Cows' milk is recognized as an important source of fat-soluble vitamins in the diet, especially vitamins A and E (McDowell, Reference McDowell2000). Milk also contains small amounts of β-carotene, which can act as a vitamin A precursor and may itself be of benefit in the diet (Burri, Reference Burri1997). The concentration of vitamins A and E and β-carotene in milk is affected by a number of factors including the season, nutrition, cow management, genetics and stage of lactation (Jensen et al. Reference Jensen, Johannsen and Hermansen1999; O'Brien et al. Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999; Lindmark-Månsson et al. Reference Lindmark-Månsson, Fondén and Pettersson2003; Nozière et al. Reference Nozière, Graulet, Lucas, Martin, Grolier and Doreau2006a; Calderón et al. Reference Calderón, Chauveau-Duriot, Martin, Graulet, Doreau and Nozière2007). As nutritional and other farm management factors differ between organic and conventional systems (Lampkin, Reference Lampkin2002) milk vitamin content may also differ between the two farming systems in the UK. Different management may also affect cows' responses to seasonal variation in nutrition and therefore the vitamin content of the milk produced by the two systems. Seasonal influences on organic milk vitamin content have not been investigated in longitudinal studies, yet the nutrient content of organically produced foods is currently an area of particular consumer interest with increasing sales of organic food in the UK, particularly in organic milk (Anon, 2005a).
To date, longitudinal studies of milk vitamin content are limited and mainly consider conventional milk from processing dairies (O'Brien et al. Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999; Lindmark-Månsson et al. Reference Lindmark-Månsson, Fondén and Pettersson2003) where only basic differences between liquid milk and manufacturing milk producers (O'Brien et al. Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999) were taken into account. One Swedish longitudinal study of organic farm bulk tank milk found equivalent β-carotene concentrations with conventional milk (Toledo & Andrén, Reference Toledo and Andrén2003). Two cross-sectional studies of processed milk and milk products, from Italy and Scandinavia, suggest that organic milk may contain a lower vitamin A (Bergamo et al. Reference Bergamo, Fedele, Iannibelli and Marzillo2003) but higher vitamin E content (Nielsen et al. Reference Nielsen, Lund-Nielsen and Skibsted2004). As limited information is available on the nutritional management on the studies from farms outside the UK, any interaction or confounding between farm type and nutrition cannot be evaluated. However, as potential differences in milk vitamin and β-carotene content between organic and conventional milk are likely to reflect effects of nutritional management with system, it is important that these factors are considered in a comparative analysis. Owing to the paucity of information on farm factors that may affect organic and conventional milk vitamin and beta carotene content in the UK, this study aimed to determine whether there is a difference in concentrations of vitamin A, vitamin E and β-carotene between the two milk types, accounting for management factors over a 12-month production cycle.
Materials and Methods
Milk sample collection
Nineteen conventional and 17 organic dairy farms in the north-west of England and in Wales were recruited for a 12-month period between 2003 and 2004. Milk samples were collected from the bulk tank of each farm and all samples were stored frozen at −80°C until vitamin analysis of samples from June, August, October and December 2003, and February and April 2004. Farm management and production data were collected by interview questionnaire and farm record analysis at each visit, and entered into a database (Microsoft Access, 2000) (Ellis et al. Reference Ellis, Innocent, Grove-White, Cripps, McLean, Howard and Mihm2006).
Laboratory analysis of milk for vitamin A, vitamin E and β-carotene
All solvents used were of HPLC grade (Rathburn Chemicals, Walkerburn, Scotland). Standard stock vitamin solutions were prepared to the following concentrations: all-trans-retinol, 1 mg/ml; dl-α-tocopherol, 5 mg/ml; δ-tocopherol, 167 μg/ml (all from Sigma, Gillingham, Dorset, UK) and were dissolved in 100% ethanol, with β-carotene, 0·1 mg/ml (Sigma, UK) dissolved in 100% chloroform. Actual concentrations of stock standard solutions were determined and checked throughout the duration of the analysis in a spectrophotometer. Specific absorbance values (A 1cm1%) for each compound analysed were as follows: retinol, 1780 at 325 nm; α-tocopherol, 75·8 at 292 nm; δ-tocopherol, 91·2 at 298 nm; β-carotene, 2396 at 465 nm (Arnaud et al. Reference Arnaud, Fortis, Blachier, Kia and Favier1991; Budavari et al. Reference Budavari, O'Neil, Smith, Heckelman and Kinneary1996). A working mixed standard solution was prepared by diluting volumes of the previous stock solutions with a volume of a methanol:hexane:acetonitrile mixture (45:12·5:42·5), to give a standard containing 0·31 μg retinol/ml, 1·66 μg δ-tocopherol/ml, 1·00 μg α-tocopherol/ml and 0·20 μg β-carotene/ml. All standard solutions were protected from light and stored at −20°C. δ-Tocopherol was chosen as the internal standard for all analyses as this isomer is not present in milk.
