Fluazifop-P-butyl, one of the aryloxyphenoxypropionate acid herbicides (APPs) (Délye Reference Délye2005), has been approved for use in Taiwan since 1982. The commercial version of this herbicide has been recommended to selectively control annual and perennial grasses in soybean fields, tea gardens, and vegetable fields in Taiwan.
In Taiwan, goosegrass is a universal annual grass that grows vigorously during the warm season in dryland fields, including among vegetables, fruit trees, and tea gardens. Following the long-term application of fluazifop-P-butyl in Taiwan, a fluazifop-resistant biotype of goosegrass was identified in 2003 (Chiang et al. Reference Chiang, Hou, Wang and Chiang2007). In general, the resistance mechanisms of a plant to graminicides can be grouped into non-target resistance, including alteration of retention, absorption, translocation, and metabolism of herbicides, and target resistance, including changes in target enzymes between resistant and susceptible plants (Hidayat and Preston Reference Hidayat and Preston1997; Menendez and De Prado Reference Menendez and De Prado1996; Ruiz-Santaella et al. Reference Ruiz-Santaella, De Prado, Wagner, Fischer and Gerhards2006).
In non-target resistance, the metabolism of the herbicide plays an important role in plant resistance (Bakkali et al. Reference Bakkali, Ruiz-Santaella, Osuna, Wagner, Fischer and De Prado2007; Bravin et al. Reference Bravin, Zanin and Preston2001; Menendez and De Prado Reference Menendez and De Prado1996). Dusky et al. (Reference Dusky, Davis and Shimabukuro1980) found that during hydrolysis of diclofop-methyl, one of the aryloxyphenoxypropionate herbicides applied to wheat (Triticum aestivum L.), diclofop acid, can convert to ring-OH diclofop through aryl hydroxylation, and this step might be catalyzed by cytochrome P450 monooxygenase (CYP; EC 1.14.14.1) (Kreuz et al. Reference Kreuz, Tommasini and Martinoia1996). Subsequently, the finding of three metabolites from diclofop-methyl, including (2, 5-dichloro-4-hydroxyl-phenoxy) diclofop, (2, 3-dichloro-4-hydroxyl- phenoxy) diclofop, and (2, 4-dichloro-5-hydroxyl-phenoxy) diclofop, in wheat (Zimmerlin and Durst Reference Zimmerlin and Durst1992) strongly supports the action of CYP on the arene oxide of the (2, 4-dichlorophenoxy) diclofop molecule.
Menendez and De Prado (Reference Menendez and De Prado1996) reported that while the CYP was inhibited by 1-aminobenzotriazole (ABT), the conjugates of diclofop could not form in a resistant (R) biotype of blackgrass (Alopecurus myosuroides Huds.), and its herbicide resistance was eliminated. Similar results were observed in ryegrass (Lolium spp.) (Bravin et al. Reference Bravin, Zanin and Preston2001) and late watergrass [Echinochloa phyllopogon] (Yun et al. Reference Yun, Yogo, Miura, Yamasue and Fischer2005). De Prado et al. (Reference De Prado, Osuna, Heredia and De Prado2005) reported that diclofop-methyl was metabolized to conjugates within 48 h in the R biotype of rigid ryegrass (Lolium rigidum Gaudin), whereas this was not so in the susceptible (S) biotype. The formation of herbicide conjugates is commonly related to two enzyme systems, CYP and glutathione S-transferase (GST; EC 2.5.1.18) (De Prado et al. Reference De Prado, Osuna, Heredia and De Prado2005), and the latter is responsible for the transfer of glutathione (GSH) to a monooxygenated herbicide molecule (De Prado and Franco Reference De Prado and Franco2004). Cocker et al. (Reference Cocker, Northcroft, Coleman and Moss2001) reported that the higher radioactivity of polar metabolites derived from [14C]diclofop-methyl, coupled with a higher activity of GST, appeared in the R biotype of Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot].
In addition, Tal et al. (Reference Tal, Romano, Stephenson, Schwan and Hall1993) reported that fenoxaprop-ethyl, another APP herbicide, in barley (Hordeum vulgare L. ‘Legér’) and wheat (‘Fredrick’) was readily hydrolyzed to its acid form, and its phenyl group was conjugated with GSH or cysteine to generate S-(6-chlorobenzoxazole-2-yl)-glutathione (CDHB-GSH) or S-(6-chloro-benzoxazole-2-yl)-cysteine. In late watergrass, Bakkali et al. (Reference Bakkali, Ruiz-Santaella, Osuna, Wagner, Fischer and De Prado2007) also found the GSH conjugate of fenoxaprop acid, S-(6-chlorobenzoxazole-2-yl)-glutathione, in the R biotype. However, though the suggestion that an enhanced metabolism of the toxophore fluazifop acid is a likely mechanism of resistance has been provided by Hidayat and Preston (Reference Hidayat and Preston1997) and Lin et al. (Reference Lin, Chiang, Wang and Wang2016), no further study of fluazifop metabolism in grasses has ever been reported.
