INTRODUCTION
Eunice sebastiani Nonato, Reference Nonato1965, is currently regarded as an endangered species (D.O.U., 2004) because of its widespread use as fish bait (Amaral & Jablonski, Reference Amaral and Jablonski2005). This species was described by using large specimens collected at Araçá beach, São Sebastião, in south-east Brazil. Nonato (Reference Nonato1965) examined ten specimens and designated one complete specimen as type material; it was 208 cm long and 2 cm wide in the anterior region. He stated that the new species resembled E. aphroditois (Pallas, 1788), and E. roussaei de Quatrefages, 1866, but that it could be distinguished by the different shape of anterior and median notopodial cirri, and by the form of the maxillary apparatus. In the latter, he noticed that the maxillae V was either uni- or bidentate. Further, although he described the subacicular hooks for the new species as unidentate, in different parts of his description he considered the species belonging to the group ‘fuscus-bidentate’, which includes those species with black bidentate subacicular hooks, not with black unidentate subacicular hooks, as is the case in E. sebastiani. Because the type materials were not available, for the monograph on Eunice, Fauchald (Reference Fauchald1992) studied and characterized the species based on two large specimens, one coming from the type locality, but he decided to use the other one for the re-description. He described the species as having maxillae V unidentate, and black unidentate subacicular hooks, but quoted that this species was originally described with bidentate subacicular hooks, and he considered that this attribute could be present in smaller specimens.
On the other hand, Eunice riojai de León-González, Reference de Léon-González1988 was described from the north-western Caribbean Sea, based upon a single specimen collected in sponges. The holotype was 12.6 cm long and was characterized with black unidentate subacicular hooks, thus belonging to the ‘fuscus-unidentate’ group, and it was further distinguished from the other species in the group because of the early starting of branchiae and subacicular hooks. Although the holotype was deposited in the Smithsonian Institution, it was not included for the monograph of the genus (Fauchald, Reference Fauchald1992: 415).
In a series of publications about the Grand Caribbean eunicids (Carrera-Parra & Salazar-Vallejo, Reference Carrera-Parra and Salazar-Vallejo1998a, Reference Carrera-Parra and Salazar-Vallejob) there were explicit or implicit indications that these two species were synonyms. The finding of specimens of E. sebastiani collected in the type locality, has allowed us to rectify this viewpoint. The comparative study of materials belonging to these species has allowed us to have a better understanding of the morphological differences and now we regard them as distinct species. Furthermore, the examinations of these specimens allowed us to re-evaluate some features described by Nonato (Reference Nonato1965), and Fauchald (Reference Fauchald1992) to have a better understanding of the species. Herein, we provide complete descriptions and illustrations for both species.
MATERIALS AND METHODS
Type and non-type materials were borrowed from the collection of the Museu de Zoologia, Universidade de São Paulo, Brazil (MZUSP); the Museu de História Natural, Universidade Estadual de Campinas, Brazil (ZUEC); the Coleccion de Referencia, ECOSUR-Chetumal, Mexico (ECOSUR); and the National Museum of Natural History Smithsonian Institution, Washington (USMN). When the maxillary apparatus was not exposed or previously dissected, an anterodorsal dissection was made to extract it, and was mounted on a glass slide to be examined. After doing this, the maxillary apparatus was returned to the original position. The morphological details follow previous formats (Carrera-Parra & Salazar-Vallejo, Reference Carrera-Parra and Salazar-Vallejo1998a) The measurements include the total length (LT), but because almost all specimens are incomplete, the measurements were standardized for length through chaetiger 10 (L10), and width at chaetiger 10 (W10).
SYSTEMATICS
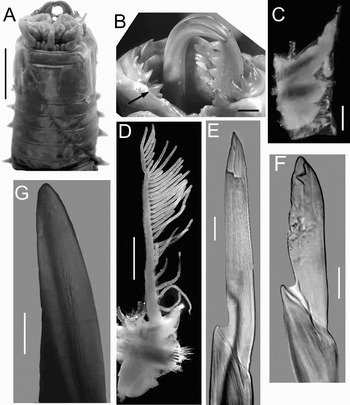
Fig. 1. Eunice sebastiani (neotype). (A) Anterior end, dorsal view; (B) maxillary apparatus, dorsal view, arrow points to maxilla V left; (C) parapodium 3; (D) parapodium 100; (E) compound falciger, chaetiger 3; (F) compound falciger, chaetiger 682; (G) subacicular hook, chaetiger 682. Scale bars: A, 7 mm; B, C, 1 mm; D, 2 mm; E, 23 µm; F, 18 µm; G, 30 µm.
