Introduction
Modern marine pharmacology starts with the work by Bergmann & Feeney (Reference Bergmann and Feeney1951) who studied the chemical activity of a Caribbean sponge and reported the first marine chemical compounds displaying antitumoural activity. This discovery shifted part of the attention from terrestrial organisms to marine organisms and expanded the research conducted in the marine environment to the pharmacological field. Since then, c. 21 500 structurally diverse natural products with different activities have been discovered from marine natural sources (MarinLit database - http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml, accessed 2009), many of them providing the basis for the investigation of new compounds for human use.
During the past 15 years, despite the promising results in the search for new natural drugs, there has been a decrease in the investment of large companies in natural products research (Lam Reference Lam2007). Against this trend in downgrading the effort invested in exploring nature, the percentage of new leads currently and over the last century with direct or indirect origins in naturally occurring compounds is still very high, always exceeding in importance the synthetically derived compounds (Paterson & Anderson Reference Paterson and Anderson2005, Mayer & Gustafson Reference Mayer and Gustafson2006, Harvey Reference Harvey2007, Lam Reference Lam2007, Newman & Cragg Reference Newman and Cragg2007). As an example, an investigation reviewing the new drugs from 1981 to 2006 stated that only 22.2% of the total number of anticancer drugs were synthetic (Newman & Cragg Reference Newman and Cragg2007). Interestingly, several of these future anticancer leads are originally from marine-derived compounds currently in clinical and preclinical trials (Simmons et al. Reference Simmons, Andrianasolo, McPhail, Flatt and Gerwick2005, Mayer & Gustafson Reference Mayer and Gustafson2006).
Marine environments are considered to be the largest potential sources of biodiversity on Earth. Experts estimate that biodiversity in certain marine ecosystems is higher than in tropical rain forests (Haefner Reference Haefner2003). This is probably due to the fact that seas cover about 70% of the Earth surface as well as that life had its origin in the primordial oceans. Furthermore, seas harbour a greater proportion of phyla - some of them exclusively marine - when compared with terrestrial habitats (Clarke & Johnston Reference Clarke and Johnston2003). This appears to be strongly correlated with the possibility of finding new compounds since when searching across phyla, the probability of finding unique classes of compounds is higher than when sampling different species within one phylum (Devlin Reference Devlin1997, Munro et al. Reference Munro, Blunt, Dumdei, Hickford, Lill, Li, Battershill and Duckworth1999).
Many marine organisms are sessile and have no physical mechanism of defence. This could have led them to develop strategies to chemically defend themselves from predators and/or competitors (Amsler et al. Reference Amsler, Iken, McClintock and Baker2001, Simmons et al. Reference Simmons, Andrianasolo, McPhail, Flatt and Gerwick2005). Evidence for the connection between marine biodiversity and the field of marine natural products are well documented. In 2005, 812 new marine compounds were described, an increase of c. 13% on the number of compounds reported the previous year. Interestingly, this increasing trend in the number of new marine chemical compounds has been steady since 1965 (Blunt et al. Reference Blunt, Copp, Hu, Munro, Northcote and Prinsep2007).
Antarctica is amongst the regions that are likely to harbour many new and promising chemical products. Evidence for chemical defensive compounds exist in many Antarctic invertebrate phyla (Blunt et al. Reference Blunt, Munro, Battershill, Copp, McCombs, Perry, Prinsep and Thompson1990, McClintock & Baker Reference McClintock and Baker1997, Lebar et al. Reference Lebar, Heimbegner and Baker2007, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). There are only a few examples in the Antarctic literature of interesting antitumoural/cytotoxic compounds in sponges (Perry et al. Reference Perry, Ettouati, Litaudon, Blunt and Munro1994, Trimurtulu et al. Reference Trimurtulu, Faulkner, Perry, Ettouati, Litaudon, Blunt, Munro and Jameson1994, Fontana et al. Reference Fontana, Ciavatta, Amodeo and Cimino1999), cnidarians (Mellado et al. Reference Mellado, Zubía, Ortega and López-González2004, Reference Mellado, Zubía, Ortega and López-González2005), echinoderms (De Marino et al. Reference De Marino, Iorizzi, Palagiano, Zollo and Roussakis1998), bryozoans (Winston & Bernheimer Reference Winston and Bernheimer1986) and tunicates (Diyabalanage et al. Reference Diyabalanage, Amsler, McClintock and Baker2006, Reyes et al. Reference Reyes, Fernández, Rodríguez, Francesch, Taboada, Avila and Cuevas2008). However, very few Antarctic specimens have been tested to date from the c. 4000 currently described invertebrate species in the Southern Ocean (Clarke & Johnston Reference Clarke and Johnston2003, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). Since it was recently predicted that there must be more than 17 000 macrozoobenthic species inhabiting the entire Antarctic Shelf in the Southern Ocean (Gutt et al. Reference Gutt, Sirenko, Smirnov and Arntz2004), it is reasonable to assume that high percentages of chemical activity may exist in these waters.