In addition to target-site resistance, more polar metabolites of fluazifop were found in the R biotype, suggesting that non-target resistance might contribute to the resistance of goosegrass in Taiwan (Lin et al. Reference Lin, Chiang, Wang and Wang2016). In the present study, the metabolic pathways of fluazifop-P-butyl are characterized to unravel the possible mechanisms for fluazifop-P-butyl resistance in goosegrass.
Materials and Methods
Metabolites of Fluazifop-P-Butyl in R and S Biotypes
Seeds of R and S biotypes of goosegrass were self-propagated from parental biotypes collected from Kaohsiung and Changhua counties, Taiwan in 2003. The R biotype was found in a guava (Psidium guajava L.) field. Both the R- and S-biotype goosegrass were pure lines propagated by selfing for at least six generations and were tested for phenotypes and stable herbicide responses (Lin et al. Reference Lin, Chiang, Wang and Wang2016).
Goosegrass seedlings at the 5-leaf stage and grown from seeds of pure lines were used as plant material. Commercial products containing fluazifop-P-butyl as an emulsion of 17.5% ai (Sinon Chemical Company, Taichung, Taiwan) were sprayed onto the seedlings in a cabinet using an orbital autosprayer at a moving speed of 0.11 km h−1. This device delivered 99 L ha−1 herbicide solution with a particle size of 25 to 30 µm (diameter), a dimension suitable for interception of herbicide droplets by the more upright leaves of seedlings.
At 7 d after treatment (DAT) with 0.1 mM fluazifop-P-butyl, the shoots from the R- and S-biotype seedlings were homogenized with 5 ml 80% acetonitrile and then centrifuged at 12,000 g for 15 min at 4 C. The supernatant was concentrated to dryness by evaporation with nitrogen gas, then weighed and redissolved in acetonitrile to bring the concentration to 15 mg ml−1. Ten microliters of this sample was injected into an ultra-high performance liquid chromatography (UHPLC) system (UltiMate® 3000, Dionex, Thermo Fisher Scientifi, Waltham, MA) system for chromatographic separation, and then the target compounds were separated using hybrid ion-trap mass spectrometry (Hybrid Ion Trap-Orbitrap Mass Spectrometer, Orbitrap Elite™, Orbitrap LC-MS, Thermo Fisher Scientific, Waltham, MA). The liquid sample molecules after UHPLC separation were converted to charged gaseous ions by electrospray ionization (ESI) techniques in an ion-trap mass spectrometry system, and the mass-to-charge ratios (m/z) were detected by a mass analyzer (Wong and Cooks Reference Wong and Cooks1997).
UHPLC conditions:
-
1. Stationary phase: EC 250/4.6 NUCLEOSIL 100-7 C18 column (Macherey-Nagel, Easton, PA)
-
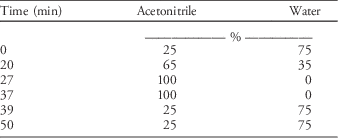
2. Gradient mobile phase (Table 1)
-
3. Flow rate: 1.0 ml min−1
-
4. Retention times of metabolites, fluazifop acid, and fluazifop-P-butyl were 3 to 4 minutes, 12 minutes, and 24 minutes, respectively.
Table 1 Gradient mobile-phase condition used in the UHPLC system for chromatographic separation.
Parameters of ESI-MS analysis:
-
1. Scanning model: ESI full scan
-
2. Scanning range of mass: mass-to-charge ratio (m/z) 70 to 1,000
-
3. Resolution: 30,000
Liquid chromatography/tandem mass spectrometry (LS/MS/MS) used in this study obtained molecular weight information while bypassing the analytical column. Daughter and parent ions formed by MS/MS provided structural information using fragmentation under collision-induced dissociation conditions. Due to the molecular structure of related metabolites or conjugates derived from fluazifop acid, which might contain either the pyridine group (MW 79) or phenoxy group (MW 93), the mass spectrometry scanning mass-to-charge ratio (m/z) from 150 to 1,000 either in positive or negative charge modes was performed. The molecules with significantly stronger signals under the MS1 system were further dissociated to smaller fragments with different m/z values in an orbitrap-based mass spectrometer, i.e., under the MS2 system. Based on the structural information of components from orbitrap-based mass spectrometers and comparisons with the Metabolite Link Metabolomics database (METLIN 2014), MassBank database (2014), and previous references, the possible metabolites were characterized.
