, as evidence presented here suggests, Ahtiella originated from Monorthis through a series of minor transformations, then the impressive morphological gap among ‘typical’ orthides and strophomenides was bridged through a brief cladogenetic event. At first, such a transition indicates that there is not a definite discontinuity between species-level evolution (processes that occur within a species or lead to a new species) and the origin of higher taxa (macroevolution). Central to this statement is the assumption that higher taxa are evolutionary entities characterized by a significant morphological disparity achieved over a long period of time, then the greater the time elapsed since their origin from a common ancestor, the larger morphological disparity. In the present case study, it would be expected that the highly plesiomorphic basal forms (i.e., ahtiellins) of a given higher taxon (i.e., strophomenides) are more similar to their putative ancestors (i.e., hesperonomiids) than they are to the more derived (apomorphic) end-members of the same clade (i.e., sowerbyellids, aegiromenids). In other words, morphological discontinuity becomes minimal at a point closer to the initial divergence of two phylogenetically related higher taxa. A consequence of this is that assignation of basal forms to one or another higher taxon can be difficult in the lack of a well-supported phylogeny. The need of a ‘shoehorn’ to classify such earliest members of a given higher taxon into a specified order or suborder has also been noted in other groups of marine benthic organisms. For instance, the clade Bivalvia includes a number of early representatives of Tremadocian-Floian age that lack certain apomorphies defining more derived crown groups. In this respect, it has been suggested that such basal taxa can be classified as plesions, i.e., paraphyletic groups having a number of symplesiomorphic traits but morphologically close to a given higher taxon (Fang and Sánchez, Reference Fang and Sánchez2012). In the case of brachiopods, as Carlson (Reference Carlson2016, p. 421) stated, numerous higher taxa had been thought to represent grade-level taxa, i.e., not clades, and our evidence indicates that this could be the case of strophomenides.
Materials and methods
Repository and institutional abbreviations
CEGH-UNC, Centro de Investigaciones en Ciencias de la Tierra CONICET and Universidad Nacional de Córdoba, Argentina; CORD-PZ, Museo de Paleontologia, Universidad Nacional de Córdoba, Argentina; MGM, Museo Geominero, Madrid, Spain.
Systematic paleontology
The systematic classification follows that of the Treatise on Invertebrate Paleontology (Cocks and Rong, Reference Cocks and Rong2000). Following Congreve et al. (Reference Congreve, Krug and Patzkowsky2015), the genus Ahtiella is referred to the ‘Plectambonitoidea’ with the quotation marks denoting that the superfamily is paraphyletic (Wiley, Reference Wiley1979).
Order Strophomenida Öpik, Reference Öpik1934
Superfamily ‘Plectambonitoidea’ Jones, Reference Jones1928
Family Taffiidae Schuchert and Cooper, Reference Schuchert and Cooper1931
Subfamily Ahtiellinae Öpik, Reference Öpik1933
Genus Ahtiella Öpik, Reference Öpik1932
Type species
Ahtiella lirata Öpik, Reference Öpik1932 from the Darriwilian (Middle Ordovician) of Tsitri, Estonia, by original designation.
Diagnosis (emended)
Shell resupinate, variably geniculate; ornamentation subequally multicostellate, ramicostellate or parvicostellate, often with posterolateral rugae. Dental plates widely spaced converging to valve floor, enclosing posteriorly subtriangular to subquadrate ventral muscle field. Interior of ventral valve with thickened margin defining disc and trail. Cardinal process simple. Dorsal median ridge broad, rounded. Dorsal platform variably developed. Saccate mantle canal system usually strongly impressed either along shell margin or whole interior.
Ahtiella argentina Benedetto and Herrera, Reference Benedetto and Herrera1986

Figure 9 Ahtiella argentina Benedetto and Herrera, Reference Benedetto and Herrera1986; Upper San Juan Formation, Cerro Viejo, Precordillera: (1) latex cast of ventral valve exterior, CEGH-UNC 27121; (2, 7) latex cast of dorsal valve exterior (2) and profile view (7), CEGH-UNC 27119; (3) posterior view of conjoined specimen showing presudodeltidium and chilidium, CEGH-UNC 16328; (4) early juvenile ventral internal mold, CEGH-UNC 27118; (5) juvenile ventral internal mold, CEGH-UNC 27153; (6) internal mold of small adult ventral valve, CEGH-UNC 27072; (8, 9) internal mold (8) and latex cast (9) of ventral valve, CEGH-UNC 27111; (10) internal mold of ventral valve, CEGH-UNC 27181; (11, 12) external (11) and internal (12) views of silicified ventral valve, CEGH-UNC 21963; (13, 14) ventral (13) and dorsal (14) views of silicified conjoined specimen, CEGH-UNC 21964; (15) internal mold of juvenile dorsal valve, CEGH-UNC 27160; (16, 17) internal mold (16) and latex cast (17) of dorsal valve, CEGH-UNC 27108; (18) internal mold of ventral valve, CEGH-UNC 21995; (19, 20) internal mold (19) and latex cast (20) of dorsal valve, CORD-PZ 8295; (21, 22) internal mold (21) and latex cast (22) of dorsal valve, CEGH-UNC 27075; (23) dorsal internal mold, CEGH-UNC 21118b. All specimens dusted with ammonium chloride. Scale bars=3 mm (3, 4); 5 mm (remainder).