We collected and analysed 770 benthic animals (corresponding to at least 290 different species) from 12 different phyla in order to investigate the antitumoural potential of the invertebrates inhabiting the Southern Ocean and adjacent waters,. In this study we present the results of an extensive antitumoural pharmacological screening performed with marine invertebrates from the eastern Weddell Sea, the South Shetland Islands (Antarctica) and the Bouvet Island (sub-Antarctic) areas. The aim of this work is to highlight the antitumoural possibilities that these geographic areas can provide, considering macrozoobenthic organisms from a wide bathymetric range.
Material and methods
Study area and field sampling
Invertebrate benthic marine samples were collected on two different Antarctic cruises: ANTXXI/2 (November 2003–January 2004) and ECOQUIM-2 (January 2006). ANTXXI/2 expedition surveyed mostly the eastern Weddell Sea area (Antarctic) but also the vicinity of Bouvet Island (sub-Antarctic waters). Sampling was performed on board the RV Polarstern, from the Alfred Wegener Institute for Polar and Marine Research (Bremenhaven, Germany), using seven different sampling devices: Agassiz trawl, bongo net, bottom trawl, epibenthic sledge, giant box corer, plankton multinet and Rauschert dredge. A total of 55 stations were sampled ranging from 0–1866 m depth (see Arntz & Brey Reference Arntz and Brey2005 for details). Sorting of the samples was carried out on deck, and invertebrates from different phyla were selected based on the availability of the specimens, the required biomass for the pharmacological tests, and the in situ observations of feasible characters related to the presence of chemical natural products (i.e. absence of physical defences, particular colour and/or smell, absence of epibionts, …). Each sample corresponded to one invertebrate species and every specimen to be chemically analysed was immediately frozen to -20°C. Some individuals from each of the corresponding samples were fixed for later taxonomic identification in the laboratory by specialists on each of the different phyla. In addition, images of live animals were taken when possible for the same purpose.
The ECOQUIM-2 cruise was carried out around Deception Island, Livingston Island and their vicinities (South Shetland Islands) on board the Spanish RV BIO-Hespérides. Two different sampling devices (Agassiz trawl and rocky dredge) were used to obtain the samples at depths from 25–215 m. Dredging range in all stations was not higher than a few metres except for a station where it started at 65 m and finished at 215 m depth. Sorting was performed as described above. Also in this case, specimens to be chemically analysed were frozen at -20°C and the procedure for the later identification of animals with the fixed material was the same as described before.
In vitro tests
All frozen samples from invertebrates collected from both expeditions were analysed by the biopharmaceutical company PharmaMar SA to search for antitumoural activity. Two grammes of frozen samples were extracted in distilled water using an ultraturrax homogenizer. The aqueous extract was decanted and stored at -30°C. The remaining solid pellet was dried using a speed-vac centrifuge and extracted in 1:1 dichloromethane/methanol. The organic extract was also decanted and stored at -30°C. To analyse the putative pharmacological potential of the extracts, equal “weight/volume” amount of each tissue homogenate was assayed in vitro, using three different final concentrations (50, 15 and 5 μg ml-1), against the following human tumour cell lines: HT-29 (ATCC HTB-38) colorectal adenocarcinoma; A-549 (ATCC CCL 185) lung carcinoma; and MDA-MB 231 (ATCC HTB-26) breast adenocarcinoma. Briefly, cells were seeded in 96-well microtitre plates and allowed to stand for 24 h in a drug-free medium before treatment with vehicle alone or test extracts for 72 h period. For viability quantification, a colorimetric assay (sulphurhodamine B, SRB) was used. Cells were washed twice with PBS, fixed for 15 min in 1% glutaraldehyde solution, rinsed twice in PBS, and stained in 0.4% SRB solution for 30 min at room temperature. Cells were then rinsed several times with 1% acetic acid solution and air-dried. SRB was then extracted in 10 mM trizma base solution and the absorbance measured at 490 nm. The cytostatic or cytotoxic effect of the compounds was estimated applying the algorithm developed by the American National Cancer Institute (NCI). Being Tz the number of control cells at time zero, C the number of cells in control wells at 72 h, and T the number of cells in the test wells at 72 h then: if Tz < T < C (no effect or growth inhibition), cell survival is 100 x ([T - Tz]/[C - Tz]); if T < Tz (net cell killing), cell survival is 100 x ([T - Tz]/Tz). Three dose response parameters were calculated for each experimental agent: i) GI50, or compound concentration that produces 50% inhibition on cell growth compared to control cells, ii) TGI, or compound concentration that produces total growth inhibition as compared to control cells, iii) LC50, or compound concentration that produces 50% net cell killing. GI50 is used as reference value. Results represented the mean of at least three independent experiments.
