Introduction
To understand emergence timing in the field, as summarized and illustrated by Merritt et al. (Reference Merritt, Turner, Clarke and Dixon2007), it is necessary to include local environmental events affecting the degree of seed dormancy, as well as circumstances actually leading to germination. Unfortunately, ‘seed dormancy’ has various definitions in the literature (e.g. Harper, Reference Harper1957, Reference Harper1977; Lang, Reference Lang1987; Baskin and Baskin, Reference Baskin and Baskin2004), referring to the circumstances that directly promote/prevent germination and, or solely, to the circumstances (including embryo and seed morphology) that make seeds delay or prevent germination even though subjected to suitable germination environments. The definition used in this paper, aimed at studying dormancy and germination from an ecological perspective, is that seed dormancy is a seed characteristic that prevents germination, even if suitable germination conditions prevail. Neither embryo nor seed morphology, nor germination mechanisms, are involved in this definition.
To describe dormancy and germination patterns of species, we use: (1) ‘dormancy pattern’, i.e. environmental events that reduce and, if applicable, induce dormancy; (2) ‘germination preferences’, i.e. environments that are (or become during dormancy reduction) suitable for germination; and (3) ‘dormancy strength’ (Karlsson and Milberg, Reference Karlsson and Milberg2007a, Reference Karlsson and Milbergb, Reference Karlsson and Milberg2008). To be ecologically meaningful, seed dormancy should be regarded as a continuous property of a seed batch (even though it is not definitely known whether or not it is a continuum or an on–off property for an individual seed). Germination preference alludes to a dynamic characteristic, not to a requirement for germination or to a theoretical optimum, e.g. light is a germination preference for several species, even though such species frequently do not have light as a requirement for germination in all circumstances (e.g. Karlsson and Milberg, Reference Karlsson and Milberg2007c). Dormancy strength, referring to the general pattern, is described as strong–weak, and the extent of dormancy present at any specific moment is referred to as ‘degree of dormancy’.
The model by Merritt et al. (Reference Merritt, Turner, Clarke and Dixon2007) addressed emergence timing of Australian species in Australia. For species occurring worldwide, one can suspect that different environmental events are important for emergence timing in different parts of the world. To evaluate the importance of different environmental circumstances, to foresee possible distributional ranges, and to compare species according to their response to specific situations and in general, we regard it as desirable to describe dormancy and germination separately through parameters that can be measured on continuous scales. The dormancy sorting systems suggested in the literature are based on discrete sorting; Harper (Reference Harper1957, Reference Harper1977) used a system where seeds could be placed in different groups depending on the circumstances instantaneously preventing germination; Lang (Reference Lang1987) regarded the within-organism location of physiological factors preventing germination or the presence of an unsuitable environment as the most important sorting factors; and Baskin and Baskin (Reference Baskin and Baskin2004) first sorted according to morphology and then into subgroups that could include species with different responses to warm and cold stratification. Vleeshouwers et al. (Reference Vleeshouwers, Bouwmeester and Karssen1995) underlined the sorting and definition problem when they attempted to integrate physiology and ecology with respect to seed dormancy, and decided simply to exclude, before beginning their reasoning, all species that have small embryos (in relation to the endosperm), and those that do not imbibe water when fresh.
The extent and rate of response to pre-treatments are important parameters for evaluating the effect of different, possibly dormancy-affecting, environmental events. These two factors can be measured by fitting germination results from tests over time to a logistic function (Karlsson and Milberg, Reference Karlsson and Milberg2007c). By condensing such measurements and plotting them together with germination preferences, species-specific patterns might arise, aiding in evaluation of emergence patterns and in comparisons of species. To evaluate this, we used six annual weedy Asteraceae that co-occur in eastern Ethiopia: Bidens pilosa L., Galinsoga parviflora Cav., Guizotia scabra (Vis.) Chiov. ssp. schimperi (Sch. Bip.) Baagoe (hereafter referred to as Guizotia scabra and included in the term ‘species’), Parthenium hysterophorus L., Tagetes minuta L. and Verbesina encelioides (Cav.) A. Gray.