Milk samples were defrosted, warmed to 40°C and thoroughly mixed to disperse the milk fat and 10 μl (167 μg/ml) of internal standard was added to all milk samples prior to vitamin and β-carotene extraction. Samples were saponified and fat-soluble vitamins were extracted following the method of Salo-Väänänen et al. (Reference Salo-Väänänen, Ollilainen, Mattila, Lehikoinen, Salmela-Molsa and Piironen2000) with the following modifications. During vitamin extraction samples were mixed for 10 min then centrifuged at 2500 g at 5°C for 10 min. From the resultant upper solvent layer, 1 ml was transferred into a clean glass tube. A repeat aliquot of 2 ml hexane:ethyl acetate was added to the original sample tubes, which were mixed for 10 min and centrifuged as before. A further 1·6 ml of the upper solvent layer was then removed and added to the previous aliquot. Samples were dried in a sample concentrator (Savant, Thermo Electron Corporation, Thermo Fisher Scientific, UK) at 40°C for 45 min, re-suspended in 1 ml of a methanol:hexane:acetonitrile mixture (45:12·5:42·5) and transferred to a silicone-capped glass vial in the HPLC system tray to await analysis.
Analysis of vitamin content was by the HPLC method of Arnaud et al. (Reference Arnaud, Fortis, Blachier, Kia and Favier1991) with the following modifications. Samples were analysed in a ThermoSeparation Products (TSP) system comprising a Spectra System SCM1000 degasser, Spectra Physics P4000 pump, Spectra Physics AS3000 auto-injector and a Spectra Focus UV detector. Chromatograms were plotted and integrated using Spectra Physics PC1000 software. Reversed-phase separations were carried out at room temperature with a Phenomenex Nemesis C18, 4 μm, 4·6×150-mm column with a pre-column. The column was eluted at a flow rate of 1 ml/min in an isocratic mode using methanol:hexane (85:15) as mobile phase. A 50-μl volume of sample was injected from each of the sample vials which were maintained at a tray temperature of 5°C. UV-detection wavelengths were changed according to the expected retention times of the vitamins and were as follows: 0–2·7 min, 325 nm (retinol); 2·7–7·0 min, 291 nm (δ-tocopherol and α-tocopherol); 7–13·5 min, 450 nm (β-carotene). During each sample run, a mixed vitamin external standard was analysed between every two unknown samples.
Milk sample total fat (butterfat) determination
Milk fat content was determined by the First Milk Laboratory (Paisley, Scotland) by means of Fourier Transform Infra-red spectroscopy analysis in the MilkoScan FT 6000 (Foss, Denmark).
Statistical analysis
The vitamin content of the samples was expressed as the amount of vitamin in μg/g milk fat and subjected to further analyses using MINITAB (Version 13.20, Minitab Inc., 2003, State College PA, USA). Summary statistics by farm system type (organic or conventional) of descriptive details of herds included in study and the mean (sd) concentration (μg/g milk fat) of vitamin A, vitamin E and β-carotene over the 12 months of sampling were calculated. A general linear mixed model (GLMM) was used to investigate effects of farming system type (organic or conventional) on milk vitamin content after accounting for a number of other herd-level and nutritional management factors which were predicted to affect milk vitamin content. Herd-level factors considered (apart from farming system type) included: cow breed, herd average annual yield, herd calving pattern. Each of the factors was divided into factor levels and coded; with breed level according to the proportions of different breed types in the herd, herd average annual yield level based on the quartile ranges of herd yield, and calving pattern based on whether there was a distinct calving pattern or year-round calving. A range of nutritional management factors were recorded at each sampling visit and assigned either binary or multi-level coding as appropriate. Individual herd-level and nutritional factors were first screened using a GLMM that also included farm identity as a random effect and month as a fixed effect to determine their significance for the vitamin content. Month was included as a fixed effect as it was intended to determine whether there were effects of specific months of the year on milk vitamin content. All factors significant at screening (P<0·2) were carried forward to a final multivariable model. This model consisted of:
Where μ is the overall mean vitamin content, Fi the individual farm effect which was nested within HLFj the herd level factors (fixed effects), Nk the nutrition variable, Ml the month effect and εijkl the residual error term. Multivariable models were developed using a backwards elimination approach, with factors removed depending on the significance of the F test for that factor. All possible, biologically plausible two-way interaction terms were then tested for significance. For main effects and for interaction terms, significance was defined as P<0·05 for the corresponding F test. For factors remaining in the model, post-hoc multiple comparisons of means for each factor level were calculated using a pairwise comparison method and the Bonferroni correction for multiple comparisons, where significance was defined as P<0·05.