Results
A total of 770 samples (corresponding to at least 290 different species) were collected, 658 from the ANTXXI/2 expedition and 112 from the ECOQUIM-2 cruise. To date, the number of species identified is 260 for the ANTXXI/2 expedition and 61 for the ECOQUIM-2 cruise. A taxonomic list of these species and the number of samples for any given species is reported in Table I. Samples consisted of benthic invertebrates belonging to 12 different phyla: Porifera, Cnidaria, Nemertina, Priapulida, Mollusca, Annelida, Arthropoda, Bryozoa, Brachiopoda, Echinodermata, Hemichordata and Tunicata. Results from the in vitro tests carried out against the three different human tumour cell lines indicated that 19 samples (corresponding to 15 different species) presented relevant antitumoural activity (Tables II & III). This represents the 2.47% of the total number of tested samples, and 5.17% when considering the number of assayed species (290) versus the number of active species (15). In every active sample detected in the study, the three tumour cell lines tested presented a similar behaviour, in the sense that a similar effect for every tumour cell line was detected, except for the tunicate Aplidium cyaneum. In this specific case, the antitumoural effects of the tested fractions were mild for A-549 lung carcinoma and strong for the other two tumour cell lines (HT-29 colorectal adenocarcinoma and MDA-MB 231 breast adenocarcinoma). For the remaining cases, there was no significant difference among the results in the different tumour cell lines assays for every analysed sample (Table II). Antitumour activity was detected in both the aqueous and organic extracts in some of the cases (five out of the 15 species showed activity in both fractions) indicating a distribution of the active metabolites between both extracts, probably due to medium polarity compounds being responsible for the bioactivity. In the rest of the cases, the bioactivity was found only in one of the fractions, most probably indicating that the antitumour properties are due to the presence of very polar compounds (activity found only in aqueous extracts) or non-polar compounds (activity found in organic extracts) (Table II).
Table I Taxonomic list of the species of this survey grouped by phylum. Number of samples used and geographic area in brackets: B = Bouvet Island, S = South Shetland Islands, W = Weddell Sea. + two species tested together, * antitumoral activity detected.

Table II Percentage of cell growth for the active samples against three human tumor cell lines (HT-29, A-549 and MDA-MB 231) at three concentrations (50, 15 and 5 μg ml-1)

aANN = Annelida, CNI = Cnidaria, ECH = Echinodermata, POR = Porifera, TUN = Tunicata.
bSee Fig. 4 for details.
cA = aqueous extract, DM = dichloromethane/methanol extract.
*active extracts are considered when the percentage of cell growth < 50% at least at two concentrations in one of the cell lines. Positive values, in the range between +100 and 0, represent samples with no activity or some degree of cytostatic activity. Negative values, in the range between 0 and -100, represent samples with cytotoxic activity (net cell death).
Samples with antitumoural activity belonged to only five phyla: Porifera, Cnidaria, Annelida, Echinodermata and Tunicata. Considering just the number of species, Tunicata is the group with the higher relative percentage of activity (13.16%), followed by Annelida with more than 9% activity. The last three phyla, in decreasing order of antitumoural activity, are Cnidaria, Porifera and Echinodermata with 7.14%, 5.26% and 4.65% activity, respectively (Fig. 1). A comparison of the relative number of active versus inactive species is shown for each phyla possessing antitumoural activity in Fig. 2. In the analysis of activity by phyla, Tunicata (with five species) contains the largest number of active species, representing more than 33% of the total activity observed in the whole screening. Porifera reaches more than 25% of the activity observed with four active species, while Cnidaria and Echinodermata are the following groups in order of importance, with three and two active species, respectively. Finally, just one Annelid species was found to show antitumoural activity (Table III; Fig. 3).

Fig. 1 Percentages of antitumoural activity (number of species with antitumoural activity respect to the total of species tested) within each active phyla.

Fig. 2 Number of active versus inactive species in phyla presenting activity (N = number of species).
Table III Total number of samples (N spls.) and species (N sps.) analysed by phylum in each surveyed area with the number of active species in brackets

aANN = Annelida, BRY = Bryozoa, CNI = Cnidaria, ECH = Echinodermata, HEM = Hemichordata, MOL = Mollusca, NEM = Nemertina, OTH = others, POR = Porifera, TUN = Tunicata
*This category includes the following phyla: Priapulida, Brachiopoda and Arthropoda
bThe total number of species for every phylum does not correspond to the sum of species for the three geographic areas since some species are shared in the different areas

Fig. 3 Relative proportions of antitumoural activity for each active phylum considering species number.
Bouvet Island (sub-Antarctica)
The four sampling stations studied from the Bouvet Island area yielded 28 different species (32 samples when considering the replicates). Specimens analysed were from seven different phyla (Porifera, Nemertina, Mollusca, Annelida, Bryozoa, Echinodermata and Tunicata) with the Echinodermata the most represented phylum in our survey, with 10 species. The rest of phyla presented five or less species each (Table III). Antitumoural activity was observed in two species: one holothurian (Psolus paradubiosus) and one sponge (Latrunculia brevis). Both samples were collected using Agassiz trawl at depths of 553 and 259 m, respectively (Table IV; Fig. 4). About 50% of the samples analysed in this area were collected at c. 260 m depth. The rest of the samples were collected at three different depths, with the highest percentage of samples concentrated at depths around 375 m and 550 m (Fig. 5).
Table IV Data from the stations where active samples were collected.