Materials and methods
Seed collection
Ripe seeds of B. pilosa, G. parviflora, G. scabra, P. hysterophorus, T. minuta and V. encelioides were collected on 7 and 8 November 2005. All species were collected at two sites: around Haramaya town (09°23′N 42°01′E) and at cultivated areas on the Haramaya University campus (09°25′N 42°02′E; previously named Alemaya University). The sites are c. 5 km apart, and are located in eastern Ethiopia, just above 2000 m above sea level (a.s.l.). All sites used were agricultural fields, including the margins. At collection, flower heads were lightly shaken in a bucket, and achenes (hereafter called seeds) that fell off were used. Seeds were used in the experiments as they are dispersed in nature, i.e. we did not remove the pappus, wings, enclosing petals or other structures. Experiments in the laboratory and in the field commenced about 10 d after collection, and until then, seeds were stored dry at natural fluctuating temperatures.
Germination experiments
Seeds were tested for germination at the beginning of experiments (‘fresh’) and after three different pre-treatments: cold or warm stratification, or dry storage, for 2.5, 5, 10, 20 or 30 weeks. Germination tests were performed both in light during daytime (‘light’) and in continuous darkness (‘darkness’). Germination tests were terminated after 4 weeks; germination in dishes in light was checked after 2 and 4 weeks, and dishes in darkness only at termination. When terminating a dish, ungerminated seeds were counted. Seeds that were soft and/or overgrown with mould were considered dead.
All pre-treatments were performed in continuous darkness. Average daily minimum–maximum temperatures were 4.5–5.4°C (SD 0.17–0.15) in the cold environment, and 22.0–23.2°C (SD 0.5–0.7) in the environment used for warm stratification and dry storage. The dry-stored seeds were subjected to a relative humidity (RH) of 28.8–31.5% (average daily min.–max., SD 3.3–4.1), which was achieved by placing open bowls with moist sand and KCl in the seed storage room to compensate for seasonal variation in indoor RH. Germination tests were performed in incubators (Rubarth Apparatebau, Laatzen, Germany) with alternating 10/10 h (day/night) temperatures of 15/5, 20/10, 25/15 or 30/20°C with a 2 h, linear transition in between. The incubators were illuminated for 12 h at a photon flux density (400–700 nm) of 28–62 μmol m− 2 s− 1 [measured with a SKP 200 photometer and SKP 215 sensor (Skye Instruments Ltd, Llandrindod Wells, Powys, Wales); measurement precision 1 μmol m− 2 s− 1] and a 660/730 nm photon fluence ratio of 3.0–3.5 [SKR 100 photometer with a SKR 110 sensor (Skye Instruments Ltd)]. Dishes in the light were randomly rearranged in the incubators at least once a week.
For germination tests, c. 100 (average 116 ± 31) seeds were distributed in 3–4 Petri dishes (50 mm diameter), depending on seed size. The substrate was 10 ml of quartz sand (0.35 mm grain size, Baskarpsand 35, AB Baskarpsand, Habo, Sweden), moistened with 3.7 ml deionized water, plus enough water for imbibition; all dishes were sealed with Parafilm™. For dark treatments, dishes were wrapped in aluminium foil, and the dishes for warm stratification treatments were also put in plastic bags.
For all four incubation temperatures, the dishes with fresh seeds tested in light were subjected to continuous incubation for an additional 30 weeks after terminating the initial test, and also a set of dishes in darkness was incubated for 34 weeks. In addition, seeds were subjected to a 40-week treatment, comprising an initial dry period of 12 weeks, followed by 8 weeks of moist incubation in light at the four temperatures, 4 weeks of dryness and an additional 16 weeks of moist incubation at the same temperature as before, resembling the dry and rainy periods in eastern Ethiopia after harvest. The dishes in light that were kept for 34 and 40 weeks were checked at least once every 6 weeks.
Seedling emergence phenology
Soil was sampled at the Haramaya University campus on 17 October 2005; the soil on the site was well-drained loam. Soil was sterilized by water vapour at 100°C for at least 8 h. Pots (9 cm diameter), with a non-woven glass-fibre sheet in the bottom, were filled with c. 250 ml soil, and 75–100 seeds, depending on seed size, were placed on the soil surface in each pot. Two pots, individually covered with mosquito netting, were used for each seed batch, and were buried in soil, in a cultivated area on the Haramaya University campus at a depth such that the soil surface in the pots was at the same level as the surrounding soil surface. Seeds were then subjected to local temperatures and precipitation. Pots were checked for seedlings at least every week, and emerged seedlings were counted and removed. Temperature and precipitation were measured at the Haramaya University weather station.
Calculations and analyses
For the seedling-emergence phenology experiment, emergence was calculated as the fraction of seeds sown in each seed batch. In germination tests, the fraction germinated was calculated from the number of seeds used, excluding seeds considered dead.