1986 Ahtiella argentina Reference Benedetto and HerreraBenedetto and Herrera, p. 114, pl. 1, figs. 4−21.
2002 Ahtiella argentina; Reference BenedettoBenedetto, p. 114, pl. 2, figs. 20, 21.
2003a Ahtiella argentina; Reference BenedettoBenedetto, p. 201, pl. 9, figs. 9–12.
2009 Ahtiella argentina; Reference Benedetto, Vaccari, Waisfeld, Sánchez and FogliaBenedetto et al., fig. 9f.
Holotype
CORD-PZ 8283, a conjoined specimen from the upper San Juan Formation, Quebrada Los Gatos, Cerro Viejo, San Juan Province, Argentina (Benedetto and Herrera, Reference Benedetto and Herrera1986).
Diagnosis
Shell transversely semielliptical (mean length/width ratio 0.39) with acute cardinal extremities; profile variably resupinate with carinate ventral median fold and corresponding subangular dorsal sulcus reaching anterior margin. Radial ornamentation unequally parvicostellate. Ventral muscle field short, triangular, extending up to 25% of valve length. Ventral valve strongly thickened marginally, defining internal disc and trail. Dorsal valve with strong rounded median ridge originating in front of notothyrial platform. Internal surface with elongated pustules coalescing to form irregular, roughly radial ridges. Both valves with deeply impressed saccate mantle canal system.
Occurrence
Uppermost levels of the San Juan Formation, Precordillera, San Juan Province, Argentina. Darriwilian (Dw1–Dw2).
Description
Shell to 23 mm width, transversely semielliptical in outline; mean length/width ratio 0.39 (N=18); profile strongly concavoconvex, mature specimens up to 45% as deep as long. Maximum width at hinge line. Cardinal extremities auriculate or alate in juvenile to medium-sized specimens, becoming acute (40–45°) in mature specimens. Ventral valve resupinate, gently convex at umbonal area, becoming broadly concave anteriorly; ventral median fold initially carinate, becoming broader and rounded toward anterior margin where it attains 15–20% of valve width, flanked by strongly concave areas. Ventral interarea planar, steeply apsacline to almost catacline, attaining height equivalent to 15% of shell length. Pseudodeltidium well developed, semiconical, covering near two-thirds of delthyrium. Dorsal valve moderately to strongly convex with deep angular median sulcus becoming wide and shallow anteriorly. Dorsal interarea anacline, planar, three to four times lower than ventral. Notothyrium covered almost entirely by large convex chilidium. Ornamentation unequally parvicostellate, with 6–8 first-order rounded costellae originating at umbonal area and 4–8 subequal finer parvicostellae intercalated between them, some of which can attain size equal to primaries on anterior third of valve; costellae 10–12 per 2 mm (counted at 5 mm growth stage). Fine, evenly spaced, concentric fila (~18 per mm). Posterolateral margins with up to six faint rugae strongly oblique to hinge line.
Ventral interior with small subtriangular teeth bearing well-developed, slit-like crural fossettes on medial faces, supported by short thick dental plates slightly converging to valve floor and diverging from each other at 80–90°. Lateral delthyrial cavities of adult specimens partially to completely filled with secondary deposits. Ventral muscle field short, triangular in outline, extending ~20–25% of valve length, enclosed posterolaterally by dental plates, delimited anterolaterally by low arcuate ridges; diductor scars elongate, almost three times longer than wide, not clearly differentiated from wider subtriangular adductor field. Mantle canal system saccate, deeply impressed in mature individuals; vascula media broad, straight, diverging forward at 60°, extending from anterior ends of diductors to approximate valve midlength where they bifurcate, with one branch directed posterolaterally and the other directed medially, each becoming repeatedly dichotomized at disc margin (Fig. 9.10, 9.18). Surface between vascular trunks covered by pustules. Gonadal areas subtriangular to suboval in outline, faintly striated radially, variably pitted. Valve margin strongly thickened, geniculated, with well-defined disc and trail deflected dorsally, forming angle of ~40° with respect to disc surface. Low rounded to subangular subperipheral rim occasionally present along entire disc margin. External ribbing not reflected on valve interior except in juvenile individuals.