References: Nonato, Reference Nonato1965: 133–138, figures 1–4. Fauchald, Reference Fauchald1992: 299, 302–303, figure 102.
Material examined
Type material: neotype MZUSP0901(1), Praia do Araça, São Sebastião, SP, Brazil; 23º48′55″S 45º24′29″W; coll. E.A. da Silva; 1996. Paraneotypes, ZUEC 0680(1), Praia do Araça, São Sebastião, SP, Brazil; 23º48′55″S 45º24′29″W; coll. E.A. da Silva; 1996. ZUEC 0681(1), Praia Saco da Ribeira, Ubatuba, Brazil; 23º30′15″S 45º07′26″W; coll. E.F. Nonato.
Characteristics
Neotype almost complete with 684 chaetigers: LT = 145 cm, L10 = 3.5 cm, W10 = 1.7 cm. Anterior part of body rounded, posteriorly depressed, flat. Prostomium distinctly shorter than peristomium, prostomial lobes frontally rounded, divided obliquely, apparently articulated; median sulcus deep. Without eyes. Prostomial appendages in a horse-shoe shaped pattern, median antenna isolated by a gap. Palps to first peristomial ring; lateral antennae to posterior end of peristomium; median antenna to second peristomial ring. Palpophore long, ceratophores short; palpostyle and ceratostyles smooth, non-articulated; pigmented with dark bands (Figure 1A). Peristomium with well-developed ventral lips; first ring five times longer than second ring, separation visible ventrally, weakly dorsally; peristomial cirri tapering, short, to posterior end of first peristomial ring, without articulation. Maxillary apparatus with five pairs and one single maxillae; MF = 1+1, 7+6, 5+0, 8+9, 2+2, 1+1. MII with large teeth tapering, distal tooth isolated, largest; MIII long behind MII; MIV left with distal five teeth small, but terminal three very short; MV bidentate, with distal tooth largest (Figure 1B); MVI reduced to edentate plate. Branchiae pectinate, distinctly longer than notopodial cirri; beginning from chaetiger 14 to last chaetiger of specimen, with up to 36 branchial filaments at about chaetiger 115 to 300. Parapodia 1–4 slightly displaced ventrally; all parapodia with inconspicuous prechaetal lobe, chaetal lobe in anterior parapodia split in two lobes, in posterior parapodia entire; postchaetal lobe rounded, as long as chaetal lobe in all chaetigers. Notopodial cirri without articulation, tapering with a swollen expansion near their bases, better developed in anterior chaetigers, decreasing gradually in median and posterior chaetigers. Ventral cirri in chaetigers 1–5 conical; from chaetiger 6 to last chaetiger with a globular base inflated and digitiform tips, better developed in anterior chaetigers (Figure 1C, D). Chaetae limbate supraciculars; pectinates heterodonts with up to 18 teeth. Compound falcigers bidentate, in anterior chaetigers with long blade, distal tooth directed upward; in posterior chaetigers with blade slightly shorter (Figure 1E, F). Aciculae black, blunt; up to five in anterior chaetigers, three in posterior ones. Subacicular hooks black, always unidentate, tapering, slightly curved (Figure 1G), starting in chaetiger 63, with up to two or three per bundle, rarely four; only one in far posterior chaetigers. Pygidium unknown.
Variation
L10 = 3.5–3.8 mm, A10 = 1.2–1.7 mm. The start of subacicular hooks varies from chaetiger 51 to 63, while the start of branchiae varies from chaetiger 8 to 13. Although there were few specimens, these features seem to be size-dependent. The number of teeth in some maxillae varies as follows: MII 6–7 + 6–8, MIII 5–7, MIV right 8–10.
Remarks
A neotype is designated because: (1) the type material was lost in a fire (Nonato, 2007, personal communication); (2) some morphological features were confused between the original and the later re-description; and (3) the species is endangered and its morphology and taxonomy is herein clarified.
Distribution
Apparently restricted to the type locality and surrounding areas, living in muddy or sandy beaches.