For all analyses, Statistica (StatSoft Inc., 2004) was used. Analyses of variance were performed on: (1) arcsine-transformed germination data; and (2) the difference between each adjacent test occasion as weekly averages. The two seed batches of each species were considered replicates of the species in both analyses. Categorical predictors were species, pre-treatment, germination test temperature and light condition. Time in pre-treatment was treated as a continuous variable in the analysis of germination. In the analysis of differences, each time span (‘moment’) between adjacent test occasions was treated as a categorical predictor. For interpretation, special attention was paid to the factor ‘species’ and interactions including ‘species’, to evaluate possible differences between species.
To investigate the general, relative, difference between seed batches and species, a principal component analysis (PCA) was done on arcsine-transformed germination results, together with absolute differences between each adjacent test occasion. In the PCA, results for seed batches were not individually standardized.
The average extent of, and time to, final response for each pre-treatment were calculated. A logistic function (equation 1) was fitted to each of the 288 series of six points in time from germination tests, i.e. one per seed batch, pre-treatment and germination test environment. In equation (1), f(t) is germination, t is time in pre-treatment, a, b, c and d are constants, where 0 ≤ d ≤ 1 and 0 ≤ (c+d) ≤ 1, and e (approximated to 2.71 828) is the base of the natural logarithm.
Calculations were performed using non-linear estimation and user-specified regression. The loss function, with quadratic difference between observed and predicted values, and the weight function (on the number of seeds tested at each occasion), with the pre-selected quasi-Newton estimation, were used. Initial values for a, b and c were set to zero and d to the actual germination of fresh seeds. Time for achieving a major response to pre-treatment was calculated as the time between t = 0 and the right extreme point (hereafter referred to as ![]() ) of the third derivative of equation (1) (equation 2; Fig. 1).
) of the third derivative of equation (1) (equation 2; Fig. 1).

The average calculated final germination [f(30) from equation 1, ± SD] and weighted average response time [equation 3, ± weighted SD (equation 4)] for the different pre-treatments were calculated.

Figure 1 An example of the logistic function ![]() fitted to germination data. f(t) is germination, t is time in pre-treatment, a, b, c, d are constants, and e is the base of the natural logarithm. To obtain the response time (i.e. the time from t = 0 to the point when a major germination response is considered achieved), the corresponding t for the shown maximum of f‴(t) can be calculated.
fitted to germination data. f(t) is germination, t is time in pre-treatment, a, b, c, d are constants, and e is the base of the natural logarithm. To obtain the response time (i.e. the time from t = 0 to the point when a major germination response is considered achieved), the corresponding t for the shown maximum of f‴(t) can be calculated.
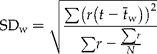
For each batch and test environment, t is the time obtained from equation (2), and r is the extent of response [i.e. the absolute difference between f(0) and f(30) from equation 1]. Because of the weighting, each calculated time to achieve a major response contributes to the weighted average time, and corresponding SD, in direct proportion to magnitude of the response. Functions without any change at all (29 and 14 functions continuously at 0% and 100%, respectively) or with ![]() outside 0 ≤ t ≤ 30 (36 functions) were excluded from calculation of weighted average time to major response.
outside 0 ≤ t ≤ 30 (36 functions) were excluded from calculation of weighted average time to major response.
Together with average germination for fresh seeds in all environments, average calculated final germination was plotted against average response time (equation 3). Further, average overall germination at all test occasions per species and test environment was calculated, and the relative average germination in each environment was plotted, for each environment, as the fraction of germination in the environment where most germination occurred for each species.
Results
Responses to test environments and pre-treatments
Fresh seeds germinated less than 50% in all test environments, with the exception of T. minuta, which germinated 100% in light at higher temperature regimes (Fig. 2). All three pre-treatments tested led to increased germination for all species, except dry storage for P. hysterophorus and warm incubation for V. encelioides, for which there was no observable effect (Fig. 2).

Figure 2 Average germination of two seed batches of six Asteraceae species. Seeds were subjected to three different pre-treatments (cold or warm stratification, or dry storage), and germination was tested at four daily fluctuating temperatures, both in light during daytime and in continuous darkness. Seeds of each species were collected from crop fields at two sites, c. 5 km apart, in eastern Ethiopia.
