Biotic and abiotic environmental factors shape the distribution of plant taxa across the globe (Reich Reference REICH2014, Schimper Reference SCHIMPER1925). The influence of environmental variation on community structure is often reflected in the distribution of plant functional traits, which capture underlying variation in a species’ ecological strategy (Westoby et al. Reference WESTOBY, FALSTER, MOLES, VESK and WRIGHT2002). Communities found in stressful environments, such as those characterized by low mean annual temperature (MAT) or low resource availability, tend to be dominated by resource-conservative functional strategies such as high wood density and low specific leaf area (SLA) (Reich Reference REICH2014). Community-wide trait shifts have been documented across various abiotic gradients including soil water content (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009) and altitude (Read et al. Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014), which captures variation across a number of environmental factors, most importantly temperature (Körner Reference KÖRNER2007).
In well-studied gradients, however, patterns of community-wide shifts in trait values are not always representative of the functional responses of all species or clades, which can exhibit counter-gradient responses (Ackerly & Cornwell Reference ACKERLY and CORNWELL2007). Understanding functional variation of communities along abiotic gradients therefore requires a better understanding of the role that individual clades play in shaping these community patterns. There are three possible relationships between clade- and community-wide trait shifts along gradients (Westoby et al. Reference WESTOBY, FALSTER, MOLES, VESK and WRIGHT2002): (1) trait variation within clades mirror community-wide trait responses (co-gradient response sensu Ackerly & Cornwell Reference ACKERLY and CORNWELL2007); (2) clades exhibit counter-gradient responses relative to the rest of the community (e.g. evergreen responses to shade, Lusk et al. Reference LUSK, REICH, MONTGOMERY, ACKERLY and CAVENDER-BARES2008); (3) all members of the clade have similar trait values, irrespective of their position along the gradient, despite shifts in community-wide means. These patterns of trait variation within a clade can be driven by a variety of patterns of taxonomic and phylogenetic variation. For example, low functional-trait variation within a clade across a gradient may be due to low taxonomic turnover, or may occur when there is high taxonomic turnover but phylogenetic conservatism restricts trait variation within the clade.
As patterns of functional-trait variation across gradients have been well-documented in plant communities (Cornwell & Ackerly Reference CORNWELL and ACKERLY2009, Read et al. Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014), here we focus on the response of Melastomataceae to a broad altitudinal gradient. This pantropical plant family contains >5000 species distributed from lowland rain forests to high-altitude shrub communities (Reginato et al. Reference REGINATO, NEUBIG, MAJURE and MICHELANGELI2016). The turnover of melastome species correlates well with the community-wide taxonomic turnover along edaphic gradients (Ruokolainen et al. Reference RUOKOLAINEN, LINNA and TUOMISTO1997), but it is unknown whether variation in melastome functional traits tracks community-wide functional-trait variation. Here, we assess whether patterns of functional variation within melastomes exhibit co- or counter-gradient responses to well-established patterns of community functional variation across altitudinal gradients in the tropics (Asner & Martin Reference ASNER and MARTIN2016, Read et al. Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014, Swenson et al. Reference SWENSON, ANGLADA-CORDERO and BARONE2011). We also explore associated patterns of taxonomic and phylogenetic variation along the gradient to better understand the drivers of community functional variation.
We measured six functional traits (leaf size, SLA, leaf dry matter content (LDMC), leaf force to punch, leaf nitrogen concentration (LNC) and stem-specific density) following standard protocols (Pérez-Harguindeguy et al. Reference PÉREZ-HARGUINDEGUY, DÍAZ, GARNIER, LAVOREL, POORTER, JAUREGUIBERRY, BRET-HARTE, CORNWELL, CRAINE, GURVICH, URCELAY, VENEKLAAS, REICH, POORTER, WRIGHT, RAY, ENRICO, PAUSAS, DE VOS, BUCHMANN, FUNES, QUETIER, HODGSON, THOMPSON, MORGAN, TER STEEGE, VAN DER HEIJDEN, SACK, BLONDER, POSCHLOD, VAIERETTI, CONTI, STAVER, AQUINO and CORNELISSEN2013) on 97 melastome species at five sites along the 2500-m altitudinal transect of Volcán Barva in Costa Rica's Cordillera Central. This transect spans a ~13°C difference in mean annual temperature (MAT, Table 1) and substantial variation in vegetation structure and composition (Clark et al. Reference CLARK, HURTADO and SAATCHI2015, Lieberman et al. Reference LIEBERMAN, LIEBERMAN, PERALTA and HARTSHORN1996). We sampled herbaceous, epiphytic and woody species where they were most likely to grow (i.e. herbs on forest edges; trees and epiphytes in forest interiors). We calculated SLA, LDMC, leaf size and leaf force to punch for each species from two fully expanded, healthy leaves from up to three individuals at each site. We measured stem density using the water displacement method on one stem per individual. LNC was measured on samples bulked by species and altitude. We also measured seed size at the species-level from herbarium material at the New York Botanical Garden collections. To generate a phylogeny of the species found in our study transect, we built a matrix including six chloroplast markers and the nuclear ribosomal spacers ETS and ITS, which have been broadly used in the family (Goldenberg et al. Reference GOLDENBERG, ALMEDA, SOSA, RIBEIRO and MICHELANGELI2015, Kriebel et al. Reference KRIEBEL, MICHELANGELI and KELLY2015). Most of the DNA sequences were downloaded from GenBank, though these data were supplemented by targeted new sequencing of several species from our study. Sequence data and phylogeny are available online (https://github.com/gauravsk/melastome_functional_traits).