Dorsal interior with small cardinalia extending ~10% of valve length. Cardinal process high, bladelike, slightly enlarged anteriorly, erected on moderately elevated slightly convex notothyrial platform merged anteriorly with strong, rounded median ridge, usually extending to valve midlegth or slightly beyond, but reaching disc margin in some specimens. Socket ridges rodlike, free distally, diverging from each other at 95–100°, bounding deep semiconical sockets excavated below and partially on interarea. Muscle field faintly impressed, quadripartite, with subcircular posterior adductor scars and larger suboval anterior adductor scars. Saccate dorsal mantle canal system with broad, well-impressed vascula media divided immediately in front of anterior extremity of muscle field in two branches, one of them running subparallel to median ridge, the other directed anterolaterally, both originating numerous small canals perpendicular to valve margin. Valve interior with elongated papillae, in large specimens coalescing to form irregular roughly radial ridges, which are more conspicuous at valve midlength on both sides and at end of median ridge. Small, closely spaced papillae between peripheral vascular trunks.
Materials
In addition to the material listed and illustrated by Benedetto and Herrera (Reference Benedetto and Herrera1986), the following new specimens have been included in the present study: CEGH-UNC 21960–21965, 21981, and 21994–21995 from Cerro Viejo (Quebrada Honda); CEGH-UNC 10561–10564, 16319–16321, 22070–22075, 27104–27130, and 27160–27162 from Cerro Viejo (Quebrada Los Gato).
Remarks
This species clearly differs from Ahtiella lirata in its more transverse shell outline and less convex, uniformly curved shell profile lacking abrupt geniculation. Moreover in A. lirata, the dorsal sulcus is very shallow and confined to the posterior region of the valve and the corresponding ventral fold is inconspicuous, whereas in A. argentina, the sulcus is deeper and always attains the anterior margin, and the ventral fold is well defined and carinate posteriorly. Internally, the Precordilleran species can be distinguished by having smaller subtriangular ventral muscle field, which in the type species tends to be subquadrate and extends to approximately one-third of the valve length; there are deeply impressed vascular trunks on the internal surface of both valves, but especially in the ventral one. The slightly older species A. baltica, from the Rögo Sandstone of Estonia, resembles A. argentina in its more transverse shell outline but differs in having a longer bilobed ventral muscle field of lirata-type. The dorsal interior, even though exhibiting features of the genus, shows a quite atypical continuous, anteriorly free dorsal platform (Öpik, Reference Öpik1933, pl. 4, fig. 6). Ahtiella arenaria Öpik, Reference Öpik1933 is a poorly known Estonian species (only a dorsal exterior and a ventral interior have been illustrated, by Öpik, Reference Öpik1933, pl. 4, figs. 7, 8) recovered from the same beds and localities as A. baltica to which it strongly resembles. Of the species from Sweden (Island of Öland) described by Hessland (Reference Hessland1949), only A. jaanussoni is known from dorsal interiors (ventral interiors are unknown). Its dorsal valve is reminiscent of A. lirata, but the shell is somewhat more transverse, the posterolateral rugae are more pronounced, and the dorsal interior possesses a long prominent median ridge. As Cocks and Rong (Reference Cocks and Rong1989) stated, the rest of the Ahtiella species described by Hessland (Reference Hessland1949) can only be questionably assigned to the genus because internal morphology remains unknown. Among them, A.? oelandica Hessland, Reference Hessland1949 is the most similar to A. argentina in its transverse shell outline, uniformly curved dorsal valve profile, and especially in the presence of a well-developed dorsal median sulcus reaching the anterior margin. The main external difference lies in the coarser and more prominent rugae in the Swedish species. It is unclear whether such differences in size, shell outline, definition of disc and trail, and strength of posterolateral rugae are taxonomically significant because these features show gradation between species and are quite variable even in individuals of the same species. Clearly, a revision of the Baltic species is needed to clarify this issue.