Species was a significant explanatory factor for both germination and for differences between test occasions (Tables 1 and 2). Overall, P. hysterophorus germinated the least and T. minuta the most (Fig. 2). B. pilosa and G. scabra had the largest, and P. hysterophorus and T. minuta the smallest, overall differences between test occasions (Fig. 2).
Table 1 ANOVA on germination (arcsine transformed) of six Asteraceae species, each collected from two sites. Seeds were subjected to germination tests in eight different environments when fresh and after three different pre-treatments. Time was treated as a continuous variable. Each seed batch was considered as one replicate in the analysis

a Bidens pilosa, Galinsoga parviflora, Guizotia scabra, Parthenium hysterophorus, Tagetes minuta or Verbesina encelioides.
b Germination tests after 0, 2.5, 5, 10, 20 and 30 weeks in pre-treatment.
c Cold or warm stratification, or dry storage.
d Germination test at 15/5, 20/10, 25/15 or 30/20°C day/night.
e Germination test in light during daytime or in continuous darkness.
Table 2 ANOVA on weekly average difference between each adjacent germination test occasion when subjected to three different pre-treatments. Six Asteraceae species were included; each was collected from two sites and each seed batch was considered one replicate. Results including the factor ‘species’ are in bold

a Bidens pilosa, Galinsoga parviflora, Guizotia scabra, Parthenium hysterophorus, Tagetes minuta or Verbesina encelioides.
b Moment in time between test occasions: 0–2.5, 2.5–5, 5–10, 10–20, or 20–30 weeks in pre-treatment.
c Cold or warm stratification, or dry storage.
d Germination test at 15/5, 20/10, 25/15 or 30/20°C day/night.
e Germination test in light during daytime or in continuous darkness.
Second-order interactions including the factor ‘species’, were significant in all cases for germination (Table 1), and in all cases except ‘species × temperature’ for difference between occasions (Table 2). For germination, this reflects species-specific differences in the extent of germination following different pre-treatments, different test temperatures and light requirements for germination (Fig. 2). For differences, the interaction ‘species × moment’ (Table 2) shows species-specific differences in time in pre-treatment before most of the changes in germination occurred. The other significant second-order interactions in the analysis of the differences resulted from species-specific differences in the extent of response to different pre-treatments, and in the extent to which a response occurred in darkness (Table 2, Fig. 2).
Third- and higher-order interactions, including the factor ‘species’, were of minor importance for explaining the overall variation (Tables 1 and 2), despite being statistically significant in some cases.
Differences between batches and species
In the PCA (Fig. 3), 57.6% and 20.1% of variation were explained by PC1 (the first Principal Component) and PC2, respectively. The two populations of each of G. parviflora, G. scabra, P. hysterophorus and T. minuta clustered close to each other, while there were larger differences between the two batches of each of B. pilosa and V. encelioides (Fig. 3).

Figure 3 Principal component analysis (PCA) of the combination of germination and weekly differences in response to pre-treatment of six Asteraceae species. Seeds were subjected to three different pre-treatments (cold or warm stratification, or dry storage), and germination was tested in eight environments after 0, 2.5, 5, 10, 20 and 30 weeks. Seeds were collected from crop fields at two sites, Haramaya University campus (open symbols) and Haramaya town (filled symbols), c. 5 km apart in eastern Ethiopia. Of the total variation, 57.6% and 20.1% are explained by PC1 and PC2, respectively.
G. scabra, P. hysterophorus and V. encelioides germinated faster and to a greater extent to cold stratification than to other pre-treatments, which either elicited a less pronounced response or no response (Fig. 4). G. parviflora responded faster and to a higher extent to warm stratification than to the other pre-treatments; B. pilosa responded fastest to warm stratification, but to the highest extent to cold stratification; and T. minuta showed a response only to dry storage (Fig. 4). G. parviflora and V. encelioides had the overall fastest responses, and B. pilosa and P. hysterophorus the slowest (Fig. 4).

Figure 4 Dormancy pattern and germination preferences of six annual weedy Asteraceae species collected in eastern Ethiopia. Graphs on the left show dormancy patterns as average germination after pre-treatment (cold or warm stratification, or dry storage) plotted against average time to a major response achieved. Average time and distribution bars on response time were weighted against the extent of each response. Graphs on the right show overall germination preferences to eight test environments (four daily fluctuating temperature regimes, with light during daytime or continuous darkness) as fractions of the environment that gave most germination. See text for detailed explanations.