aAT = Agassiz trawl, BT = Bottom trawl, ES = Epibenthic sledge, RD = Rocky dredge.
bANN = Annelida, CNI = Cnidaria, ECH = Echinodermata, POR = Porifera, TUN = Tunicata.

Fig. 4 Map representing the stations that presented active samples at the three different surveyed areas: a. Bouvet Island (B1-2), b. South Shetland Islands-Livingston Island (S1-2), and c. eastern Weddell Sea (W1-9).
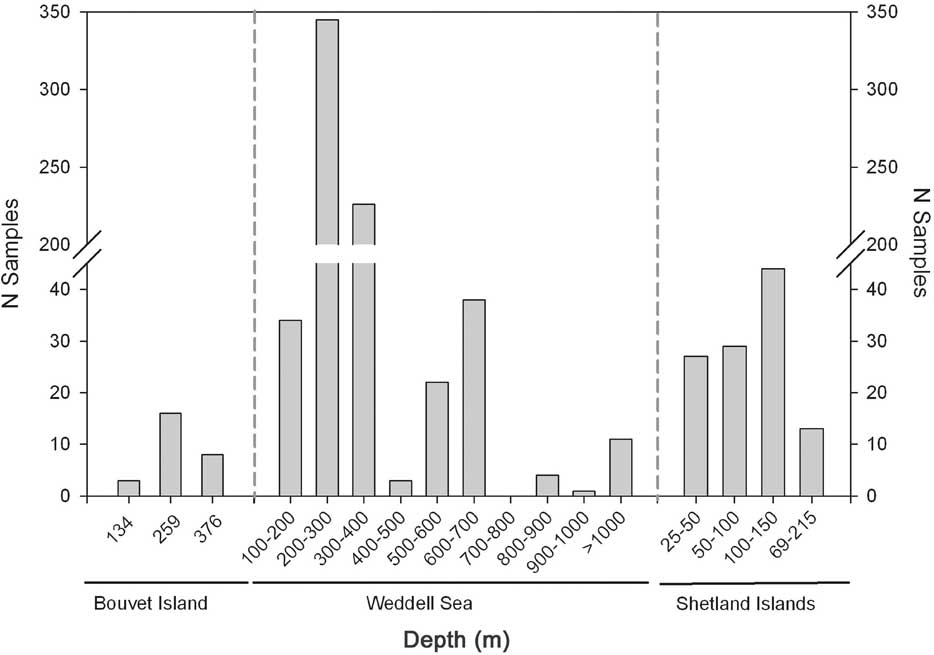
Fig. 5 Number of samples analysed in the stations from the Bouvet Island, the Weddell Sea and the South Shetland Islands area at different depths.
Eastern Weddell Sea (Antarctica)
The eastern Weddell Sea was the largest area sampled, as well as being the most surveyed region. A total of 232 different species (626 samples when considering the replicates) were collected from 51 different sampling stations. Specimens from ten phyla were tested (Porifera, Cnidaria, Nemertina, Mollusca, Annelida, Bryozoa, Brachiopoda, Echinodermata, Hemichordata, and Tunicata). From these, Porifera (4 active species), Cnidaria (3), Tunicata (2), Echinodermata (1) and Polychaeta (1) presented antitumoural activity (Table III). Although the bathymetry of stations ranged from 0 to more than 1800 m, most of the active samples were collected from depths c. 300 m. Only in one deeper station, more than 900 m deep, the cnidarian Fannyella mawsoni presented antitumoral activity (Table IV; Fig. 4). Most samples in this area (> 80%) were collected at depths ranging between 200–400 m (Fig. 5).
South Shetland Islands (Antarctica)
Sampling in the South Shetland Islands yielded 61 different species (112 samples when considering the replicates) belonging to 11 phyla (Porifera, Cnidaria, Nemertina, Priapulida, Mollusca, Annelida, Arthropoda, Bryozoa, Brachiopoda, Echinodermata and Tunicata). A total of 13 sampling stations, ranging from a few metres to more than 200 m depth were surveyed. Only two stations - both in the vicinity of Livingston Island - presented organisms with antitumoural activity; in particular three different tunicate species: Polysyncraton trivolutum, Tylobranchion speciosum and Aplidium falklandicum. These sampling stations were at relatively shallow depths, in the very first 100 m depth (Table IV; Fig. 4). Most of the samples from the South Shetland Islands area were obtained from the first 150 m depth. Only 13 samples were collected from a deeper station that ranged from 69–215 m deep (Fig. 5).
Discussion
The present work is, to the best of our knowledge, the largest pharmacological study ever carried out on Antarctic and sub-Antarctic marine benthic invertebrates. Different studies conducted on sessile marine invertebrates from other areas of the world have proved these organisms to have the highest probability of providing compounds with cytotoxic properties (Schmitz et al. Reference Schmitz, Bowden and Toth1993, Munro et al. Reference Munro, Blunt, Dumdei, Hickford, Lill, Li, Battershill and Duckworth1999). In this sense, our results from the Antarctic and sub-Antarctic areas are consistent with this general trend, since the majority of the pharmacologically active hits (80%) correspond to strict sessile invertebrates belonging to the phyla Porifera, Cnidaria and Tunicata.