Ahtiella concava from the Bob Deiniol Formation of Anglesey (Wales) resembles A. argentina in its moderately convex and uniformly curved dorsal valve profile, acute cardinal angles, and well-developed, carinate ventral fold, but differs from the Precordilleran species in having a catacline to procline ventral interarea and a less transverse shell; the width/length ratio in the specimens measured by Bates (Reference Bates1968, p. 168) is 0.63. According to Bates (Reference Bates1968, p. 167), the ornamentation in the Welsh specimens is “too fine to be observed,” and posterolateral rugae are not evident. Internally, A. concava has a subquadrate rather than subtriangular ventral muscle field. The dorsal valve shares with A. argentina a high rounded median dorsal ridge extending beyond the valve midlength, but in the Welsh species, the muscle field is strongly elongated and bounded by a pair of conspicuous ridges, a feature atypical of the genus. Ahtiella quadrata from the Torllwyn Formation of Anglesey is represented by fragmentary material, but judging from the two illustrated complete ventral valves (Bates, Reference Bates1968, pl. 8, figs. 2, 3), the shell is slightly wider than long and coarsely costellate (~6 costellae per 2 mm) than A. argentina.
Ahtiella zarelae from the San José Formation of Peru can readily be distinguished from A. argentina by its less transverse, nonauriculate shell, and its ramicostellate radial ornamentation. Internally, the Peruvian species possesses a subpentagonal rather than triangular ventral muscle field, and a higher, septum-like dorsal median ridge. In addition, the inner surface of both valves is almost entirely covered by radial ribs lacking vascular impressions.
Most similar to the Precordilleran species is Ahtiella paucirugosa from the volcaniclastic Summerford Group of Newfoundland. Given that the North American specimens are tectonically distorted, shell outline is difficult to compare (A. paucirugosa seems to be approximately twice as wide as long). However, they have in common such external features as a well-defined dorsal sinus and ventral fold, and moderate shell convexity, although posterolateral rugae are less marked in the North American species. Internally, the dorsal valve resembles that of A. argentina in having a broad median ridge almost reaching the margin, rows of elongate pustules and septules forming radial crests, and deeply marked mantle canals of the saccate type. The main difference lies in the ventral muscle field of A. paucirugosa, which is bilobed with longer diductor scars extending approximately to the valve midlength.
Ahtiella tunaensis new species
urn:lsid:zoobank.org:act:C9CF5CB8-E169-4777-8C0B-ADEF34A52181

Figure 10 (1−8) Ahtiella tunaensis n. sp.; Las Chacritas Formation, Sierra de la Trampa, Precordillera: (1−3, 5) paratype, conjoined specimen, CEGH-UNC 21084, in ventral (1), dorsal (2), and profile (3) views, and detail of interarea (5) showing pseudodeltidium and chilidium; (4) holotype, interior of ventral valve, CEGH-UNC 21083; (6) paratype, ventral view of conjoined specimen, CEGH-UNC 27172; (7) detail of ventral valve, CEGH-UNC 27174, showing pseudopunctae; (8) paratype, dorsal internal mold, CEGH-UNC 27171. (9−24) Ahtiella famatiniana n. sp.; Punta Pétrea Member of the ‘Suri’ Formation (Chaschuil), and volcaniclastic beds exoposed at Las Pircas anticline (Central Famatina Range): (9) paratype, latex cast of ventral valve exterior, CEGH-UNC 27149; (10, 11) external mold (10) and latex cast (11) of ventral valve, CEGH-UNC 27131; (12−14) ventral (12) and dorsal (13) views of conjoined specimen, CEGH-UNC 27163, and detail of pseudodeltidium (14); (15) juvenile ventral internal mold, CEGH-UNC 27158; (16, 17) paratype, internal mold (16) and latex cast (17) of ventral valve, CEGH-UNC 27135a; (18, 21) holotype, internal mold (18) and latex cast (21) of ventral valve, CEGH-UNC 27137; (19, 20) internal mold (19) and latex cast (20) of dorsal valve, CEGH-UNC 27135b; (22) internal mold of ventral valve, CEGH-UNC 27140; (23, 24) internal mold (23) and latex cast (24) of dorsal valve, CEGH-UNC 27141, showing detail of cardinalium. All specimens dusted with ammonium chloride. Scale bars=1 mm (7); 3 mm (5, 14); 5 mm (remainder).
2003a Ahtiella n. sp.; Reference BenedettoBenedetto, p. 201, pl. 9, figs. 16–18.
Type specimens
Holotype, CEGH-UNC 21083, a ventral valve. Paratypes: CEGH-UNC 27171, an internal mold of a dorsal valve; CEGH-UNC 21084 and 27172, two conjoined specimens.