Germination preferences differed between species (Table 1). B. pilosa and G. parviflora germinated most at the highest temperatures tested, G. scabra and P. hysterophorus at 25/15°C, V. encelioides at the lower temperatures, and T. minuta had no clear temperature preference within the studied range (Fig. 4).
Seedling emergence and germination over time
After some rain at sowing in November, there was a dry period of nearly 3 months (Fig. 5). Most emergence during the 11-month observation period occurred during the middle of February, i.e. following the first precipitation (Fig. 5). Only G. scabra did not emerge substantially following the first precipitation, but had an emergence peak during intense rains in late March and early April, as did B. pilosa and P. hysterophorus (Fig. 5). G. scabra and B. pilosa were the only species that emerged during May or later (Fig. 5).

Figure 5 Cumulative average emergence of six Asteraceae species. Seeds were collected from crop fields at two sites, c. 5 km apart, in eastern Ethiopia. Seeds were sown in pots outdoors in Ethiopia, at c. 2000 m a.s.l. on 20 November 2005, and were subjected to natural temperature and precipitation. Pots were checked at least every week; the first emergence was observed on 21 February 2006. Daily precipitation (bars), and maximum and minimum temperature (crosses) are shown.
When continuously moist-incubated in the light, T. minuta germinated to nearly 100% at all temperatures within 8 weeks, and the few remaining seeds at 15/5°C germinated during 10 additional weeks (Fig. 6). V. encelioides did not germinate at 30/20°C, and more slowly at 25/15°C than at 20/10 or 15/5°C, and P. hysterophorus germinated mostly at 25/15°C (Fig. 6). G. scabra and P. hysterophorus germinated little at 15/5°C (Figs 4 and 6), while B. pilosa and G. parviflora germinated mostly after 18 weeks of incubation at 15/5°C; B. pilosa had its germination peak after 14 weeks of incubation at all temperatures (Fig. 6). When incubated for 34 weeks in darkness, only B. pilosa subjected to 30/20°C had germinated more than after 4 weeks of incubation (data not shown).

Figure 6 Average germination of two seed batches of six Asteraceae species that were subjected to continuous incubation or to dry periods, alternating with incubation at different temperatures. Germination was checked at least every 6 weeks. Seeds of each species were collected from crop fields at two sites, c. 5 km apart, in eastern Ethiopia.
When subjected to alternating periods of dryness and incubation, total germination after 24 weeks was higher than after continuous incubation for 34 weeks (Fig. 6). Compared to continuous incubation, G. scabra and V. encelioides increased germination at 30/20°C, and G. scabra also increased at 15/5°C, after the second dry period.
Discussion
Intra-species variation
Differences in dormancy and germination patterns within species may depend on genetic differences, local weather during growth of the mother plants, soil quality, seed position on the mother plant, or differences between seeds with different morphology. By collecting seeds available at a specific point in time, irrespective of variation within fruit heads, we describe the natural variation of two populations that have grown relatively close to each other in similar environments.
For G. parviflora, G. scabra, P. hysterophorus and T. minuta, the overall relative difference within species was relatively small, while B. pilosa and V. encelioides differed along PC1 (Fig. 3). Despite the occurrence of intra-species differences, ‘species’ was an important explanatory factor for both germination and differences between test occasions (Tables 1 and 2). Consequently, we conclude that it was meaningful to compare the general dormancy patterns and germination preferences of the species.
Comparison of species
When attempting to study differences in germination ecology between closely related species, groups within species and/or species with similar emergence patterns in the field (e.g. Keller and Kollman, Reference Keller and Kollman1999; Copete et al., Reference Copete, Herranz and Ferrandis2005; Bischoff et al., Reference Bischoff, Vonlanthen, Steinger and Müller-Schärer2006), dormancy classification systems are usually not used, but differences have been detected by ANOVA with seed batches as replicates (e.g. Tables 1 and 2; Pezzani and Montaña, Reference Pezzani and Montaña2006; Karlsson and Milberg, Reference Karlsson and Milberg2007c), by ordination (e.g. Fig. 3; Keller and Kollman, Reference Keller and Kollman1999) or by comparing germination using variations within batches (e.g. Copete et al., Reference Copete, Herranz and Ferrandis2005; Bischoff et al., Reference Bischoff, Vonlanthen, Steinger and Müller-Schärer2006). These methods can detect differences and the magnitude of differences between compared units, but are not suitable tools for general, species/ecotype-specific descriptions. For making comparisons that are not dependent on specific testing points in time, logistic functions can be used to generalize germination results; this method allows descriptions of the principal response over time, rather than paying attention to seemingly random variations (e.g. Kettenring et al., Reference Kettenring, Gardner and Galatowitsch2006; Karlsson and Milberg, Reference Karlsson and Milberg2007c). The selected point for measuring time to achieve a major response (Fig. 1) is the point where f(t) has the highest ‘un-curving rate’, i.e. when major change is fulfilled and the curve begins to flatten toward its upper asymptote. Thus, the amount of germination does not limit the calculation of response time.