Fig. 2. Eunice riojai (ECOSUR). (A) Anterior end, frontal view; (B) anterior end, lateral view; (C) maxillary apparatus, dissected, arrow points to maxilla V left; (D) parapodium 3; (E) parapodium 49; (F) compound falciger, chaetiger 3; (G) compound falciger, chaetiger 179; (H) subacicular hook, chaetiger 179; (I) subacicular hooks, chaetiger 162 (holotype). Scale bars: A, 1 mm; B, 3 mm; C, 0.7 mm; D, 0.6 mm; E, 1.5 mm; F, G, 21 µm; H, 25 µm; I, 6 µm.
References: de León-González, 1988: 75–78, figures 1 & 2. Carrera-Parra & Salazar-Vallejo, Reference Carrera-Parra and Salazar-Vallejo1998b: 1505, figure 10a–f (as Eunice sebastiani non Nonato).
Material examined
Type-material: holotype USNM105342, Isla Pérez, Yucatán, Mexico, June 1979, in sponge (damaged after previous dissection). Non-type material: ECOSUR EUNI-24 Quintana Roo: AVE5(4), Playa Aventura, in drifting wood cast ashore, 23 March 1996. Xahuayxol(1), in coralline rock, 27 November 2005, 1 m. XCA2(1), Xcacel, in drifting wood cast ashore, 16 April 1996 (used for re-description). Playa Paraíso, Cozumel(2), in coralline rock, 27 November 1996, 2 m.
Characteristics
Best specimen complete, with 189 chaetigers; L10 = 1.4 cm, W10 = 0.85 cm, LT = 16 cm. Prostomium as long as peristomium, prostomial lobes frontally rounded, divided obliquely, seem to be biarticulated (Figure 2A); median sulcus shallow. Eyes large, rounded, behind palps. Prostomial appendages in a horse-shoe shaped pattern, median antenna isolated by a gap. Palps to half of peristomium; lateral and median antennae to chaetiger 1. Palpophore long, ceratophores short; palpostyle and ceratostyles smooth, non articulated; all pigmented with dark bands (Figure 2B). Peristomium with first ring five times longer than second ring, separation between rings dorsal and ventral; peristomial cirri short, to half of first peristomial ring, without articulation, pigmented with dark bands. Maxillary apparatus with five pairs and one single maxillae; MF = 1+1, 7+5, 6+0, 5+8, 1+1, 1+1; MIII part of distal arc; MVI reduced to an edentate plate (Figure 2C). Branchiae pectinate, longer than notopodial cirri; present on chaetigers 6 to 187, with up to 20 branchial filaments at about chaetiger 49. All parapodia with inconspicuous pre- and postchaetal lobes, and entire, truncated chaetal lobe. Notopodial cirri without articulation, tapering with a swollen expansion near their bases, better developed in anterior chaetigers, decreasing gradually in median and posterior chaetigers. Ventral cirri in chaetigers 1 to 4 short, conical; from chaetiger 5 to the end of body with a globular base inflated and digitiform tips (Figure 2D, E). Chaetae limbate supracicular; pectinates heterodonts with up to 19 teeth. Compound falcigers bidentate, in anterior chaetigers with long blade, in posterior chaetigers with stout, short blade; distal tooth larger than proximal, curved (Figure 2F, G). Aciculae black, blunt; stout in posterior chaetigers. Subacicular hooks black, unidentate, distally curved (Figure 2H), starting in chaetiger 32, with up to three per bundle, usually two; in far posterior chaetigers (beyond the 177th) with subacicular hook bidentate (Figure 2I). Pygidum with two pairs of anal cirri, without articulation; dorsal cirri as long as the last six chaetigers, ventral cirri very short, barely visible.
Variation
L10 = 5.0–8.5 mm, A10 = 3.0–7.0 mm. The start of subacicular hooks varies from chaetiger 28 to 35 (32 in holotype) and it is size-dependent. Only the smallest specimen has branchiae from chaetiger 6. All complete specimens have bidentate subacicular hooks in at least the last 15–20 chaetigers (last 16 in holotype). The number of teeth in some maxillae varies as follows: MII 6–8+6–7, MIII 6–7, MIV right 9–11, (FM holotype: 1+1, 8+7, 7+0, 5+11, 1+1, 1+1).
Distribution
Associated with sponges on shallow subtidal rocky bottoms or in drifting wood cast ashore around the Yucatan Peninsula, north-western Caribbean Sea, and southern Gulf of Mexico.
