Introduction
The oriental armyworm Mythimna separata Walker (Lepidoptera: Noctuidae) is a major migratory pest in East Asia, South Asia and Australia (Chen and Bao, Reference Chen and Bao1987; Chen et al., Reference Chen, Bao, Drake, Farrow, Wang, Sun and Zhai1989; Sharma et al., Reference Sharma, Sullivan and Bhatnagar2002). It severely damages cereal crops and pasture grasses which yields great losses (Han and Gatehouse, Reference Han and Gatehouse1991; Wang et al., Reference Wang, Zhang, Ye and Luo2006). This pest has caused six nationwide outbreaks in 1970s in China (Wang et al., Reference Wang, Zhang, Ye and Luo2006; Jiang et al., Reference Jiang, Luo, Zhang, Sappington and Hu2011) and several local outbreaks in North China since 2010 (Jiang et al., Reference Jiang, Zhang, Yang, Sappington, Cheng and Luo2016; Xiao et al., Reference Xiao, Fu, Liu and Wu2016). Its powerful flight capability (Chen et al., Reference Chen, Bao, Drake, Farrow, Wang, Sun and Zhai1989; Feng et al., Reference Feng, Zhao, Wu, Wu, Wu, Cheng and Guo2008), high fecundity (Li et al., Reference Li, Gong and Wu1992, Reference Li, Xu, Ji and Wu2018) in the adult stage, and high feeding levels in the late larval stages (Lin, Reference Lin1990) make outbreaks difficult to predict and prevent.
The oriental armyworm undertakes four large-scale long-distance-migrations in the east part of China: two northward displacements in spring and early summer, and two southward displacements in summer and autumn (Li et al., Reference Li, Wang and Hu1964; Jiang et al., Reference Jiang, Luo, Zhang, Sappington and Hu2011). Migration of insects requires mobilization of energy and synchronization with other life functions such as reproduction (Dingle, Reference Dingle2014). The substances and energy for migration in adults are accumulated in immature stages and are affected by biotic and abiotic factors during pre-adult developmental stage. Temperature is one of the most important abiotic factors that determines adaptive strategies of migratory insects (Dingle, Reference Dingle2014). For M. separata, temperature limits their geographic distribution, and they cannot overwinter in the northern part of China (north of 33°N). Escaping from high temperatures in South China in spring and summer, and from low temperatures in North China in autumn and winter, is considered to be an important pressure driver of the annual roundtrip migration of M. separata (Jiang et al., Reference Jiang, Luo, Zhang, Sappington and Hu2011).
Life tables provide comprehensive information on the survival, development and reproduction of a population under various conditions (Yang and Chi, Reference Yang and Chi2006). Previous laboratory experiments have studied the life history traits of M. separata under different temperatures (Li et al., Reference Li, Gong and Wu1992, Reference Li, Xu, Ji and Wu2018; Jiang et al., Reference Jiang, Luo, Zhang, Sappington and Hu2011). The insects showed the highest adult survival rate and fecundity at 24°C, and the fecundity decreased at 32°C (Li et al., Reference Li, Gong and Wu1992). Life table and flight mill studies have suggested that moths have the highest flight potential at 27°C, but reproductive development and longevity declined compared with 24°C. Both flight potential and reproduction were suppressed at 30 and 33°C (Jiang et al., Reference Jiang, Liu, Luo and Hu1998, Reference Jiang, Luo and Hu2000). The fecundity and population parameters decreased at 20 and 30°C before, after pupation, and through the whole life cycle, compared with constant 25°C. Massive mortalities were observed in the pupal stage when eggs and larvae were reared at 30°C (Li et al., Reference Li, Xu, Ji and Wu2018). However, it was suggested that developmental times were distinct between those predicted from constant temperatures and those under alternating temperatures around the same mean or natural fluctuating temperatures (Mironidis and Savopoulou-Soultani, Reference Mironidis and Savopoulou-Soultani2008; Carrington et al., Reference Carrington, Armijos, Lambrechts, Barker and Scott2013; Kjærsgaard et al., Reference Kjærsgaard, Pertoldi, Loeschcke and Blanckenhorn2013); therefore, life table studies under constant temperature are not realistic enough to reflect physiology under natural fluctuating conditions. Although increasing attention has been paid to life history traits under diurnal fluctuating temperatures, rarely have outdoor experiments been undertaken due to the inherent difficulties of field experiments (Tuan et al., Reference Tuan, Lee and Chi2014; Chen et al., Reference Chen, Xu, Li, Wu and Xu2019).