There is only one previous study in a comparable geographic area dealing with pharmacological activity in marine invertebrates. Blunt et al. (Reference Blunt, Munro, Battershill, Copp, McCombs, Perry, Prinsep and Thompson1990) investigated a different region (Ross Sea) and restricted their bathymetry range of study to shallow waters (SCUBA diving). Although in their analysis they considered jointly the incidence of antiviral and cytotoxic activity of the different Antarctic phyla, and the number of surveyed benthic species was relatively small (59), it is remarkable that the main active phyla are coincident with our results.
In our survey, two main geographical areas were sampled: sub-Antarctic (Bouvet Island) and Antarctic (eastern Weddell Sea and South Shetland Islands). In the sub-Antarctic area, two different species out of the 28 analysed (7.4%) presented antitumoural properties. In contrast, the percentage of active samples in the Antarctic area reached 5.1% (14 active species out of the 277 species analysed). However, these differences cannot lead us to hypothesize any trend, since there is a significant difference in the sampling effort when comparing both areas. Further analysis should be conducted in order to compare, from the pharmacological point of view, Bouvet Island with the rest of Antarctic samples. This could be especially relevant since this island, situated just south of the Polar Front, is a transitional area considered to be a linking point between the High Antarctic and the adjacent temperate Atlantic ecosystems (Arntz et al. Reference Arntz, Thatje, Linse, Avila, Ballesteros, Barnes, Cope, Cristobo, De Broyer, Gutt, Isla, López-González, Montiel, Munilla, Ramos Esplá, Raupach, Rauschert, Rodríguez and Teixidó2006).
As stated above, the majority of active species (c. 90%) came from the Antarctic area (eastern Weddell Sea and the South Shetland Islands) and this area included also the largest number of bioassayed species (> 90%). The Antarctic benthos is commonly characterized by presenting a very rich and diverse community of sessile suspension feeders (Arntz et al. Reference Arntz, Brey and Gallardo1994, Orejas et al. Reference Orejas, Gili, Arntz, Ros, López, Teixidó and Filipe2000, Clarke & Johnston Reference Clarke and Johnston2003). This community has been quite well surveyed in our case, despite the fact that our sampling was qualitative; the Porifera, Cnidaria, Bryozoa and Tunicata in our study represent > 70% of the whole survey. The environment, below the area of ice scouring, believed to be very old and stable and with a high degree of physical environment predictability, is postulated to be ruled by biological factors (Dayton et al. Reference Dayton, Robilliard, Paine and Dayton1974). Accordingly, it could be expected that marine benthic Antarctic invertebrates (mostly sessile) develop chemical means to defend themselves from predation, inhibition of settling, and prevention of fouling and overgrowth of other species (Amsler et al. Reference Amsler, Iken, McClintock and Baker2001, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). These chemical compounds could be hypothesized to be involved in the antitumoural activity described here.
In our study, Porifera yielded the highest number of pharmacological hits (seven), although it is also true that it was the group with the highest percentage of tested samples (c. 30% of our samples were sponges) and the highest percentage of tested species (c. 30%) (Table II & III; Fig. 2). Among the species found to possess antitumoural activity, there are two species from the genus Latrunculia (L. biformis and L. brevis). Analysis of the biochemical composition of one of our L. brevis specimens (Table IV, PS/65-265-1 station code) confirmed the occurrence of discorhabdins A, C and G and also tsitsikammamine A (Fig. 6; unpublished results from the authors), an alkaloid firstly described in a South African latrunculid sponge (Hooper et al. Reference Hooper, Davies-Coleman, Kelly-Borges and Coetzee1996). Similarly, specimens of L. brevis with its origin in New Zealand and Argentinean waters yielded some antitumoural alkaloids, discorhabdins A, D, L and I (Perry et al. Reference Perry, Blunt, Munro, Higa and Sakai1988, Reyes et al. Reference Reyes, Martín, Rueda, Fernández, Montalvo, Gómez and Sánchez-Puelles2004). In addition, an Antarctic congeneric species, Latrunculia apicalis, was found to possess discorhabdin G located preferentially in the outermost layer of the sponge, where it could cause deterrence against predatory sea stars (Furrow et al. Reference Furrow, Amsler, McClintock and Baker2003). Apart from the latrunculids, two specimens of the genus Rossella (Rossella sp. 1 and sp. 2), still under taxonomic study, also displayed antitumoural activity. To the best of our knowledge, this is the first time that any specimen from the class Hexactinellida (glass sponges provided with long siliceous spicules that can act as a physical defence) is reported to show antitumoural activity. Porifera are one of the major targets of chemical investigations in marine environments due to their high biomass and their well-documented ability to possess interesting natural products (McClintock et al. Reference McClintock, Amsler, Baker and van Soest2005, Blunt et al. Reference Blunt, Copp, Hu, Munro, Northcote and Prinsep2007, Avila et al. Reference Avila, Taboada and Núñez-Pons2008, Peters et al. Reference Peters, Amsler, McClintock, van Soest and Baker2009). There