When investigating dormancy and germination ecology, especially in cases when differences between batches are relatively small, we perceive a need for a method that summarizes results from many tests (combinations of pre-treatment and incubation) and highlights possible differences. From an ecological point of view, such differences in responses between species as shown in Fig. 2 are of ecological interest, but this kind of result is difficult to use for comparisons and interpretations. Our intention was to visualize dormancy patterns and germination preferences in a condensed way, using continuous scales (Fig. 4). Positions on the x-axis show the weighted averages of response time, and positions on the y-axis average f(30), i.e. the final magnitude of germination from equation (1). In addition, average germination data for fresh seeds were plotted on the y-axis. The weighting of response time data (equation 3) was done to allow the inclusion of all response times without giving too much influence to the cases where the response was weak (e.g. a change from 1% when fresh to 10% final germination was considered less important than a change from 1% to 100%). The dispersal bars shown are not a tool for statistical comparisons, but are a way to give an impression of variation in the data. Points are included regardless of the extent of change in y-level; of course, if there is no obvious difference in relation to fresh seeds, the time to response is of no or minor ecological importance. For this kind of description to be meaningful, it is necessary that the experimental set-up includes test environments where germination is possible, but does not always lead to full germination, thus allowing expressions of dormancy changes for all species. As shown in Fig. 2, this requirement was fulfilled for all species in the present study.
The dormancy patterns of B. pilosa and G. parviflora were quite similar regarding positive response to all pre-treatments, and they both responded faster to warm stratification than to the other pre-treatments (Fig. 4). However, they differed in that G. parviflora reduced dormancy much quicker, B. pilosa germinated relatively less at the two lowest temperatures tested and G. parviflora germinated exclusively when provided with light (Fig. 4).
G. scabra, P. hysterophorus and V. encelioides all responded more quickly and more distinctly to cold stratification than to other pre-treatments (Fig. 4). However, there were considerable differences in dormancy patterns among these species: G. scabra responded nearly as well to dry storage and warm stratification as to cold stratification, P. hysterophorus did not respond to dry storage, and V. encelioides did not respond to warm stratification (Fig. 4). Both G. scabra and P. hysterophorus germinated the most at 25/15°C, while V. encelioides had a clear preference for the two coolest test environments (Fig. 4).
T. minuta differed from the other species in that dormancy was only substantially reduced when subjected to dry pre-treatment. The species was strongly favoured by light for germination, but, in contrast to the other species, germinated to the same magnitude at all temperatures within the range studied (Fig. 4).
Germination and emergence ecology
Because five out of six species responded to two or three pre-treatments (Fig. 4), we conclude that time per se is as important as specific environmental events for dormancy reduction for these species. Furthermore, considering the relatively small seasonal temperature differences in the study area and in the areas of origin for the species, it is noteworthy that all but T. minuta clearly responded to cold pre-treatment (Fig. 4). This suggests that this dormancy reduction pattern is an evolutionary conservative character. The response could remain from hypothetical ancestral populations for which dormancy reduction during cold periods was favourable. The response could also be a result of evolution of a seed dormancy pattern with distribution of germination of each cohort over time, regardless of current environment. Thus, dormancy reduction during a cold period may have been a response neither useful nor harmful during the evolution of these species.