Metabolic Enzyme Activity of Fluazifop in Goosegrass Biotypes
Cytochrome P450 Monooxygenase
To detect the metabolism of fluazifop-P-butyl in goosegrass controlled by CYP, phenyl-[U-14C] fluazifop-P-butyl (Syngenta Crop Protection, Basel, Switzerland), with a specific activity of 5,469 MBq mg−1, was used as a stock and diluted with 0.1 mM nonisotopic fluazifop-P-butyl to prepare a [14C]fluazifop-P-butyl solution with a radioactivity of 3,094 Bq μl−1.
Goosegrass seedlings were cultivated in hydroponic solution (Kimura B nutrient solution) (Ma et al. Reference Ma, Goto, Tamai and Ichii2001) to the 5-leaf stage, and a nutrient solution was replaced by the same solution with/without 0.7 mM ABT, an inhibitor of CYP, for 24 h (Hidayat and Preston Reference Hidayat and Preston1997). Then, the whole seedling was evenly treated with 0.1 mM fluazifop-P-butyl. When the herbicide application was almost dry, 1 µl of [14C]fluazifop-P-butyl solution was spread evenly on the middle part of the fifth leaf in an area of approximately 0.5 cm2. The treated seedlings were incubated under a light intensity of 200 μE m−2 s−1, and the middle part of the treated leaf was harvested at 1, 3, 5, and 7 DAT. The collected leaf segments were placed in 6-ml liquid scintillation counting vials containing 1 ml 20% methanol and 0.5% Tween-20 and shaken vigorously for 30 s to wash out the [14C]fluazifop-P-butyl solution remaining on the leaf surface. Leaf segments were ground in liquid nitrogen and homogenized with 5 ml 80% acetonitrile, and the homogenate was centrifuged at 12,000 g for 15 min at 4 C. The supernatant was concentrated to 100 µl with a vacuum decompression concentrator (Speed Vac SC 2000, Savant Instruments, Holbrook, NY) for analysis of fluazifop metabolites.
For 14C-metabolite separation, an aliquot of 50 µl of concentrate was injected into an isocratic HPLC (L-7100 HPLC System, Hitachi, San Diego, CA) system consisting of a stationary phase of EC 250/4.6 Nucleosil 100-7 C18 column (Macherey-Nagel, Easton, PA) and a mobile phase of 1% (v/v) acetic acid in water/1% (v/v) acetic acid in acetonitrile equivalent to 350/650 (v/v); eluate was collected consecutively in 1-ml increments into counting vials at a rate of 1.0 ml min−1 for 30 min (Yu et al. Reference Yu, Friesen, Zhang and Powles2004). Subsequently, a 4-ml cocktail solution (LS-273, Ecoscint A, National Diagnostics, Atlanta, GA) was mixed with the collected eluate for 24 h before the radioactivity was read by a liquid scintillation counter (LSC, LS-6000IC, Beckman, Brea, CA). Retention times of metabolites, fluazifop acid, and fluazifop-P-butyl were at 3 to 4, 5 to 6, and 16 to 17 min, respectively. Radioactivity of the 14C isotope as read by LSC (i.e., count per minute) was transformed through quenching correction to disintegrations per minute (Wilkinson Reference Wilkinson1981). Differences in the radioactivities of the metabolites in goosegrass pretreated with and without ABT reveal the activity of CYP.
Glutathione S-Transferase
The activity of GST (EC 2.5.1.18) was determined based on the method of Milner et al. (Reference Milner, Reade and Cobb2001). After treatment with 0.1 mM fluazifop-P-butyl on the 5-leaf plants of goosegrass, 0.5 g of each shoot were harvested at 0, 1, 3, 5, and 7 DAT. Shoot tissues were ground in liquid nitrogen and homogenized with 5 ml 0.1 M potassium phosphate buffer (pH 7.0) containing 5 mM dl-dithiothreitol, 10 mM sodium ascorbate, and 0.2 g polyvinylpyrrolidone. The mixture was centrifuged at 15,000 g for 15 min at 4 C, and the supernatant was desalted by passing it through a SephadexTM (Healthcare Bio-Sciences, Pittsburgh, PA) G-25 column to obtain the enzyme extract.