are several examples in the literature providing evidence of pharmacologically active compounds from sponges presenting relevant antitumoural effects from tropical (e.g. Bergmann & Feeney Reference Bergmann and Feeney1951) and temperate waters (e.g. Burres & Clement Reference Burres and Clement1989). As shown in this work, a similar pattern can be expected in the Southern Ocean since this group of invertebrates constitutes a basic element in the benthic ecosystem, both in terms of abundance and in number of described species (Orejas et al. Reference Orejas, Gili, Arntz, Ros, López, Teixidó and Filipe2000, Clarke & Johnston Reference Clarke and Johnston2003). Actually, Antarctic sponges represent the group of invertebrates with the highest number of natural compounds described to date and have been extensively studied in terms of chemical compounds, when compared with the rest of invertebrate groups (McClintock et al. Reference McClintock, Amsler, Baker and van Soest2005, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). Interestingly, some Antarctic sponges have been previously found to present antitumoural activity, with variolin-B, a new alkaloid described from Kirkpatrickia variolosa, (Perry et al. Reference Perry, Ettouati, Litaudon, Blunt and Munro1994, Trimurtulu et al. Reference Trimurtulu, Faulkner, Perry, Ettouati, Litaudon, Blunt, Munro and Jameson1994), and flabellatene A, a new antiproliferative cembrane isolated from Lissodendoryx flabellata (Fontana et al. Reference Fontana, Ciavatta, Amodeo and Cimino1999), the most remarkable ones.

Fig. 6 Chemical compounds found in the samples analysed in the present survey.
In our pool of tested cnidarians three different species were active against the tumour cell lines assayed. The Gorgonacea Fannyella mawsoni is reported for the first time as possessing interesting pharmacological activity. The other two cnidarians (order Gorgonacea) presenting activity are still under taxonomic study. As in sponges, cnidarians also play an important ecological role in Antarctic marine benthic ecosystems (Orejas et al. Reference Orejas, Gili, Arntz, Ros, López, Teixidó and Filipe2000). Although very few species have been studied so far from the chemical point of view (Avila et al. Reference Avila, Taboada and Núñez-Pons2008), there are examples of two different species pertaining to the orders Gorgonacea and Alcyonacea with compounds presenting cytotoxic activity against human tumour cell lines (Mellado et al. Reference Mellado, Zubía, Ortega and López-González2004, Reference Mellado, Zubía, Ortega and López-González2005). Cnidarians are one of the major sources of marine natural products in other geographical areas as well (Schmitz et al. Reference Schmitz, Bowden and Toth1993, Munro et al. Reference Munro, Blunt, Dumdei, Hickford, Lill, Li, Battershill and Duckworth1999).
After the results of our survey, Antarctic Tunicata represent a much more important potential source for pharmacological purposes than previously. In fact, this is the group with the highest percentage of activity in our tests (Fig. 1). Among these interesting results there is one that stands out from the rest: aplicyanins A–F (Fig. 6), new compounds from the ascidian Aplidium cyaneum yielding strong antitumoural activity (Reyes et al. Reference Reyes, Fernández, Rodríguez, Francesch, Taboada, Avila and Cuevas2008). Other examples with a similar relevance such as didemnin B (Rinehart et al. Reference Rinehart, Gloer, Hughes, Renis, McGovern, Swynenberg, Stringfellow, Kuentzel and Li1981) or Ecteinascidin (Rinehart et al. Reference Rinehart, Holt, Fregeau, Stroh, Keifer, Sun, Li and Martin1990), both derived from tropical ascidians, highlight the importance of these animals in the context of the marine drug discovery field. In our study, two of the tunicates found to be active against tumour cell lines, belong to the genus Aplidium (A. cyaneum and A. falklandicum). This genus has been widely recognized as a source of antitumoural compounds in different areas of the world (McKee et al. Reference McKee, Galinis, Pannell, Cardellina, Laakso, Ireland, Murray, Capon and Boyd1998, Le Tourneau et al. Reference Le Tourneau, Raymond and Faivre2007). Another species to highlight is Polysyncraton trivolutum, from the family Didemnidae. This family is also recognized as a source of chemical products with potent biological properties (e.g. Rinehart et al. Reference Rinehart, Gloer, Hughes, Renis, McGovern, Swynenberg, Stringfellow, Kuentzel and Li1981) and a congeneric species from the Fiji Islands, P. lithostrotum, also displays relevant antitumoural effects (McDonald et al. Reference McDonald, Capson, Krishnamurthy, Ding, Ellestad, Bernan, Maise, Lassota, Discafani, Kramer and Ireland1996). On the other hand, this is the first time that the ascidian Tylobrachion speciosum is reported as a source for antitumoural activity. Tunicates, together with bryozoans and the above-mentioned sponges and cnidarians, all of them being sessile suspension feeders, conform the basis of the Antarctic benthic ecosystems (Orejas et al. Reference Orejas, Gili, Arntz, Ros, López, Teixidó and Filipe2000). Nevertheless, little chemical work has been conducted to date in tunicates from the Southern Ocean (Avila et al. Reference Avila, Taboada and Núñez-Pons2008). It is also worth mentioning the antitumoural activity described in the Antarctic ascidian Synoicum adareanum (Diyabalanage et al. Reference Diyabalanage, Amsler, McClintock and Baker2006).