Table 1. Description of the five surveyed sites sampled along an altitudinal gradient in Cordillera Central, Costa Rica. Mean annual temperature data were interpolated from data reported in Clark et al. (Reference CLARK, HURTADO and SAATCHI2015).

We sampled 286 individuals from 101 taxa (97 species, 4 subspecies), which represents a third of the melastome diversity in Costa Rica (Hammel et al. Reference HAMMEL, GRAYUM, HERRERA and ZAMORA2007). Only 25 of the 101 taxa occurred at more than a site. Species richness decreased monotonically with altitude, ranging from 45 species found at the lowland site to only six at the top of Volcán Barva (Table 1).
We found significant phylogenetic signal in leaf size, LDMC, leaf force to punch and seed size using Blomberg's K (Blomberg et al. Reference BLOMBERG, GARLAND and IVES2003). Traits were remarkably variable within communities. At 30 m asl, melastome leaf size ranged from ~19 cm2 to ~608 cm2. To test for trait variation with altitude, we performed linear regressions of site-specific species trait means against altitude. We also used Mantel tests to assess whether dissimilarity in each functional trait between a pair of sites is correlated to their altitudinal dissimilarity.
Using linear regressions, we found weak but significant shifts in mean functional trait values with altitude for SLA, leaf size and leaf force to punch (Figure 1). In accordance with established community-wide predictions, leaf force to punch increased with altitude, whereas leaf size and SLA decreased with altitude. These patterns held when we restricted the analysis to just the woody taxa (leaf force to punch: R2 = 0.23, P <0.01; leaf size: R2 = 0.10, P <0.01; SLA: R2 = 0.08, P <0.01), and were robust to a permutation test that shuffled species traits across altitude (999 simulations with R2 as the statistic of interest; P <0.05 for leaf force to punch, leaf size and SLA). We also performed a bootstrap analysis to account for the unequal sample sizes (number of species) across the gradient. To do this, we fitted linear regressions to 1000 randomly generated subsets of the data that included six randomly sampled species from each site. The proportion of bootstrapped regressions with significant R2 were 93% for leaf force to punch, 70% for leaf size, 25% for SLA, 11% for LDMC, 3% for leaf nitrogen, 5% for seed size and <1% for stem density.
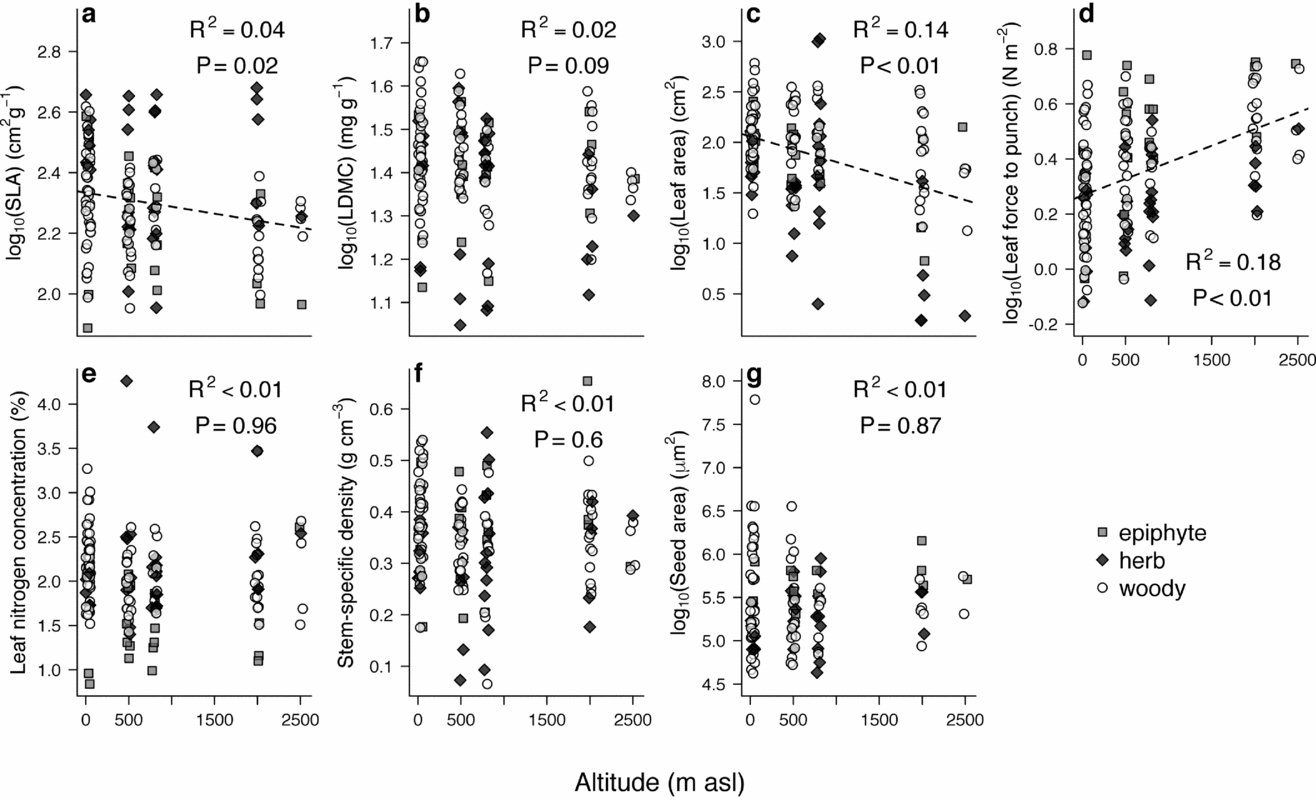
Figure 1. Variation of melastome functional traits along the altitudinal transect of Volcán Barva in Cordillera Central, Costa Rica. Specific leaf area decreases with increasing altitude (a); no evidence for leaf dry matter content variation with altitude (b); leaf size decreases with increasing altitude (c); leaf force to punch increases with altitude (d); no evidence for leaf nitrogen concentration (e), stem density (f) or seed mass (g) varying with elevation.