For an assay of GST activity, 40 µl enzyme extract was mixed with 160 µl 0.1 M potassium phosphate buffer (pH 6.5) containing 0.2 mM dl-dithiothreitol, 1.5 mM reduced GSH, and 1.5 mM 1-chloro-2,4-dinitrobenzene (CDNB). The absorbance of the mixture was read by an enzyme-linked immunosorbent assay reader at 340 nm (Stat Fax 3200, Awareness Technology, Palm, FL) within 15 min. The protein content of the enzyme extract was determined by reacting with Bio-Rad protein assay dye reagent concentrate, #5000006 (Bio-Rad Life Science Research, Hercules, CA) (Bradford Reference Bradford1976). Specific activity was presented as micromole per milligram of protein per minute.
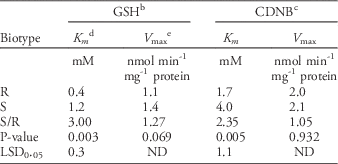
For characterization of enzymatic kinetics of GST, various concentrations of either GSH or CDNB were used (Chronopoulou et al. Reference Chronopoulou, Madesis, Asimakopoulou, Platis, Tsaftaris and Labrou2012; Milner et al. Reference Milner, Reade and Cobb2001; Reade and Cobb Reference Reade and Cobb1999). In this assay, enzyme extract was prepared from the shoot part of 5-leaf goosegrass at 7 d after 0.1 mM fluazifop-P-butyl application, following the same preparation process previously described. The concentrations of GSH used were 0.7, 0.8, 0.9, 1.1, 1.3, 1.5, 1.7, and 2.0 mM, while that of CDNB was maintained at 1.5 mM. Concentrations of CDNB used were 0.40, 0.46, 0.50, 0.56, 0.60, 0.80, 1.00, and 1.50 mM, while that of GSH was kept at 1.5 mM. All observed data were calculated by the Lineweaver-Burk equation in SigmaPlot software (v. 12.0, Systat Software, San Jose, CA) for obtaining K m and V max values.
Statistical Analysis
All data were subjected to ANOVA, followed by Fisher’s least significant difference (LSD) test, at 5% probability. The results from three independent experiments are presented as the mean and standard error of the sample mean.
Results and Discussion
Metabolites of Fluazifop-P-Butyl in R and S Biotypes
To unravel the differences in fluazifop-P-butyl metabolism between the R and S biotypes of goosegrass, metabolite profiles were first determined using LS/MS/MS. Because the molecular structure of fluazifop acid consisted of pyridine (MW 79) and phenoxy groups (MW 93), the mass-to-charge ratios (m/z), ranging from 70 to 1,000 for the metabolite fragments, were detected. In a previous study, a higher proportion of metabolites of fluazifop were found in the R biotype of goosegrass (Lin et al. Reference Lin, Chiang, Wang and Wang2016), suggesting a key role of herbicide metabolism and therefore a non–target resistance mechanism.
The molecules remaining in the eluates after LC separation were transformed from the liquid to the gas phase via ion-trap mass spectrometry and were ionized by ESI. Then, the neutral molecules were converted to either anions or cations during the ionization process, and the m/z value of each fragment was determined by a mass analyzer set to either a positive (POS) or negative (NEG) ion model (Wong and Cooks Reference Wong and Cooks1997). Accordingly, the molecular weight of each metabolite fragment was calculated by either adding 1 to or subtracting 1 from the m/z value. Experimental results showed that the m/z signal 384 in POS mode was identified as fluazifop-P-butyl (MW 383) (Table 2), and the relative intensities of the other six signals, including m/z 512, 432, 423, 415, 314, and 160, i.e., MW 511, 431, 422, 414, 313, and 159 (unknown compounds 1 to 6, Table 2), in the R biotype were all higher than those in the S biotype. Under the NEG ion model, a signal m/z 326 was identified as fluazifop acid (MW 327), and three other metabolites with signals m/z 788, 623, and 593, i.e., MW 789, 624, and 594 (unknown compounds 7 to 9, Table 2), all had higher signal intensities in the R biotype, especially signal m/z 788, with R/S 26.52. However, the signal intensity of m/z 162, i.e., MW 163 (unknown compound 10, Table 2) in the R biotype was lower than that in S biotype, with an R/S of only 0.49.