Only bryozoans seem not to follow the suggestion that sessile marine invertebrates have a high probability of showing cytotoxic activities (Schmitz et al. Reference Schmitz, Bowden and Toth1993, Munro et al. Reference Munro, Blunt, Dumdei, Hickford, Lill, Li, Battershill and Duckworth1999), since none of the 53 species assayed here showed any activity (this group was the second in number of tested species and also the second in number of samples analysed; see Table III). Although they are very speciose and abundant in Antarctic waters (Orejas et al. Reference Orejas, Gili, Arntz, Ros, López, Teixidó and Filipe2000, Clarke & Johnston Reference Clarke and Johnston2003), they have been little studied from a chemical perspective (Avila et al. Reference Avila, Taboada and Núñez-Pons2008) and, to the best of our knowledge, there is only one reported case of Antarctic cytotoxic activity (haemolytic activity against erythrocytes from man and dog) in the bryozoan Carbasea curva (Winston & Bernheimer Reference Winston and Bernheimer1986). Nevertheless, there are examples from other marine geographical areas such as the cosmopolitan Bugula neritina, which possesses bryostatin 1, one of the strongest naturally derived antitumoural compounds known to date (Pettit et al. Reference Pettit, Herald, Doubek and Herald1982).
Non-sessile invertebrates are usually considered less likely groups in which to find cytotoxic compounds (Munro et al. Reference Munro, Ludibrand and Blunt1987). However, in our survey, there were some vagile invertebrates showing interesting antitumoural activity. Two echinoderm species (holothurians), Psolus paradubiosus and Taenyogytus contortus, presented antitumoural activity. These are not exceptional cases for these slow and soft-bodied organisms since echinoderms have been reported to have a remarkable incidence in cytotoxic activity in other areas of the world (Schmitz et al. Reference Schmitz, Bowden and Toth1993, Munro et al. Reference Munro, Blunt, Dumdei, Hickford, Lill, Li, Battershill and Duckworth1999). This highly diverse group of Antarctic invertebrates (Clarke & Johnston Reference Clarke and Johnston2003) has also been extensively studied for their chemical ecology (Amsler et al. Reference Amsler, Iken, McClintock and Baker2001, Avila et al. Reference Avila, Taboada and Núñez-Pons2008) yielding a large number of natural products. Among them, at least one of the species analysed (an unidentified sea star from the family Asteriidae) has been observed to possess compounds with cytotoxic activity against human carcinoma cells (De Marino et al. Reference De Marino, Iorizzi, Palagiano, Zollo and Roussakis1998).
Annelids are the other group of invertebrates presenting antitumoural activity in this study. The active species belongs to the family Terebellidae. Terebellids are sessile deposit feeders that live attached to the substrate protected by a tube. We know of only one precedent of an annelid with antitumoural activity: the case of Terebella sp. (also from the family Terebellidae) showing a mild antitumour activity against P388 murine leukaemia cell line (Battershill et al. Reference Battershill, Blunt, Barns and Dale1989). As reported for echinoderms, annelids also represent a high percentage of the invertebrate biodiversity in Antarctica; actually, they are the most speciose group in the Antarctic benthos (Clarke & Johnston Reference Clarke and Johnston2003). However, they have been barely studied from the chemical point of view (Lebar et al. Reference Lebar, Heimbegner and Baker2007, Avila et al. Reference Avila, Taboada and Núñez-Pons2008), and it seems probable that further positive results may appear for this group.
Other phyla have also displayed antitumoural activity in other marine areas. Examples such as dolastatins in molluscs (Pettit et al. Reference Pettit, Kamano, Herald, Tuinman, Boettner, Kizu, Schmidt, Baczynskyj, Tomer and Bontems1987) or cephalostatins in pterobranchs (Pettit et al. Reference Pettit, Xu, Williams, Christie, Doubeck and Schmidt1994) are just two of the many examples that can be found in the literature. Thus, we may hypothesize that the chances of finding interesting active chemicals in these and other groups in future analyses in Antarctica are reasonably high.
Sampling depth is also an important variable to take into account when bioprospecting. It was mentioned that there seems to be a greater probablility of finding cytotoxicity in animals at depths greater than 30 m (Munro et al. Reference Munro, Ludibrand and Blunt1987). In our survey, samples showing antitumoural activity were predominately found in depths ranging from 250–500 m in the eastern Weddell Sea area and the Bouvet Island vicinities, and c. 100 m depth in the South Shetland Islands area (Fig. 5). Since our study was qualitative and the sampling effort was clearly biased to some depths (Fig. 5), in our case no further inferences can be drawn when evaluating depth as a factor related to bioactivity.