Results
HPLC analytical performance
The limit of quantification (LOQ) for each compound analysed in the milk samples was determined by reference to the external standard (ES) and were as follows: retinol, 0·03 μg/ml (ten times lower than ES); α-tocopherol, 0·05 μg/ml (20 times lower than ES); β-carotene, 0·01 μg/ml (20 times lower than ES); and δ-tocopherol (IS), 0·08 μg/ml (20 times lower than ES). All LOQ values were 10–20 times lower than the actual vitamin values determined from the milk samples.
Descriptive statistics for farms included in study
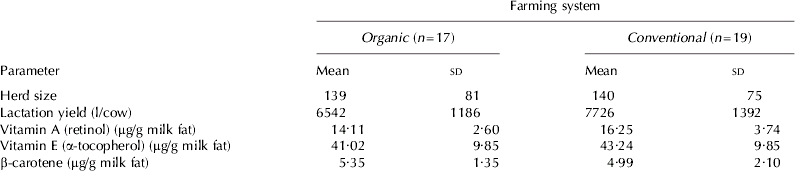
Summary statistics by farming system type of herds included in the study are shown in Table 1, including mean (sd) milk fat vitamin and β-carotene concentration. Following univariable screening, a number of factors predicted to potentially affect vitamin A, vitamin E and β-carotene were not further considered if they were either co-linear or were non-significant at screening (P>0·2). Those factors still considered for inclusion in the multivariable analyses for either vitamin A, vitamin E and β-carotene are thus also shown, distributed by farm system type in Table 1. As the nutritional management varied on individual farms each month, the percentage of total responses by farming type for each factor level over the 12-month study are shown.
Table 1. Summary descriptive statistics by farm system type (organic or conventional) of descriptive details of herds included in study, the mean concentration of vitamin A, vitamin E and β-carotene in milk over the 12 months of sampling for each milk type and distribution of herds within those factor levels significant at univariable screening (P<0·2)


† High=>6 kg concentrate/cow per day
Factors affecting the vitamin content of milk
All coefficients for significant factors for each of the multivariable models are summarized in Table 2. For each model, the effect of individual farm was highly significant (P<0·001; coefficients not shown). There were no significant interactions for any models.
Table 2. Coefficients and significance from final Generalized Linear Mixed Model output of factors affecting milk vitamin A, vitamin E and β-carotene content

† Farm identity was highly significant in all models (individual farm coefficients not listed)
‡ Yield quartile levels 1=lowest to 4=highest
§ IP=in-parlour
¶ G=Grass, GRC=Grass and Red Clover, GWC=Grass and White Clover, GMC=Grass and Mixed Clover
ab Factor level groups with common superscript lettersare not significantly different at the 5% level
* P<0·05, ** P<0·01, *** P<0·001
Vitamin A
Farming system remained significant, even with other factors accounted for, with conventional milk fat having a higher concentration of vitamin A than organic milk fat, where least squared mean values were 16·26 and 14·12 ug/ml respectively (P<0·05). There were highly significant effects (P<0·001) of both individual farm and month (Fig. 1a) on the content of vitamin A in milk, with the main difference in concentration found between April and October (P=0·001). Feeding higher levels of concentrate in the parlour increased vitamin A concentration (P<0·05). Whilst the overall effect of type of conserved forage fed affected milk fat vitamin A concentration (P<0·05), factor level comparison found differences only between no-silage feeding and grass and mixed clover silage feeding (P<0·05). Overall the effect of grazing type on milk fat vitamin A concentration remained significant (P<0·001) although factor level comparisons found differences only between no-grazing and grass and mixed clover silage grazing (P<0·05).