Diagnosis
Shell semielliptical to subrectangular, moderately to strongly convexoconcave (mean length/width ratio 0.63) with subrectangular cardinal extremities; ventral median fold low, almost indistinct near commissure; dorsal sulcus shallow posteriorly, impersistent at anterior margin. Radial ribbing subequally to unequally parvicostellate. Ventral muscle field bilobed with suboval diductor scars extending 40–45% of valve length. Valve margin strongly thickened with peripheral rim separating disc and trail. Dorsal median ridge initially low and highest at approximately two-thirds valve length.
Occurrence
Type specimens and other materials considered in this study come from the Las Chacritas Formation exposed at Quebrada La Tuna, Cordón de La Trampa, San Juan Province, Argentina; middle Darriwilian.
Description
Shell to 16.5 mm in width, semielliptical to subrectangular in outline, with mean length/width ratio 0.63 (N=15); lateral profile moderately convexoconcave, slightly resupinate, typically 38% as deep as long. Cardinal extremities subrectangular, slightly acute in juvenile specimens. Ventral valve broadly concave with low carinate median fold originating at umbonal area, becoming almost indistinct near commissure that is slightly sulcate or rectimarginate. Ventral interarea planar, catacline to gently procline, twice as high as dorsal. Arched imperforated pseudodeltidium covering one-half to two-thirds of delthyrium. Dorsal valve moderately to strongly convex, uniformly curved in lateral profile, with broad rounded median sulcus on its posterior third, becoming shallow to impersistent at anterior margin. Dorsal interarea planar, steeply anacline to orthocline. Notothyrium entirely covered by strongly convex chilidium. Ornamentation varying from subequally parvicostellate to unequally parvicostellate; accentuated costellae better defined on anterior half of largest individuals, with 6–8 finer parvicostellae between them; costellae 11 or 12 per 2 mm (counted at 5 mm growth stage). Fine, evenly spaced concentric fila present on entire shell surface. Posterolateral rugae poorly defined or absent.
Ventral interior with small, transversely triangular teeth supported by short strongly diverging dental plates partially masked by secondary deposits. Muscle field bilobed extending anteriorly near 40–45% of valve length; diductor scars suboval, bounded by ridges arising from anterior ends of dental plates; adductor field shorter than diductors, not raised, unbounded anteriorly. Valve margin strongly thickened with rounded subperipheral rim separating disc and trail. Vascula media straight, initially subparallel, subdivided toward valve margin. Gonadal areas poorly defined, striated. Areas between vascular trunks finely pustulose. External ribbing not reflected on valve interior.
Dorsal interior with high, bladelike cardinal process erected on moderately elevated subtriangular notothyrial platform. Socket ridges thickened distally, diverging anteriorly from each other at ~100°, bounding semiconical sockets. Median ridge initially low, becoming highest at approximately two-thirds valve length, fading near anterior margin. Muscle field indistinct in single available internal mold. Dorsal mantle canal system faintly impressed with straight inner branchs of vascula media almost subparallel to median ridge. Internal surface with coarse papillae near margin.
Etymology
Named after the original locality, Quebrada La Tuna.
Materials
CEGH-UNC 27172–27183, 12 conjoined specimens; CEGH-UNC 27168–27170, three fragmentary ventral valves.
Remarks
This new species is readily distinguished from Ahtiella argentina by the less transverse subrectangular shell outline, nonalate cardinal extremities, catacline to procline ventral interarea, and larger bilobed ventral muscle field. In addition, the mantle vascular system in A. tunaensis n. sp. is barely impressed, although this could be due to the lack of well-preserved internal molds. Its shell outline is reminiscent of A. quadrata, from which it differs mainly in its much finer radial ribbing. The large bilobed ventral muscle field of A. tunaensis n. sp. is comparable to that of the Baltic species A. lirata, but differs from the latter species in its less convex and smoothly geniculated profile, better defined ventral fold and dorsal sinus, and indistinct rugae. The elongate oval ventral diductors of the Precordilleran species, although rather shorter, resemble those of A. paucirugosa. However, the Precordilleran species can be distinguished by its subrectangular shell, broad dorsal median ridge, and more deeply impressed mantle canals. The Peruvian species A. zarelae differs in having uniformly costellate radial ribbing, a more prominent ventral fold and deeper dorsal sulcus, a proportionally shorter and wider subpentagonal ventral muscle field, and strongly marked internal radial ornamentation at all growth stages.