As explained above, the collection of our samples was supported by a qualitative sampling design in order to maximize the return for the effort invested. Although the sampling effort was, therefore, clearly biased to some particular groups, we believe that results in terms of pharmacological activity are similar to what could be expected after a quantitative sampling, since samples tested were, in general, the most representative organisms in each station. In a similar way, the different number of hits registered in the three major areas studied are proportionally correlated with the sampling effort (eastern Weddell Sea > South Shetland Islands > Bouvet Island); this leads us to hypothesize that the study area was not a decisive factor in our survey.
In our study, two samples identified as the same species (Latrunculia brevis) had a similar pharmacological behaviour although they were collected in different areas - Bouvet Island and eastern Weddell Sea (Table II). This species has also been reported to display antitumoural effects in other nearby geographical areas such as South America and New Zealand (Perry et al. Reference Perry, Blunt, Munro, Higa and Sakai1988, Reyes et al. Reference Reyes, Martín, Rueda, Fernández, Montalvo, Gómez and Sánchez-Puelles2004). It is common that individuals from the same species possess similar activity regardless of the geographical area, as it is the case of the ascidian Ecteinascidia turbinata (Munro et al. Reference Munro, Ludibrand and Blunt1987). However, occasionally, individuals of the same species but from different geographical locations may possess distinctly different activity. An example is Bugula neritina, a bryozoan only found to present bryostatin 1 in certain geographical areas (Pettit Reference Pettit1991). In our survey, we also found species that showed antitumoural activity in one sampling station and did not display any antitumoural effect in the rest of the stations where they were collected. These are the cases of the holothurian Taeniogytus contortus (only one out of four replicates analysed showed antitumoural activity), and the tunicates Aplidium falklandicum (only the sample collected in the South Shetland Islands displayed antitumoural activity), Polysincraton trivolutum (one replicate out of the three with activity) and Tylobranchion speciosum (one replicate out of the two with activity) (Table I). Whether this situation is common or rare in nature is still to be established, and it could be related, among other reasons, to the presence of symbionts (Faulkner et al. Reference Faulkner, Harper, Haygood, Salomon and Schmidt2000, König et al. Reference König, Kehraus, Seibert, Abdel-Lateff and Müller2006). We suggest, therefore, that it is important to bioprospect different areas even when sampling similar or the same species, since unexpected results may be obtained.
Since the beginnings of marine pharmacological studies in the 1950s, this discipline has mainly focused on tropical areas and, to a lesser extent, on temperate regions (Bergmann & Feeney 1951, Dietzman Reference Dietzman1996, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). Polar regions have received much less attention, in part due to the difficulties of prospecting in these remote areas and in part also due to the traditional and incorrect belief that they hold low marine chemical diversity (challenged by Amsler et al. Reference Amsler, McClintock and Baker2000). Results of this and previous works in the field of chemical ecology are uncovering a very promising future in the search for new leads in the Southern Ocean (Lebar et al. Reference Lebar, Heimbegner and Baker2007, Avila et al. Reference Avila, Taboada and Núñez-Pons2008). Since only c. 25% of Antarctic fauna has been described so far (Gutt et al. Reference Gutt, Sirenko, Smirnov and Arntz2004) and just a tiny part of it has been tested for biological activity (Avila et al. Reference Avila, Taboada and Núñez-Pons2008), it can be assumed that natural products in this area will continue providing novel bioactive chemical structures. Furthermore, due to the particular characteristics of the Southern Ocean, which has been physically isolated from the surrounding oceans for 34 million years (Tripati et al. Reference Tripati, Backman, Elderfield and Ferretti2005), the chances of finding totally novel natural products seem to be higher in this area than in other parts of the world. Natural products are proving to be the most reliable way to find solutions to current and future human diseases (Amsler et al. Reference Amsler, Iken, McClintock and Baker2001, Newman & Cragg Reference Newman and Cragg2007) and many new compounds wait to be discovered. As in an iceberg, which we believe to be very appropriate in this context, for chemical studies in general and pharmacological studies in particular, one could say that only the tip has been discovered so far.
Acknowledgements
Thanks are due to the crews of the two research vessels used for sampling: RV Polarstern and BIO-Hespérides, as well as to W. Arntz and the Bentart team. Thanks are also due to the taxonomists helping in the identification of samples: F.J. Cristobo and P. Ríos (Porifera), L. Núñez-Pons, B. Figuerola and M. Edo (Cnidaria and Bryozoa), A. Ramos and M. Varela (Tunicata), and M. Ballesteros, A. Bosch and N. Campanyà (Echinodermata). The useful comments of J. Blunt, A. Riesgo and F. Reyes, and the help in the creation of Fig. 4 by F.J. Cristobo are also gratefully acknowledged. Financial support of ECOQUIM and ACTIQUIM projects (REN2003-00545, REN2002-12006E/ANT, CGL2004-03356/ANT, CGL2007-65453/ANT) is acknowledged as well. This manuscript has greatly benefited from the very helpful comments of an anonymous reviewer.