G. scabra originates from East Africa (Baagøe, Reference Baagøe1974), and seems not to have been dispersed from that area. V. encelioides originates from the region of Mexico and the southern USA (Parsons and Cuthbertson, Reference Parsons and Cuthbertson2001), P. hysterophorus from the Caribbean (Parsons and Cuthbertson, Reference Parsons and Cuthbertson2001) and the other species from South America (Holm et al., Reference Holm, Doll, Holm, Pancho and Herberger1997; Bromilow, Reference Bromilow2001; Parsons and Cuthbertson, Reference Parsons and Cuthbertson2001). The six species co-occur in various combinations as annual weeds in crop fields in highland, eastern Ethiopia (personal observation). With the exception of V. encelioides, the species were among the 15 most common of 102 weedy species investigated in eastern Ethiopia in 1998 (Tamado and Milberg, Reference Tamado and Milberg2000), among the more abundant species in sugarcane in central Ethiopia (Firehun and Tamado, Reference Firehun and Tamado2006), and were included among 107 important weeds in Ethiopia as a whole (Stroud and Parker, Reference Stroud and Parker1989). V. encelioides, which was rare in the area in 1998, had increased considerably by 2005 (personal observation). B. pilosa, G. parviflora, P. hysterophorus, T. minuta and V. encelioides occur as serious, principal or common weeds around the southern hemisphere and north to Mexico, and G. parviflora and T. minuta are also present in the USA (Holm et al., Reference Holm, Pancho, Herberger and Plucknett1979, Reference Holm, Doll, Holm, Pancho and Herberger1997; Parsons and Cuthbertson, Reference Parsons and Cuthbertson2001). G. parviflora, T. minuta and V. encelioides are reported from 60°N in Norway, but only G. parviflora is established there (Lid et al., Reference Lid, Lid and Elven2005).
Despite the different origins and present distribution of the species, they co-occur as weeds in highland eastern Ethiopia, with similar periods for seed dispersal and emergence (Fig. 5). The weather during the study period in Ethiopia (Fig. 5) was normal for that time of the year; thus, seeds dispersed in autumn are normally subjected to a relatively long dry period before being imbibed for a number of consecutive days. Most emergence in pots occurred soon after the first rains in February (Fig. 5), similar to the germination after 12 weeks of storage before the onset of incubation (Fig. 6). In the field, there was a second peak of emergence in late March to early April (Fig. 5). From comparisons of continuous incubation to incubation interspersed with dry periods (Fig. 6), that response seems to be more an effect of time than of the actual dry periods per se, even though G. scabra and V. encelioides increased germination at 15/5 and 30/20°C, respectively, when dry periods were present.
In the field, in climates without pronounced temperature differences during the year, emergence in irrigated fields and in fields subjected to dry periods is probably about the same, according to the total amount of seedlings for these species, but emergence is more extended in time when fields are continuously irrigated (Fig. 6). Dry periods can increase the fraction of seeds germinating in darkness [buried seeds in the field, e.g. T. minuta (Fig. 4)], thus reducing the soil seed bank and momentarily increasing the number of seedlings, compared to continuously irrigated fields. Seed germination of these species was generally stimulated by light (Fig. 4), and therefore, soil disturbance affects emergence by bringing seeds to soil surface (favouring germination), or by burying seeds, preventing germination.
The emergence timing in the field where these seeds were collected and sown can be explained by dormancy reduction during a dry period for B. pilosa, G. parviflora, G. scabra and V. encelioides, even though a dry period was not the most efficient environmental circumstance for dormancy reduction for any of them (Fig. 4). For imbibed T. minuta, the emergence pattern was a result of germination that readily occurred in the light at the temperatures studied, and for P. hysterophorus, it was mostly a matter of elapsed time from first imbibition (Fig. 6). Thus, the six investigated species have different dormancy patterns (Fig. 4), but achieve similar emergence timing in the field following a dry period after dispersal (Fig. 5), leading to possible co-occurrence in environments similar to those studied.
The different dormancy patterns (Fig. 4) indicate differences in possible distribution ranges or emergence timing in environments with larger temperature differences and less pronounced dry periods than at the collection site (Fig. 5). From the dormancy patterns, one can suspect that both P. hysterophorus and B. pilosa, and other species with similar dormancy pattern and germination preferences, are able to establish in colder climates than their present range without any further adaptation. Because of a relatively strong dormancy, a reduction of dormancy in response to cold periods, and restricted germination at lower temperatures (Fig. 4), a substantial part of a seed cohort, produced from an initial successful plant flowering during summer, could disperse in autumn, but not germinate at the prevailing low temperatures. The degree of dormancy would be reduced during winter, and seeds would germinate in early summer, thus becoming established.
Acknowledgements
We thank Abdulrazak Abdulahi, Almaz Alemu and co-workers, Daniel Wondimu, Mekonnen Takaro and Peter Reneby, for assistance. Funding was provided by Formas (The Swedish Research Council for Environment, Agricultural and Spatial Planning).