Ahtiella famatiniana new species
urn:lsid:zoobank.org:act:9AB173B1-73FE-4142-A2AF-A2DF1FD3ED9C
2003a Ahtiella sp.; Reference BenedettoBenedetto, p. 210, pl. 18, figs. 18–20.
Type specimens
Holotype, CEGH-UNC 27137, internal mold of ventral valve. Paratypes: CEGH-UNC 27135, internal mold of dorsal valve; CEGH-UNC 27149, conjoined specimen.
Diagnosis
Shell transversely semielliptical (mean length/width ratio 0.48), alate to mucronate; ventral valve with strongly concave posterolateral areas and prominent carinate median fold; corresponding dorsal sulcus narrow and deep posteriorly, broader and wider at anterior margin. Ornamentation subequally multicostellate to incipiently parvicostellate, with primary costellae slightly more accentuated. Ventral muscle field almost pentagonal, extending to ~32% of valve length, with elongate diductor scars bounded laterally by curved ridges and shorter adductor field. Broad rounded dorsal median ridge fading anteriorly. Usually well-defined discontinuos dorsal platform. Vascular system deeply impressed only on disc margin and trail.
Occurrence
Cerro Morado Group, Las Pircas anticline, central Famatina Range, La Rioja Province. Northern margin of Chaschuil River (Loma del Médano), Catamarca Province. Dapingian.
Description
Shell transversely semielliptical, resupinate in lateral profile, up to 18 mm in width (mean 13 mm, N=26), mean length/width ratio 0.48 (N=20). Cardinal extremities alate, often projecting in rodlike mucrons (usually incompletely preserved). Ventral valve strongly concave with greatest concavity at posterolateral areas; carinate median fold stronger posteriorly, becoming wider and rounded anteriorly; anterior commissure broadly sulcate. Ventral interarea planar, procline or occasionally catacline, approximately twice as high as dorsal. Delthyrium covered in apical two-thirds by semiconical pseudodeltidium. Dorsal valve moderately convex, 30–35% as deep as long, uniformly curved (nongeniculated) in lateral profile, with median sulcus initially narrow and deep, becoming broader and wider at anterior margin where it attains ~25% of valve width. Dorsal interarea planar, orthocline to steeply anacline, with triangular notothyrium covered posteriorly by convex chilidium. Radial ornamentation subequally multicostellate, with rounded primary costellae often slightly more accentuated than second order ones giving ribbing a parvicostellate aspect; costellae increasing in number mainly by interpolation in dorsal valve and by interpolation and dichotomy in ventral valve, typically 9 per 2 mm (counted at 5 mm growth stage). Ribs crossed by prominent, closely and evenly spaced fila (~20 per mm) and by few prominent growth discontinuities. Posterolateral rugae weakly defined or absent. Shell substance apparently impunctate.
Ventral interior with strong triangular teeth supported by short dental plates diverging at ~120°; umbonal cavities partially filled by secondary deposits. Muscle field proportionally large, subpentagonal in outline, generally wider than long, typically extending 32% of valve length (maximum 36%); diductor scars elongate subtriangular, enclosed laterally and anterolaterally by curved ridges originating in front of dental plates; adductor field shorter and more deeply impressed than diductors, suboval to subrectangular in outline, not enclosed anteriorly by diductors. Valve margin strongly thickened, defining internal disc and trail separated by low, rounded peripheral rim. Vascula media broad, weakly impressed, slightly divergent; distal region of vascular system coinsisting of numerous radially arranged distal branches deeply cutting disc margin and prolonged on trail; small papillae between vascular trunks. External ribbing weakly impressed only on juvenile interiors. Gonadal areas large, occupying most posterolateral areas, sculptured by roughly radial anastomosing ridges.
Dorsal interior with simple bladelike cardinal process slightly enlarged anteriorly, erected on elevated subtriangular notothyrial platform. Socket ridges strong, slightly thickened and free distally, divergent anterolaterally at ~80º, bounding deep semiconical sockets partially excavated under dorsal interarea. Broad, rounded median ridge arising in front of notothyrial platform, fading anteriorly at intersection with peripheral rim. Muscle field weakly impressed, slightly wider than long, extending anteriorly for 30% of valve length, bounded laterally by low ridges; posterior adductor scars subcircular, smaller than suboval anterior pair. Dorsal mantle canal system indistinct or feebly impressed; vascula media broad, divergent, branching toward valve margin. Coarse ridge-like pustules or endospines coalescing to form well-defined discontinuous platform, which extends along entire valve margin except posterolateral extremities.