Table 2 Possible metabolites of fluazifop-P-butyl in resistant (R) and susceptible (S) biotypes of goosegrass 7 days after treatment with 0.1 mM fluazifop-P-butyl.Footnote a

a Ten similar metabolites were found in two biotypes but with stronger signal intensity in the R biotype than in the S biotype, except the unknown compound 10.
b Ratio of each signal intensities between R- and S-biotypes.
Further analysis by LC/MS/MS revealed that molecules of compounds 1, 2, 7, 8, and 9 could be collided into smaller fragments of MW 255, as fragments degraded from the fluazifop acid molecule (Table 2). These fragments, either 4-(5-trifluoromethyl-2-pyridyl) oxyphenol or 2-(4-hydroxyphenoxy)-5-trifluoro-methyl pyridine (compound F, Table 3), have been found previously (Nègre et al. Reference Nègre, Gennari, Andreoni, Ambrosoli and Celi1993). Laganà et al. (Reference Laganà, Fago, Marino and Penazzi2000) also reported that these molecular structures consisted of pyridine and benzene groups, the basic structure of fluazifop acid (Table 3); thus, it is suggested that compounds 1, 2, 7, 8, and 9 must be related to the metabolites derived from fluazifop acid. In addition, in a comparison of information from the METLIN database, the MassBank database (2014), and previous studies, the reduced form of fluazifop acid, 2-[4-(5-trifluoromethyl-2-pyridyloxy) phenoxy] propanol (MW 313), was also found (compound 5, Table 2; compound H, Table 3) in a previous study (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004). This compound can be broken into smaller fragments of MW 144, which was part of compound 4 (MW 414).
Table 3 Compounds identified from metabolic studies on fluazifop-P-butyl.

In the R biotype, the signal strength of compound 10 (MW 163; Table 2) was lower than that in the S biotype. This compound is either 5-trifluoromethyl-2-hydroxyl-pyridine (compound B, Table 3) or 5-trifluoromethyl-2-pyridone (compound C, Table 3) (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004; Nègre et al. Reference Nègre, Gennari, Andreoni, Ambrosoli and Celi1993). However, there are two compounds, i.e., compounds 3 and 6 (Table 2), that cannot be determined due to the limited information at present; these two compounds might be new, previously undiscovered metabolites.
Metabolic Pathway of Fluazifop-P-Butyl in Goosegrass Biotypes
According to the molecular mass obtained by LC/MS/MS, the metabolic pathway of fluazifop-P-butyl in goosegrass was described (Figure 1). After being absorbed by the leaf tissue, the herbicide fluazifop-P-butyl was hydrolyzed to fluazifop acid immediately, and then it was either reduced to 2-[4-(5-tri-fluoromethyl-2-pyridyloxy) phenoxy] propanol (MW 313) (compound 5, Table 2; compound H, Table 3; Figure 1; Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004) or oxidized to 2-[4-(3-hydroxy-5-trifluoromethyl-2-pyridyloxy) phenoxy] propionic acid (MW 343) (compound I, Table 3; Figure 1) by CYP (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004), although the latter was not found in our sample with our detection system.

Figure 1 Proposed metabolic pathway of fluazifop-P-butyl in resistant (R) and susceptible (S) biotypes of goosegrass found in Taiwan. Numbers in parentheses indicate molecular mass of deionized fragment from parental compound.
The fluazifop acid was then degraded to a compound of MW 255 (compound F, Table 3), called 4-(5-trifluoromethyl-2-pyridyl) oxyphenol (Nègre et al. Reference Nègre, Gennari, Andreoni, Ambrosoli and Celi1993) or 2-(4-hydroxy-phenoxy)-5-trifluoro-methyl pyridine (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004). According to the molecular structure of compounds B, C, and F found by researchers (Table 3), it is reasonable to predict that compound F (MW 255) could be further degraded to either B or C (MW 163). Kinard et al. (Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004) reported that compound B could be conjugated with GSH to generate N-[1-carboxy-2-(5-trifluoromethyl-2-pyridylthio) ethyl] malonamic acid (MW 304, compound G, Table 3) by GST, although this conjugate was not found in our detection system.