In the current study, we collected life table data for M. separata reared outdoors from 15 April to 17 October 2018. We compared the demographic parameters of M. separata in four consecutive generations using the age-stage, two-sex life table program (Chi, Reference Chi2018), which shows the stage overlaps governed by developmental rate among individuals and the contribution of males to the population. This research will provide supportive data for analyzing population fluctuations of M. separata under seasonally fluctuating abiotic conditions.
Materials and methods
Insect rearing
Larvae of the oriental armyworms were collected from maize fields in Xingping, Shaanxi Province, China in early July 2014. The population was successively reared in growth chambers (MGC-450HP-2, Shanghai Yiheng Science Instruments Ltd, Shanghai) at 25 ± 1°C, 75 ± 10% RH, and a photoperiod of 14:10 (L:D) (Li et al., Reference Li, Xu, Ji and Wu2018). The parent generations of larvae were reared on fresh wild oats (Avena fatua L.). The parent pupae were moved to outdoor in late March in 2018 in Yangling, Shaanxi, China. We collected the emerged adults, paired them, and provided them with 5% honey solution from 6 to 8 April. We used the eggs produced on 15 April for the life table studies.
Life table study
Fresh eggs laid within 24 h for life table studies were collected on 15 April, 7 June, 10 July, and 15 August in spring, early summer, summer, and autumn, respectively. Newly hatched larvae in different seasons were counted. A total of 49, 100, 98, and 89 larvae on 22 April, 10 June, 12 July, and 17 August, respectively, were selected for life table studies. The insects were reared in transparent plastic cups (dbottom = 5.0 cm, dtop = 6.5 cm, and h = 8.0 cm). Cups were covered with a transparent plastic film with fine needle holes. Larval density was less than 10 per cup from first to forth instars and less than 5 per cup for the fifth to sixth instars. All larvae were fed from 14:30 to 16:00, except for the sixth instar larvae that were additionally fed at 8:00 to ensure sufficient feeding. The mature larvae were transferred to new cups for pupation with peat soil covering the bottom. We distinguished the sex of 2 day old pupae and weighed them on a 1/10,000 analytical balance (AYU220, Shimadzu, Japan). Dark pupae that did not react to touch were considered dead. Adults were paired within 24 h after eclosion. Each pair was placed in a new plastic cup and covered with a plastic film with needle holes as were the larvae. Cotton balls soaked in a 5% honey solution were supplied for food for the adults and folded paper for oviposition. Survival and fecundity were recorded daily until all individuals died. If a moth died after pairing, then another young adult in the same sex was recruited. The data of recruited individuals were not analyzed. Daily temperature was checked from http://www.weather.com.cn/.