Etymology
Named after one of the original localities, the Famatina Range.
Materials
CEGH-UNC 27131–17159, 29 specimens from the volcanosedimentary unit exposed at Anticlinal Las Pircas, north of Cachiyuyo River, central Famatina Range, La Rioja Province; CEGH-UNC 27163–27167, five specimens from the Chaschuil River, north Famatina Range, Catamarca Province.
Remarks
In its size and shell convexity, nearly uniform costellate ornamentation, high carinate ventral fold, and well-developed dorsal peripheral rim, this species strongly resembles A. zarelae from the upper Floian of Peru, but differs in having a proportionally larger ventral muscle field reaching approximately one-third of the valve length; broader, rounded, nonseptiform dorsal median ridge; and vascular trunks strongly impressed on the margin of the ventral disc and especially on the trail. The Peruvian Ahtiella sp., which is known only by its dorsal valve, can be distinguished from the Famatina species by its finer and uniform ribbing, higher and shorter median ridge, and more continuous, anteriorly excavated platform. Ahtiella argentina can readily be distinguished from A. famatiniana n. sp. by having a more transverse shell outline, unequally parvicostellate ornamentation, a smaller triangular ventral muscle field, and a strongly impressed vascular system. The ventral muscle field of A. famatiniana n. sp. is reminiscent of that of A. tunaensis n. sp., but in the latter it is more definitely bilobed. Moreover, the Precordilleran species differs in having subrectangular cardinal extremities, a less pronounced ventral median fold, and unequally parvicostellate ornamentation. Ahtiella lirata resembles the Famatinan species in its alate or mucronate cardinal extremities and its large subrectangular ventral muscle field, but can be distinguished by its unequally parvicostellate ornamentation, poorly defined (or absent) dorsal sulcus and corresponding ventral fold, and a strongly convex gibbous dorsal valve. Ahtiella paucirugosa externally resembles A. famatiniana n. sp. in having a well-developed dorsal sinus and ventral fold, moderate shell convexity, and almost indistinct posterolateral rugae, but differs internally in its elongate ventral diductor scars extending to near the valve midlength, its deeply impressed mantle canals, and its high, septum-like dorsal median ridge. As far as can be judged from the incomplete material, A. quadrata resembles A. famatiniana n. sp. in its shell outline and morphology of its ventral muscle field, but the Welsh species differs in its coarser radial ornamentation. Ahtiella concava clearly differs in having a strongly impressed and longer dorsal muscle field and impersistent (much finer? smooth?) ribbing.
Ahtiella coloradoensis (Benedetto, Reference Benedetto1998b)

Figure 11 (1−15) Ahtiella coloradoensis (Benedetto, Reference Benedetto1998b); ‘Green Member of the Sepulturas Formation,’ Los Colorados, northwestern Argentina: (1−4) holotype, external mold (1), latex cast (2), internal mold (3), and latex cast (4) of ventral valve, CEGH-UNC 13780; (5, 6) latex cast of exterior (5) and internal mold (6) of dorsal valve, CEGH-UNC 13817; (7) latex cast of ventral exterior, CEGH-UNC 13797; (8) ventral internal mold, CEGH-UNC 13802; (9) latex cast of ventral valve, CEGH-UNC 27187, showing incipient delthyrial cover; (10) latex cast of dorsal interior, CEGH-UNC 13808; (11) internal mold of juvenile dorsal valve, CEGH-UNC 13786; (12) internal mold of juvenile ventral valve, CEGH-UNC 13795; (13) dorsal internal mold, CEGH-UNC 13826; (14, 15) dorsal internal mold (14) and latex cast (15) of dorsal valve, CEGH-UNC 13823, showing detail of cardinalium. (16−18) Ahtiella zarelae Villas in Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007; San José Formation, Inambari River, Peru (illustrations from Gutiérrez-Marco and Villas, Reference Gutiérrez-Marco and Villas2007, p. 552, figs. 4D, F, and J, reproduced under the Creative Commons Attribution License CC BY 4.0): (16) latex cast of dorsal valve exterior, MGM 5944X; (17) dorsal internal mold, MGM 5945X; (18) latex cast of ventral interior, MGM 5926X. All specimens dusted with ammonium chloride. Scale bars=5 mm.
?1980 Valcourea sp.; Reference Havlíček and BranisaHavlíček and Branisa, p. 23, pl. 2, fig. 7.
1998b Monorthis coloradoensis Reference BenedettoBenedetto, p. 11, pl. 2, figs. 15–28.