DISCUSSION
The species of Eunice have been traditionally organized in different groups mainly based upon the colour and number of teeth of subacicular hooks (Hartman, Reference Hartman1944; Fauchald, Reference Fauchald1970). As stated above, Nonato (Reference Nonato1965) indicated that in E. sebastiani subacicular hooks were black unidentate, but he attributed the species to the flavus-bidentate group, which includes species with black bidentate subacicular hooks. Fauchald (Reference Fauchald1992) considered that this kind of hook appears to be present in smaller specimens. The materials examined by Fauchald (Reference Fauchald1992) were two large specimens and he only described the presence of unidentate subacicular hooks; herein, we have also examined large specimens, but in all cases, there are no bidentate subacicular hooks, even in the more posterior chaetigers. In some Eunice species that might become very large, the shape of the subacicular hooks tend to change during growth, being bidentate in juveniles and unidentate in adults; but for E. sebastiani, the adults retain unidentate subacicular hooks even in the most posterior chaetigers. A different pattern is found in E. riojai since in all the complete specimens there are bidentate subacicular hooks in the more posterior chaetigers. There are five other Eunice species with black unidentate subacicular hooks: E. fucata Ehlers, 1887 (Florida), E. unidentata Rioja, 1962 (Baja California Sur, Mexican Pacific), E. sonorae Fauchald, Reference Fauchald1970 (Sonora, Mexican Pacific), E. donathi Carrera-Parra & Salazar-Vallejo, Reference Carrera-Parra and Salazar-Vallejo1998b (Mexican Caribbean) and E. marenzelleri Gravier, 1900 (Red Sea). However, E. riojai and E. sebastiani differ from the others by having prostomial lobes obliquely divided, seeming articulated, while all the other species have entire prostomial lobes.
Nonato (Reference Nonato1965) described a very early starting of subacicular hooks (from chaetiger 7), but this is actually starting in more posterior chaetigers since we found they start by chaetigers 51–63, and Fauchald (Reference Fauchald1992) likewise found them in chaetigers 51 and 56. On the other hand, Nonato indicated that eyes were inconspicuous and Fauchald (Reference Fauchald1992) made no reference to them; we have concluded that there are no eyes in this species, or at least on the largest members of it.
Some major discrepancies stem from the details for the maxillary apparatus. Thus, Nonato (Reference Nonato1965) indicated eight teeth on the left maxilla IV, but Fauchald only reported three teeth. We conclude that there are eight teeth on the left maxilla IV, but the discrepancy is due to their relative size since the five basal teeth are very small. Further, Fauchald (Reference Fauchald1992) reported that the maxilla V was unidentate, but it is bidentate, with a very large proximal tooth and a smaller distal one. This bidentate condition is only known for this species; all described Eunice species have unidentate maxillae V.
Carrera-Parra & Salazar-Vallejo (Reference Carrera-Parra and Salazar-Vallejo1998a,b) based upon the re-description by Fauchald (Reference Fauchald1992), and following the idea that E. sebastiani might have bidentate subacicular hooks in smaller specimens, as those found in the Mexican Caribbean, considered E. riojai as a junior synonym of E. sebastiani. This deserves rectification. Herein, after the examination of Brazilian specimens, we conclude that both species are different. Thus E. riojai can be easily distinguished from E. sebastiani because the former has a pair of large eyes, the maxilla III is short, forming part of the distal arc, the maxillae V are unidentate; and compound falcigers with distal tooth curved; while E. sebastiani has no eyes, the maxilla III is long and placed behind maxillae II, the maxillae V are bidentate, compound falcigers with distal tooth directed upward, and the body is dorsoventrally flattened posteriorly. Furthermore, the presence of bidentate subacicular hooks is only confirmed for juveniles of E. riojai and retained in more posterior chaetigers in the adults, but completely missing in E. sebastiani. Then, the distribution of E. sebastiani is restricted to the south-east region of Brazil, and E. riojai is distributed along the Mexican Caribbean shores.
ACKNOWLEDGEMENTS
We thank A. Cecilia Z. Amaral (Unicamp), Edmundo F. Nonato (IO/USP) and Kristian Fauchald (USNM) for making available the materials that made this study possible. We also thank Humberto Bahena-Basave (ECOSUR) for his help in the processing and editing of photographs. Funding for this research was supplied by CONACYT (SEMARNAT-2004-01-C01-00066).