Considering the molecular mass of fragments degraded from compounds 1, 2, 7, 8, and 9 (Table 2), a common fragment of MW 255 was found; thus, it is reasonable to assume that these five compounds might be conjugates of the intermediate metabolite (MW 255) of fluazifop acid (Figure 1). In addition, it is interesting to find that compound 2 (MW 431) could be degraded to a fragment of MW 176 and compound 4 (MW 414), and the latter could be degraded to a fragment of MW 144. Both fragments of MW 144 and 176 were also parts of compound 5. According to the molecular mass data from LC/MS/MS analysis and information from previous studies and the databases, the putative metabolic pathway of fluazifop-P-butyl in goosegrass is proposed in Figure 1.
The LC/MS/MS analysis also revealed that differences in signal intensities of all compounds in goosegrass appeared between the R and S biotypes; the R/S ratio of signal intensity reflected the relative amount of each compound in the two biotypes. Experimental data showed that most of the metabolic compounds of fluazifop acid in the R biotype were higher than that in the S biotype, with R/S ratios ranging from 1.2 to 26.5, except the ratio of 0.49 for compound 10 (Table 2; Figure 1). Compound 7 (MW 789) accumulated in the R biotype as a major conjugate (Figure 1).
Comparing metabolites derived from fluazifop acid, which have been identified in several non-Gramineae plants, it is clear that 2-(4-hydroxy-phenoxy) propionic acid, N-[1-carboxy-2-(5-trifluoromethyl-2-pyridylthio) ethyl] malonamic acid, 2-[4-(3-hydroxy-5-trifluoro-methyl-2-pyridyloxy) phenoxy] propionic acid, and 5-trifluoromethyl-2-(1H) pyridine (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004) are all absent in goosegrass, except 5-trifluoromethyl-2-pyridone (compound B, Table 2). Although most of the [14C]fluazifop-P-butyl was hydrolyzed to fluazifop acid in large crabgrass [Digitaria sanguinalis (L.) Scop.], and more polar metabolites were found in its R biotype (Hidayat and Preston Reference Hidayat and Preston1997), detailed studies on fluazifop metabolism in Gramineae plants are rare.
In this study, the metabolic pathway of fluazifop acid in goosegrass apparently differs from that of non-Gramineae plant species, and two metabolic enzymes, CYP and GST, might be involved in this process (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004).
Metabolic Enzyme Activity of Fluazifop in Goosegrass Biotypes
Cytochrome P450 Monooxygenase
In general, the monooxygenation catalyzed by CYP is the first and most important detoxification process in herbicide-resistant plants, providing herbicide molecules an oxygen bond for the subsequent conjugation with GSH (De Prado et al. Reference De Prado, Osuna, Heredia and De Prado2005; Yuan et al. Reference Yuan, Tranel and Stewart2006). The latter process is catalyzed by GST. This conjugate is then delivered to the vacuole or extracellular space for further degradation (Yuan et al. Reference Yuan, Tranel and Stewart2006). In goosegrass, we found that fluazifop acid was reduced to compound 5; this reduction might be catalyzed by CYP (Guengerich and Johnson Reference Guengerich and Johnson1997; Werck-Reichhart et al. Reference Werck-Reichhart, Hehn and Didierjean2000). It is likely that fluazifop acid was converted to 2-[4-(3-hydroxy-5-trifluoromethyl-2-pyridyloxy) phenoxy] propionic acid (MW 343; compound I, Table 3) by CYP (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004).
In the CYP activity assay, ABT was used as an inhibitor for CYP activity (Sun et al. Reference Sun, Harper, Dierks, Zhang, Chang, Rodrigues and Marathe2011). At 24 h after ABT uptake via the root system, 5-leaf seedlings of goosegrass were treated with fluazifop-P-butyl and smeared with [14C]fluazifop-P-butyl on the middle part of the fifth leaf. Radioactivity determination showed that the relative radioactivity of 14C metabolites in the S biotype was reduced to 40% at 3 DAT and maintained at 45% to 50% within 7 DAT, whereas it moderately declined by approximately 15% at 7 DAT by ABT (Figure 2). However, the relative radioactivity of 14C metabolites in the R biotype was reduced to 50% at 3 DAT and significantly increased to 60% at 5 DAT, which is higher than in the S biotype. The relative radioactivity of 14C metabolites decreased by approximately 10% at 5 DAT, and even by approximately 20% at 7 DAT, revealing the greater contribution of CYP to the fluazifop metabolism in the R biotype rather than in the S biotype.

Figure 2 Changes in the relative radioactivity of 14C metabolites derived from [14C]fluazifop-P-butyl with or without pretreatment with 0.7 mM aminobenzotriazole (ABT) on the roots of both resistant (R) and susceptible (S) biotypes of goosegrass at the 5-leaf stage. ABT was applied 24 h before [14C]fluazifop-P-butyl application on the central part of the fifth leaf.