Data analysis
The raw life data of M. separata were analyzed using the TWOSEX-MSChart program (Chi, Reference Chi2018) based on the age-stage, two-sex life table theory (Chi and Liu, Reference Chi and Liu1985; Chi, Reference Chi1988). Following this program, the following parameters were calculated: age-stage-specific survival rate (sxj) (where x is the age and j is the stage), age-stage-specific fecundity (fxj), age-specific survival rate, age-specific fecundity (mx), and demographic parameters including intrinsic rate of increase (r), finite rate of increase (λ), net reproduction rate (R 0), and mean generation time (T). lx, which is the survival rate from age 0 to x, was calculated as:
 $$l_x = {\rm \;}\mathop \sum \limits_{\,j = 1}^\beta s_{xj}$$
$$l_x = {\rm \;}\mathop \sum \limits_{\,j = 1}^\beta s_{xj}$$where β is the number of stages. mx, the average number of eggs produced by an individual at age x, was calculated as:
 $$m_x = \displaystyle{{\mathop \sum \nolimits_{\,j = 1}^\beta s_{xj}f_{xj}} \over {\mathop \sum \nolimits_{\,j = 1}^\beta s_{xj}}}$$
$$m_x = \displaystyle{{\mathop \sum \nolimits_{\,j = 1}^\beta s_{xj}f_{xj}} \over {\mathop \sum \nolimits_{\,j = 1}^\beta s_{xj}}}$$The net reproductive rate (R 0) was defined as the total number of offspring that an individual can produce during its lifetime and was calculated as:
The intrinsic rate of increase (r) was estimated using the iterative bisection method and the Euler–Lotka equation with the age indexed from 0 (Goodman, Reference Goodman1982):
The finite rate (λ) was then calculated as:
The mean generation time (T) is the length of time that a population needs to increase to R 0-fold of its size approaching infinity and the population reaches to a stable age-stage distribution. Mean generation time was calculated as:
Age-stage-specific life expectancy (exj) represents the time that an individual of age x and stage j is expected to live (Chi and Su, Reference Chi and Su2006), which was calculated as:
 $$e_{xj} = \mathop \sum \limits_{i = x}^\infty \mathop \sum \limits_{y = j}^\beta s^{\prime}_{iy}$$
$$e_{xj} = \mathop \sum \limits_{i = x}^\infty \mathop \sum \limits_{y = j}^\beta s^{\prime}_{iy}$$The contribution of an individual of age x and stage j to the future population (vxj) (Tuan et al., Reference Tuan, Lee and Chi2014) and was calculated as:
 $$v_{xj} = \displaystyle{{{\rm e}^{r( {x + 1} ) }} \over {s_{xj}}}\mathop \sum \limits_{i = x}^\infty {\rm e}^{-( {i + 1} ) }\mathop \sum \limits_{y = j}^\beta s^{\prime}_{iy}f_{iy}$$
$$v_{xj} = \displaystyle{{{\rm e}^{r( {x + 1} ) }} \over {s_{xj}}}\mathop \sum \limits_{i = x}^\infty {\rm e}^{-( {i + 1} ) }\mathop \sum \limits_{y = j}^\beta s^{\prime}_{iy}f_{iy}$$The means and standard errors of stage means and demographic parameters were calculated by using the paired bootstrap method (Bootstrap = 100,000). Differences between treatments were calculated using the bootstrap test (Efron and Tibshirani, Reference Efron and Tibshirani1993). These procedures were carried out in the TWOSEXMSChart program (Chi, Reference Chi2018).
Results
The duration in each stage of M. separata in four seasons is listed in table 1. The duration of the larval stage in spring and autumn was significantly longer than in the other two seasons. The duration of the pupal stage in early summer was significantly shorter than in spring and autumn, and longer than in summer (table 1). The temperature and duration of different stages of M. separata during outdoor life table studies are shown in fig. 1.

Figure 1. Temperature and different stages of M. separata during outdoor life table studies in 2018. The red line, black line, and dark green line represents the maximum temperature, the mean temperature, and the minimum temperature daily, respectively.
Table 1. Means and SE of the developmental durations, adult longevity, APOP, TPOP, fecundity, female proportion in cohort (Nf/N), and pupal weight of M. separata reared in four seasonal periods

APOP, adult pre-oviposition period; TPOP, total pre-oviposition period; L1–L6, larval instars 1–6.
Means within a row followed by the same lowercase letter are not significantly different according to the paired bootstrap test at 5% level.
The age-stage-specific survival rate curves (sxj) of M. separata reared in four consecutive seasons are shown in fig. 2. The sxj represents the probability that a newly laid egg will survive to age x and stage j. Overlapping between stages was observed and resulted from variable developmental rates among individuals and stages. The curves for both sexes in spring and early summer were much higher than those in summer and autumn, reflecting the higher pre-adult survival rates in the former two seasons.

Figure 2. Age-stage-specific survival rate (sxj) of M. separata in different seasons. L1–L6, larval instars 1–6.