Fig. 1. Mean values for (a) vitamin A (retinol) (b) vitamin E (α-tocopherol) and (c) β-carotene (μg/g milk fat) in organic (○) and conventional (●) milk for the 12 month study. Mean values are taken across all farms of each type with SEM bars shown.
Vitamin E
Farming system type (organic or conventional) did not affect the milk vitamin E content and was not significant even at screening (P>0·2). There were highly significant effects (P<0·001) of both individual farm and month on the content of vitamin E in milk, with vitamin E concentration increasing between February and April (P<0·001), with all months associated with higher concentrations compared with the months of February and December (P<0·05) when the concentration was lowest (Fig. 1b). Vitamin E concentrations decreased as herd average yield increased (P<0·01), thus the lowest yielding herds had a higher milk vitamin E content than all other yield groups (P<0·001 for all comparisons), and herds in yield groups 2 and 3 still had higher milk vitamin E concentrations than herds in group 4 (P<0·001 and P<0·05 respectively). Higher level of concentrate feeding in the parlour appeared to increase vitamin E concentrations compared with lower level concentrate feeding (P<0·05).
β-Carotene
Farming system type (organic or conventional) did not affect the milk β-carotene content and was not significant even at screening (P>0·2). There were highly significant effects (P<0·001) of individual farm and month on the content of β-carotene in milk, with an increase in β-carotene in April (P<0·001), following a nadir in February (Fig. 1c). Highest concentrations were seen in June with a subsequent drop in August (P<0·05) and a more significant decrease was also observed in December (P<0·001). β-Carotene concentrations decreased as herd average yield increased (P<0·05), thus the lowest yielding herds had a higher milk β-carotene content than all other yield groups (P<0·001 for all comparisons), and herds in yield groups 2 and 3 still had higher milk concentrations of β-carotene than herds in group 4 (P<0·05 and P<0·01 respectively). Feeding higher levels of concentrate in the parlour appeared to increase milk β-carotene content (P<0·05). Whilst the overall effect of type of conserved forage fed affected milk fat β-carotene concentration (P<0·05), factor level comparison found differences only between no-silage feeding and grass and mixed clover silage feeding (P<0·05).
Discussion
This study is the first year-long longitudinal study of organic and conventional milk vitamin content in the UK. Similar values to the vitamin A and β-carotene concentrations in the current study are reported in Irish milk by O'Brien et al. (Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999) and Toledo & Andrén (Reference Toledo and Andrén2003) report similar β-carotene concentrations in Swedish organic milk samples. The higher vitamin E concentrations obtained in the current study compared with other studies (O'Brien et al. Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999; Bergamo et al. Reference Bergamo, Fedele, Iannibelli and Marzillo2003; Lindmark-Månsson et al. Reference Lindmark-Månsson, Fondén and Pettersson2003) could be a reflection of a different population of herds, different nutritional intake or differences in the analytical methods used. In the current study, the same recovery percentage for analysis of all compounds was assumed, although work by Salo-Väänänen et al. (Reference Salo-Väänänen, Ollilainen, Mattila, Lehikoinen, Salmela-Molsa and Piironen2000) suggests that this may vary from 82–91%. However, in terms of comparing the milk types the relative amounts would be constant and, although there may be an underestimate in the milk fat content of vitamin A and β-carotene, results were similar to other published data on milk vitamin A and β-carotene content.