Although studies on fluazifop metabolism are lacking, many studies on the metabolism of diclofop-methyl have been reported. In the R biotype of blackgrass and rigid ryegrass, CYP was reported to be involved in the metabolism of diclofop-methyl (Bravin et al. Reference Bravin, Zanin and Preston2001; Menendez and De Prado Reference Menendez and De Prado1996). Shimabukuro et al. (Reference Shimabukuro, Walsh and Hoerauf1979) found that diclofop-methyl can be hydrolyzed to diclofop acid in a short time in resistant wheat and wild oat (Avena fatua L.), and the major metabolites were ring-OH diclofop and glycosyl ester conjugates. Further analysis suggested that diclofop acid in wheat can be converted to ring-OH diclofop through aryl hydroxylation and a subsequent phenolic conjugate (Dusky et al. Reference Dusky, Davis and Shimabukuro1980; Shimabukuro et al. Reference Shimabukuro, Walsh and Hoerauf1979). In resistant wheat, three metabolites, (2,5-dichloro-4-hydroxy-phenoxy) diclofop, (2,3-dichloro-4-hydroxy-phenoxy) diclofop, and 2,4-dichloro-5-hydroxyphenoxy) diclofop, of diclofop-methyl have been identified that might result from the action of multiple isomerases of CYP on the arene oxide of (2,4-dichlorophenoxy) diclofop molecule (Zimmerlin and Durst Reference Zimmerlin and Durst1992).
In goosegrass, due to absence of 2-[4-(3-hydroxy-5-trifluoromethyl-2-pyridyloxy) phenoxy] propionic acid (MW 343; compound I, Table 3; Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004) and the higher activity of CYP, coupled with a 3.5-fold accumulation of compound 5 in the R biotype, it is reasonable to speculate that the function of CYP might contribute to the production of compound 5 via a reduction process (Figure 1) (Guengerich and Johnson Reference Guengerich and Johnson1997; Werck-Reichhart et al. Reference Werck-Reichhart, Hehn and Didierjean2000).
Glutathione S-Transferase
In the S biotype of goosegrass, the GST activity was maintained at 70% of control from the first day after treatment with fluazifop, but the activity in the R biotype increased linearly from 3 to 7 DAT, with a 2-fold increase (Figure 3), revealing that the higher GST activity in the R biotype was induced largely by fluazifop in 7 d. The role of GST in the detoxification of APP herbicides has been postulated, and studies have focused on the metabolism of fenoxaprop-ethyl in late watergrass, barley, large crabgrass, oat, and wheat (Bakkali et al. Reference Bakkali, Ruiz-Santaella, Osuna, Wagner, Fischer and De Prado2007; Tal et al. Reference Tal, Romano, Stephenson, Schwan and Hall1993) and diclofop-methyl in ryegrass (Cocker et al. Reference Cocker, Northcroft, Coleman and Moss2001). The phenyl group of fenoxaprop or diclofop is able to conjugate with GSH or cysteine via GST (Bakkali et al. Reference Bakkali, Ruiz-Santaella, Osuna, Wagner, Fischer and De Prado2007; Cocker et al. Reference Cocker, Northcroft, Coleman and Moss2001; Tal et al. Reference Tal, Romano, Stephenson, Schwan and Hall1993).

Figure 3 Changes in glutathione S-transferase (GST) activity in resistant (R) and susceptible (S) biotypes of goosegrass seedlings at the 5-leaf stage after application of 0.1 mM fluazifop-P-butyl. The original GST activities of the R and S biotypes were 0.051 and 0.104 nmol CDNB mg−1 protein min−1, respectively.
In non-Gramineae plants, the GST activity in the common bean (Phaseolus vulgaris L.) was induced by fluazifop (Chronopoulou et al. Reference Chronopoulou, Madesis, Asimakopoulou, Platis, Tsaftaris and Labrou2012), and the pyridine group of fluazifop acid could be conjugated with GSH to produce N-[1-carboxy-2-(5-trifluoromethyl-2-pyridylthio) ethyl] malonamic acid in celery (Apium spp.) (Kinard et al. Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004). In Gramineae plants such as goosegrass, though lack of evidence of N-[1-carboxy-2-(5-trifluoromethyl-2-pyridylthio) ethyl] malonamic acid has been found by Kinard et al. (Reference Kinard, Anderson, Eckel, Nielsen and Ollinger2004), the R/S 0.49 of compound 10 and the highly increased GST activity strongly support the notion that the degradation of compound 10 might be catalyzed by GST in the R biotype.