The stage-specific mortality is shown in table 2. The mortality of L1 in autumn was significantly higher than in the other seasons. From L2 to L4, the mortality in spring was significantly lower than that in the other seasons. The mortalities of L5 and L6 were extremely high in summer, over 8.5-fold and 5.2-fold greater than the mortalities of L5 and L6 in the other seasons, respectively. Pupal mortality was also the highest in summer, which was 4.4-fold, 2.1-fold, and 6.0-fold greater than that in spring, early summer and autumn, respectively. The survival rate of the total pre-adult stage was 65.31, 65.00, 14.29, and 49.44% in spring, early summer, summer, and autumn, respectively.
Table 2. Stage-specific mortality (±SE) of M. separata reared in four seasonal periods

L1–L6, first to sixth larval instars.
Means within a row followed by the same lowercase letter are not significantly different according to the paired bootstrap test at 5% significance level.
The longest adult longevity (13.0 days) and adult pre-oviposition period (APOP) (8.4 days) occurred in autumn, while the shortest adult longevity (4.5 days) and APOP (4.7 days) occurred in summer. The insects had the shortest total pre-oviposition period (TPOP) when reared in summer (35.0 days) and those had the longest TPOP when reared in spring (44.4 days) (table 1). Mean fecundity (F) of female adults (Nf) was significantly different among the four generations. The lowest mean fecundity was observed in summer (40.6 eggs), and the greatest in spring (782.1 eggs). Oviposition days (Od) in early summer (5.1 days) and autumn (5.9 days) were longer than those for the two other seasons. The mean number of eggs laid within each oviposition day (Ed) was also lowest in summer (6.5 eggs), which was only 4% of the number laid in spring (159.5 eggs). The pupae were heavier in spring (342.5 mg) and early summer (353.6 mg) than in summer (238.8 mg) and autumn (243.5 mg).
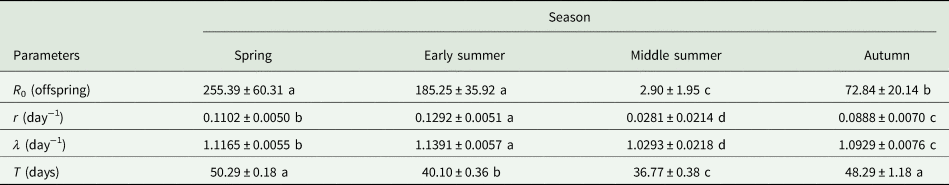
The demographic parameters are listed in table 3, including the net reproductive rate (R 0), the intrinsic rate of increase (r), the finite rate of increase (λ), and the mean generation time (T). The R 0 values were significantly higher in spring and early summer than in the latter two seasons. The highest intrinsic rate of increase (r) and finite rate of increase (λ) appeared in early summer (r = 0.1292 day−1, λ = 1.1391 day−1), which was significantly higher than the other three seasons, while the lowest r and λ appeared in summer (r = 0.0281 day−1, λ = 1.0293 day−1). The mean generation time (T) was between the shortest (36.7 days) in summer and the longest (50.3 days) in spring.
Table 3. Mean (±SE) of demographic parameters of M. separata reared in four seasonal periods: the reproductive rate (R 0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T)

Means within a row followed by the same lowercase letter are not significantly different according to the paired bootstrap test at 5% significance level.
The age-specific survival rate (lx), female age-stage-specific fecundity (fx 7), age-specific fecundity (mx), and age-specific maternity (lxmx) of M. separata in the four seasons are shown in fig. 3. The lx curve is a simplified version of all curves in fig. 1. The age-stage-specific fecundity of female adults (fx 7) represents the mean number of fertilized eggs laid by the female at age x (fig. 2). The peak fx 7 values were 300.20, 132.75, 35.5, and 58.0 offspring in spring, early summer, summer and autumn, respectively. The highest age-specific maternity values (lxmx) were 91.9, 33.8, 1.0, and 10.0 offspring, respectively.

Figure 3. Age-specific survival rate (lx), age-stage specific fecundity (fx 7), age-specific fecundity of total population (mx), and age-specific maternity (lxmx) of M. separata in different seasons.
The age-stage specific life expectancy (exj) of M. separata reared in four seasons is shown in fig. 3, which gives the expected length of time that an individual of age x and stage j will survive. The life expectancy of a newborn egg (e 01) equals the mean longevity. e0 1 was 35.27, 33.56, 23.86, and 34.24 days in spring, early summer, summer, and autumn, respectively (table 1; fig. 4).