Analysis of the factors affecting milk vitamin content in the current study is complex owing to the inter-related nature of many farm-level factors. Farming system and milk yield, although not co-linear in our statistical analysis, indicates an effect of farming system on yield where organic farms tended to lower yields. Additionally, it is difficult to tease out the nutritional differences and confounding when some practices are reported predominantly, but not solely in different systems, for example grazing on clover and grass. However, despite these limitations, the current study suggests that conventionally produced milk had a higher mean Vitamin A content with this effect significant even after accounting for a number of important management and nutritional factors in the analyses, although farming system was not associated with a difference in the vitamin E or β-carotene content of milk. The higher vitamin A content in conventional milk supports the findings of Bergamo et al. (Reference Bergamo, Fedele, Iannibelli and Marzillo2003) with respect to conventional buffalo milk compared with organic buffalo milk. However, in the same study, no difference was reported between conventional and organic cows’ milk products (not liquid milk) with respect to vitamin A content. No details of other factors that may affect the milk vitamin content were included, such as feeding and time of year of sampling; so direct comparison with the current study should only be made with caution. Bergamo et al. (Reference Bergamo, Fedele, Iannibelli and Marzillo2003) also reported a higher vitamin E concentration in milk fat in both organic buffalo milk and organic cows’ milk products when compared with conventional milk samples as well as higher β-carotene concentration in organic cows’ milk. Additionally, Nielsen et al. Reference Nielsen, Lund-Nielsen and Skibsted2004 (not peer reviewed) reported a higher vitamin E concentration in processed Danish organic milk compared with conventional milk, and suggested a higher concentration of natural vitamin E in organic milk, rather than synthetic vitamin E from feedstuffs. However, whether these effects are maintained when including confounding management factors such as nutrition and herd yield in the data analysis is unknown. The vitamin analysis method in the current study utilized saponification to ensure a more complete release of vitamin for extraction. However, this hydrolyses the vitamins to their alcohol forms, eliminating determination and quantification of the form (natural v. synthetic) in which the vitamins are present in the sample (Hewavitharana et al. Reference Hewavitharana, Van Brakel and Harnett1996; Strobel et al. Reference Strobel, Heinrich and Biesalski2000). Methods of analysis that do not break down vitamin structure have been developed (Hewavitharana et al. Reference Hewavitharana, Van Brakel and Harnett1996) but involve the use of larger volumes of more volatile and dangerous solvents than the method used in this study. Further studies may wish to investigate whether UK organic milk does indeed contain a different content of natural rather than synthetic vitamins compared with conventional milk. Whether this is of nutritional significance to the consumer, however, is unclear.
The concentration of vitamin A, vitamin E and β-carotene increased in milk fat in summer in this study, which supports the findings of others (Lindmark-Månsson et al. Reference Lindmark-Månsson, Fondén and Pettersson2003; Toledo & Andrén, Reference Toledo and Andrén2003). This increase is associated with the grazing season when cows have access to fresh pasture, which has a higher vitamin E and β-carotene content than conserved forages (Marks, Reference Marks1968; Chamberlain & Wilkinson, Reference Chamberlain and Wilkinson1998; McDowell, Reference McDowell2000; Shingfield et al. Reference Shingfield, Salo-Vaananen, Pahkala, Toivonen, Jaakkola, Piironen and Huhtanen2005; Nozière et al. Reference Nozière, Graulet, Lucas, Martin, Grolier and Doreau2006a) and accordingly, grazing increased the milk vitamin content in the current study, independent of farming system. Overall, grazing and conserved forage type remained significant factors affecting vitamin A and β-carotene content in the current study. However, factor level differences affecting milk vitamin A or β-carotene content were largely non-significant and may reflect the relatively small number of farms undertaking some of the feeding practices. The addition of silage feeding would be expected to reduce milk vitamin content compared with a ration composed of fresh forage, as forage crop conservation is reported to decrease the carotene content (Nozière et al. Reference Nozière, Graulet, Lucas, Martin, Grolier and Doreau2006a), which acts as the principal precursor of vitamin A for ruminants (McDowell, Reference McDowell2000). It can be hypothesized that the effect of feeding clover silages may be less marked because clover silages contain more carotene than grass silage owing to the increased β-carotene-rich leaf to stem ratio (McDowell, Reference McDowell2000); this would require further study to elucidate.
In the current study, feeding higher levels of concentrate in-parlour was associated with an increase in milk vitamin A, vitamin E and β-carotene concentration. This closely supports the findings of O'Brien et al. (Reference O' Brien, Lennartsson, Mehra, Cogan, Connolly, Morrissey and Harrington1999), where retail Irish milk had a higher vitamin A concentration compared with manufacturing milk, with herds producing retail milk being fed more concentrates. Many conventional dairy farm concentrate feeds are supplemented with additional synthetic vitamins and this can increase the vitamin A and E concentrations in milk. Concentrate feeds are thought to be a relatively poorer source of β-carotene than forage (Nozière et al. Reference Nozière, Graulet, Lucas, Martin, Grolier and Doreau2006a), but the results in this study may reflect the fact that some feeds may contain raw materials with higher β-carotene content, for example grass nuts, and warrant further investigation.