2003a Monorthis coloradoensis; Reference BenedettoBenedetto, p. 211, pl. 24, figs. 5–9.
Holotype
CEGH-UNC 13780, internal mold of ventral valve from the ‘Green Member of the Sepulturas Formation,’ northwest of Los Colorados village, Cordillera Oriental, Jujuy Province, Argentina.
Diagnosis
Shell semielliptical, convexoplanar to gently convexoconcave (mean length/width ratio 0.63) with right-angled cardinal extremities in adult specimens; ventral valve almost planar with low median fold and well-defined corresponding dorsal sulcus. Ornamentation equally multicostellate, occasionally ramicostellate in ventral valve; ribs 10–12 per 2 mm. Ventral muscle field subrectangular to subpentagonal, slightly wider than long, extending to ~31% of valve length, bounded by strong ridges in adult specimens; variably developed platform in dorsal valve. Vascular system indistinct excepting on periphery of each valve.
Occurrence
‘Green Member of the Sepulturas Formation’ (sensu Astini et al., Reference Astini, Toro, Waisfeld and Benedetto2004a), Los Colorados area, western slope of Cordillera Oriental, Jujuy Province, Argentina. Middle-late? Darriwilian.
Description
See Benedetto (Reference Benedetto1998b).
Materials
In addition to the type material listed by Benedetto (Reference Benedetto1998b), new specimens CEGH-UNC 27187–27190 were collected from Quebrada Chamarra, northwest of Los Colorados village, Jujuy Province, Argentina.
Remarks
The overall morphology of the Los Colorados species, in particular its convexoplane shell profile, carinate ventral fold, equally multicostellate ornamentation, acute cardinal extremities, and orthoid cardinalia led Benedetto (Reference Benedetto1998b) to refer it to the hesperonomiid genus Monorthis. This assignment was further supported by the lack of evidence of pseudopunctae and pseudodeltidium, both considered diagnostic features of the plectambonitoids. As discussed previously, however, the differences between Monorthis and the basal species of Ahtiella are subtle and exhibit transitional characteristics, so that assignement to one or the other genus depends largely on the weight accorded to each character. The species M. coloradoensis is reassigned here to Ahtiella mainly because of the incipient internal geniculation in the ventral valve defining a low disc and a trail, the large subquadrate ventral muscle field bounded by widely splayed dental plates, and the presence of a faint discontinuous platform in the dorsal valve. In addition, a revision of the type material and newly collected specimens of M. coloradoensis revealed an incipient apical delthyrial cover, like some specimens of M. cumillangoensis (Figs. 6.15 and 11.9).
Havlíček and Branisa (Reference Havlíček and Branisa1980) referred to Valcourea sp. a few ventral valves from supposedly Darriwilian sandstones exposed along the road Sucre-Potosí at Tambo Acachila, Bolivia. The single illustrated interior is nearly indistinguishable from Ahtiella coloradoensis but without dorsal valves and the lack of information of external ornamentation, assignment to this species must remain provisional.
Ahtiella coloradoensis resembles A. famatiniana n. sp. in its large subrectangular (sometimes subpentagonal) ventral muscle field extending anteriorly to near one-third of the valve length, its well-defined peripheral ventral thickerning, and its incipient dorsal platform. Ornamentation is multicostellate in both species but in A. coloradoensis it is more uniform tending to be ramicostellate in the ventral valve. Ahtiella coloradoensis mainly differs from the Famatinan species in its planar to gently concave ventral valve profile, its less transverse shell outline, its rectangular cardinal extremities, and its indistinct pseudodeltidium. It can be distinguished from A. zarelae by its lower and rounded ventral fold, less pronounced geniculation, larger ventral muscle field, and the presence of a conspicuous peripheral rim in the ventral valve. In addition, internal ribbing in the Peruvian species is strongly marked.
Acknowledgments
I am especially indebted to my colleagues F. Dávila and R. Astini who collected material from the Famatina Range (Las Pircas anticline), and B. Waisfeld, who collected specimens of Ahtiella coloradoensis, making them available for this study. The helpful comments of the journal reviewer D.A.T. Harper and the Associate Editor R.-b Zhan greatly improved the original manuscript. The TNT program for phylogenetic analysis was made available with sponsorship of the Willi Hennig Society. Research has been financially supported by CONICET (grant PIP 112-201101-00803). This paper is a contribution to the International Geoscience Programme (IGCP) Project 653 ‘The onset of the Great Ordovician Biodiversification Event.’