Figure 4. Life expectancy of each age-stage group of M. separata in different seasons. L1–L6, larval instars 1–6.
The reproductive value (vxj) describes the expected contribution of an individual of age x and stage j to the future population (Tuan et al., Reference Tuan, Lee and Chi2014) (fig. 5). The reproductive curve peaks for individuals in the four seasons appeared at 49 days (v 49,7 = 691.8 day−1), 37 days (v 37,7 = 427.4 day−1), 35 days (v 35,7 = 64.3 day−1), and 43 days (v 43,7 = 179.5 day−1), respectively (fig. 4). The peak reproductive values were higher in spring and lowest in summer than the other seasons, whereas the peak values occurred earlier in early summer and summer than spring and autumn.

Figure 5. Reproductive value (vxj) of each age-stage group of M. separata in different seasons. L1–L6, larval instars 1–6.
Discussion
As insects are ectothermal animals, their physiological progresses are highly sensitive to ambient temperature (Taylor, Reference Taylor1981; Beck, Reference Beck1983). In our study, the duration of M. separata in the immature stage was the longest in spring when the average daily temperature was 18.8°C, and was the shortest in summer when the average daily temperature was 28.9°C. The results correspond to the trend of the developmental rates increasing with temperature within a certain temperature range. However, the duration in summer did not shorten compared with that in early summer, although the average temperature in summer (29.3°C) was much higher than the average temperature in early summer (24.9°C) during the larval stage. Similarly, a previous laboratory study showed that the larval development of M. separata at 33 and 35°C was slowed compared with 30°C (Jiang et al., Reference Jiang, Liu, Luo and Hu1998). This may be attributed to the intolerance of this pest to daytime temperatures of mid-summer.
The sxj curves (fig. 2) clearly characterized overlaps among stages induced by different developmental rates among individuals, which could not be observed in traditional lx curves (fig. 2). High mortalities of M. separata were observed in 5th and 6th instars and the pupal stage in summer (table 2), largely owing to the high temperatures in this period. From 23 July to 9 August, the daily mean temperature was 29.6°C. In an earlier life table study of M. separata under different constant temperatures, the mortalities in L4–L6 instars, pre-pupal, and pupal stages at 32°C were much higher than those at 16–28°C (Li et al., Reference Li, Gong and Wu1992). The results of the above two experiments indicated that high temperature has a detrimental effect on the survival of this migratory lepidopteran insect. The low survival associated with rapid development under high temperatures could be attributed to severe energy limitation, because high temperature increased the basal metabolic rate. This leaves lower resources and less energy for metamorphosis during the pupal stage when larval tissues degrade and adult tissues establish (Lee and Roh, Reference Lee and Roh2010; Lemoine and Shantz, Reference Lemoine and Shantz2016).
The APOP of M. separata was longest in autumn due to low and decreasing ambient temperature from late September (table 1). Analogously, the APOP of Mythimna convecta, a closely related species in Australia, was inversely proportional to temperature (Smith, Reference Smith1986). Pre-calling period of M. (Pseudaletia) unipuncta was extended for 11 days at 10°C compared with 25°C with a given photoperiod (Delisle and McNeil, Reference Delisle and McNeil1987). Considering the lack of external phenotype differentiation between migratory and resident individuals in most lepidopteran migratory insects, APOP provides an indication of the time available for migratory flight (Hill and Gatehouse, Reference Hill and Gatehouse1992; Colvin and Gatehouse, Reference Colvin and Gatehouse1993). In this research, the long APOP of the autumn generation of M. separata reflected that these armyworms tended to delay maturation and probably undergo a long-distance migration, which coincides with the fact that this pest hardly causes damage during autumn in Shaanxi and the other Yellow-Huai river basin areas.