Increasing herd yield was associated with a decrease in β-carotene concentration in the milk fat; an effect previously reported by Larson et al. (Reference Larson, Wallen, Owen and Lowry1983) and a decrease in vitamin E content. Vitamin content in this study has been reported as concentrations per gram of milk fat, which would control for variation in total milk fat content. The dynamics of vitamin and β-carotene transfer to milk fat are not well understood, although the association of increasing herd yield with decreasing milk fat vitamin E and β-carotene concentration is a phenomenon for which an explanation was suggested by Jensen et al. (Reference Jensen, Johannsen and Hermansen1999). Secretion of both vitamin E and β-carotene into milk is limited in quantity and is independent of yields of milk and milk fat; thus selection of genotypes for increased yield may be associated with a decrease in the milk content of these vitamins. If vitamin secretion is of a finite capacity and independent of total fat yield, this could have accounted for some of the decrease in vitamin concentration seen in high production herds in the current study. Certainly, breed variation in the secretion of β-carotene in milk fat is well recognized, with lower yielding Channel Island breeds more readily absorbing and excreting β-carotene in milk fat compared with higher yielding Holstein cows (McDowell, Reference McDowell2000; Nozière et al. Reference Nozière, Graulet, Lucas, Martin, Grolier and Doreau2006a). Recent work has shown that secretion of vitamins A and E and β-carotene into milk varies only slightly with stage of lactation (Calderón et al. Reference Calderón, Chauveau-Duriot, Martin, Graulet, Doreau and Nozière2007) but is affected by the energy status of the cow (Nozière et al. Reference Nozière, Grolier, Durand, Ferlay, Pradel and Martin2006b), with lower energy intake associated with decreased milk vitamin and β-carotene content. Additionally, vitamin requirements, particularly for vitamin E, are higher for animals that are lactating, eating diets with high concentrations of polyunsaturated fatty acids, stressed or deficient in selenium (McDowell, Reference McDowell2000). It is conceivable that high-yielding dairy cows with an associated high metabolic rate could be included in all of these groups and be energy deficient, thus potentially producing milk with lower vitamin E content. Finally, the higher milk vitamin E and β-carotene content seen with lower yielding herds may be a result of being given a ration with a forage base higher in vitamin and β-carotene content compared with a ration that contained more maize silage or other lower carotene containing feeds such as those typically fed to higher yielding herds in a total mixed ration (TMR).
It was outwith the limits of this study to analyse all feedstuffs used on each farm in each management group of cows; particularly, the vitamin content and use of supplementation are very difficult to ascertain as many compound feeds are supplemented, with some farmers adding vitamin and mineral premixes and differing diets being fed to different production groups that contribute to the same bulk tank. Further field studies may attempt to record detailed feed vitamin content and production group response. In the current study it is interesting that lower yielding herds and those herds with higher concentrate supplementation were associated with higher milk vitamin E concentrations. This apparent paradox highlights that the supply and metabolism of vitamins is complex, possibly owing in part to the body's ability to store vitamins to some extent. Therefore, determination of requirements and the establishment of the need for supplementation are difficult.
This study shows that farming system only affected the milk content of vitamin A, where there was a lower concentration in organic milk. Additionally, this study supports the findings of previous studies on the effects of season, herd yield and access to fresh pasture on milk vitamin content, and no interaction with farming system could be detected. The higher milk fat vitamin A concentration from the conventional farms may be due to increased concentrate feeding with vitamin A-supplemented dairy cow feeds. Organic regulations stipulate that concentrate feeds should not be routinely supplemented with vitamins and allow only limited use of vitamin and mineral supplements in organic farms when additional requirements are necessary (Anon, 2005b). This study suggests that organic farms are able to supply at least as much vitamin E and β-carotene to their cows as the conventional farms in the absence of additional feed supplements. The difference in mean milk vitamin A concentrations of 15% was small, and the nutritional significance to a calf or a human consumer would be hard to estimate. However, if it reflects a potential undersupply of vitamin A to the cow, further investigations in organic dairy cows could include blood and liver analysis to determine the body stores, and also should include feed analysis to determine the amounts in the ration.
The financial support of the Organic Milk Suppliers Co-operative is gratefully acknowledged. The authors also acknowledge the access given to farms by all producers who participated in the study and the skilled technical services of Alex Holme in the University of Liverpool. Giles Innocent was supported by the Wellcome Trust funded International Partnership Research Award in Veterinary Epidemiology (IPRAVE) project and is presently a DEFRA VTRI fellow.