Due to the increased GST activity in the R biotype of goosegrass within 7 d, the kinetics of this enzyme were further characterized. The kinetic analysis used the GSH substrate as the variable and showed that the K m of GST reacting with GSH was 0.4 mM in the R biotype; this was 3-fold lower than that in the S biotype (Table 4), reflecting the higher affinity of GST to GSH in the R biotype. In addition, the lack of difference in V max of GST or GSH between the two types revealed that under the lower concentration of GSH, GST in the R biotype could more strongly bind GSH (Cocker et al. Reference Cocker, Northcroft, Coleman and Moss2001; Kreuz et al. Reference Kreuz, Tommasini and Martinoia1996). Labrou et al. (Reference Labrou, Karavangeli, Tsaftaris and Clonis2005) reported that a point mutation of a GST-encoding gene in maize (Zea mays L. ssp. parviglumis Iltis & Doebley var. huehuetenangensis Iltis & Doebley) resulted in a 5-fold increase in K m for GSH; the Phe-35-Leu substitution diminishes the binding ability of GST with GSH.
Table 4 Michaelis constants of enzymatic kinetic analysis for crude enzyme extract of GST extracted from resistant (R) and susceptible (S) biotype of goosegrass at the 5-leaf stage.Footnote a
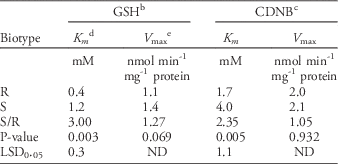
a Abbreviations: CDNB, 1-chloro-2,4-dinitrobenzene; GSH, glutathione; GST, glutathione S-transferase.
b The reduced GSH was used as a substrate for GST enzyme action.
c CDNB was used as a substrate for GST enzyme action.
d The Michaelis constant K m is defined as the concentration at which the rate of the enzyme reaction is half V max.
e The enzyme’s maximum reaction rate.
The GST enzyme consists of homodimer or heterodimer subunits, and each subunit contains a GSH binding site at the N-terminus and hydrophobic sites at the C-terminus (Dixon et al. Reference Dixon, Lapthorn and Edwards2002, Reference Dixon, Skipsey and Edwards2010) that catalyze the nucleophilic displacement of sulfur on GSH with the herbicide molecule, and this reaction leads the herbicide to lose bioactivity (Cocker et al. Reference Cocker, Northcroft, Coleman and Moss2001; Kreuz et al. Reference Kreuz, Tommasini and Martinoia1996). The hydrophobic site at the C-terminus is able to interact with many hydrophobic substrates, such as certain herbicides and CDNB (Chronopoulou et al. Reference Chronopoulou, Madesis, Asimakopoulou, Platis, Tsaftaris and Labrou2012; Dixon et al. Reference Dixon, Lapthorn and Edwards2002; Edwards et al. Reference Edwards, Dixon and Walbot2000), thus CDNB is usually used as a target of GSH in a GST assay (Reade and Cobb Reference Reade and Cobb1999; Zhang et al. Reference Zhang, Shibata, Ito, Shuto, Ito, Mannervik, Abe and Morgenstern2011).
In the kinetics study of GST, we found that the K m of GST reacting with CDNB was 1.7 mM in the R biotype, 2.35-fold lower than that in the S biotype (Table 4), reflecting a higher affinity to CDNB in the R biotype. Similar results were reported for fenoxaprop-ethyl-resistant blackgrass (Reade and Cobb Reference Reade and Cobb1999). Considering that there is no significant difference in the V max of GST for CDNB between the two biotypes (Table 4), it is believed that under the lower concentration of CDNB (or herbicide), GST binds the herbicide more strongly. Therefore, both the N- and C-termini of the GST protein in the R biotype have higher affinities for GSH and herbicide (or CDNB), respectively, likely elevating GST activity in resistant goosegrass.
In summary, the fate of fluazifop in both R and S biotypes of goosegrass from Taiwan was studied. Different metabolite profiles and enzyme activities between the two biotypes confirmed that the higher metabolic activity of fluazifop in the R biotype plays an important role in the non–target resistance mechanism of fluazifop herbicide.
Acknowledgments
The authors thank MOST (project no. 103-2313-B-005- 003-MY3) for financial support and C. N. Sun, Department of Entomology, National Chung-Hsing University, for her suggestions for improving this paper.