Pupal weight can be an indirect but easily measured index of fitness in Lepidoptera (Leuck and Perkins, Reference Leuck and Perkins1972; Liu et al., Reference Liu, Gong, Wu and Li2004; Chen et al., Reference Chen, Xu, Li, Wu and Xu2019). Fecundity of insects is usually positively correlated with body size (Honěk, Reference Honěk1993; Nylin and Gotthard, Reference Nylin and Gotthard1998). In the current study, both the pupal mass and female fecundity in spring and early summer were significantly greater than those in summer and autumn (table 1). Smaller body size of organisms typically results from development at higher temperature (Atkinson, Reference Atkinson1994; Kingsolver and Huey, Reference Kingsolver and Huey2008). For example, Manduca sexta larvae were smaller when reared at 30°C than those reared at 20 and 25°C (Davidowitz et al., Reference Davidowitz, D'Amico and Nijhout2003). The wing length of Drosophila subobscura in the northwest USA was shortest in summer (Kari and Huey, Reference Kari and Huey2000). The field captured females of Asobara tabida, a parasitoid of frugivorous Drosophila larvae, were larger at the start and at the end of the field season from early June to early October in the Netherlands (Ellers et al., Reference Ellers, Bax and van Alphen2001). Although the growth rate and developmental rate were positively related to temperature, the thermal sensitivity of the development rate outperformed that of the growth rate for most ectothermal organisms (Walters and Hassall, Reference Walters and Hassall2006; Kingsolver and Huey, Reference Kingsolver and Huey2008). This could explain why M. separata pupae tended to be smaller in summer.
Three factors could determine the intrinsic rate of increase (r) according to equation 4: x, lx, and mx. These factors reflect the effects of the survival rate of individuals that successfully oviposited, the first reproductive age, the peak of reproduction, and the length of reproductive period (Akca et al., Reference Akca, Ayvaz, Yazici, Smith and Chi2015; Liu et al., Reference Liu, Li, Yang, Chi and Chen2018). A large population increase requires a rapid development of immature stages, a high adult survival rate, and high female fecundity. In our study, although the immature stage of M. separata was the shortest, the extremely poor adult survival rate and fecundity resulted in the lowest r in summer. In contrast, the longest immature stage occurred in spring, but the high adult survival and fecundity resulted in the second highest r. From the above results, either one of these three factors, namely x, lx, and mx, could not solely, but could integrate to determine the intrinsic rate of increase. r could comprehensively reflect the effect of biotic and abiotic factors including temperature on the population fitness.
Various biotic and abiotic factors impact life tables of insect populations. Therefore, life tables based on naturally fluctuating environmental conditions are commonly more variable than those obtained from indoor controlled conditions (Tuan et al., Reference Tuan, Lee and Chi2014). Compared to our laboratory constant temperature life table studies (Li et al., Reference Li, Xu, Ji and Wu2018), the fitness at 25°C (r = 0.1438 day−1) was higher than that in early summer with almost the same mean daily temperature (r = 0.1292 day−1), probably because the insects under laboratory controlled conditions received better care (Tuan et al., Reference Tuan, Lee and Chi2014). The much lower r in summer (0.0281) than the population at 30°C (0.1024) was due to stressfully high daytime temperatures, although the summer population developed on a slightly lower mean daily temperature (Tmean = 28.9°C). The maximum daily temperature reached or exceeded 35°C from 17 July to 25 July, and from 1 August to 13 August. Interestingly, the fitness of M. separata in spring was superior to that in autumn, when the Tmean of these two seasons was ~ 20°C. It seems that temperature closer to adult stage had a more powerful effect on the reproduction of moths. This was supported by the experiment on Plutella xylostella under heat stress at different life stages (Zhang et al., Reference Zhang, Chang, Hoffmann, Zhang and Ma2015). In the current study, the spring generation experienced a stepwise warming environment, whereas the autumn generation experienced a stepwise cooling environment. The warm weather in early June was beneficial to the reproduction of M. separata, while the cool weather after late September delayed their maturation.
We concluded that the spring and early summer generation had a larger population growth potential, causing the larvae of early summer and summer generations to have a more serious damage potential in the Central Shaanxi Plain area. The autumn generation possessed a long APOP, which was beneficial to a long distance southward migration. This life table study under naturally fluctuating temperatures will be helpful to learn more about the population dynamics of M. separata in this region.
Acknowledgements
This study was supported by the National Key R&D Program of China (2017YFD0201807), and the Special Fund for Agro-scientific Research in the Public Interest of China (201403031).