Using Mantel tests, we found that only dissimilarity in leaf force to punch between sites was significantly correlated with dissimilarity in altitude (Mantel r = 0.88, P <0.01). To test for multivariate patterns of functional variation across all traits, we computed a single community distance matrix using all seven principal component axes constructed from all measured traits. We found no evidence for variation in aggregated trait patterns along altitude (Mantel r = 0.61, P = 0.08).
We also tested the correlation between taxonomic and phylogenetic dissimilarity and altitudinal dissimilarity. We used the Jaccard dissimilarity index between each community pair to calculate taxonomic dissimilarity, and two metrics to compute phylogenetic dissimilarity: UniFrac (Lozupone & Knight Reference LOZUPONE and KNIGHT2005) and Dpw (Swenson Reference SWENSON2011). Variation in UniFrac is driven primarily by variation between communities in the terminal tips of the phylogeny, whereas Dpw emphasizes basal phylogenetic variation (Swenson Reference SWENSON2011). We found that taxonomic dissimilarity was strongly correlated with differences in altitude (Mantel r = 0.86, P <0.01). The phylogenetic dissimilarity was well correlated with altitudinal dissimilarity when calculated using UniFrac (Mantel r = 0.74, P = 0.03), but not when calculated using Dpw (Mantel r = 0.14, P = 0.40).
Taken together, we found mixed evidence that melastome species growing in higher-altitude communities have more resource-conservative traits than species in lower-altitude communities (Figure 1). There is no evidence of trait variation in four of the seven measured functional traits, weak evidence that SLA and leaf size decrease with altitude (significant correlation in linear regression only), and strong evidence that species at higher altitudes have tougher leaves than those at lower altitudes (significant correlation in both linear regression and Mantel tests). Even for leaf force to punch, though, altitude is a poor predictor of how this trait varies with altitude (R2 = 0.18). The observed variation in SLA and the lack of variation in LNC with altitude are consistent with commonly reported community-wide trends with altitude, although the altitudinal variation in SLA among melastomes is much weaker than global community-wide variation reported by Read et al. (Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014). Although such patterns are well-documented for some commonly measured traits, little is known about traits like leaf force to punch.
The lack of altitudinal variation in four of the measured traits may in fact mirror a low variation of community-wide means along the Volcán Barva transect, but this is unlikely given the significant change in MAT along the gradient (Table 1). Low species turnover cannot explain low functional variation, as community taxonomic dissimilarity was well correlated with altitudinal dissimilarity. As there is low deep phylogenetic variation (Dpw) with altitude, the observed low functional variation may be explained by phylogenetic trait conservatism.
The strong turnover of melastome species across the gradient, coupled with low variation in functional traits across the gradient, may be explained by dispersal limitation shaping this assemblage. Indeed, wind-dispersed melastome species are restricted to lower altitudes (Renner Reference RENNER1986), whereas melastomes with endozoochoric seeds may have short dispersal distances due to the low gut retention time (~20 min) of their passeriform dispersers (Ribeiro et al. Reference RIBEIRO, FIGUEIREDO, PICORELLI, OLIVEIRA and SILVEIRA2016). Alternatively, the integrative functional traits that we measured may obscure some key physiological differences between species that are the targets of abiotic filtering. Tolerance of environmental extremes (e.g. freezing temperatures) may restrict species ranges more strongly than the traits measured in this study.
While general trends in plant functional traits along environmental gradients are well studied (Read et al. Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014), the underlying drivers of these patterns are poorly understood (Violle et al. Reference VIOLLE, ENQUIST, MCGILL, JIANG, ALBERT, HULSHOF, JUNG and MESSIER2012). We found evidence for co-gradient variation in some traits across altitude, but not for others, which may indicate a counter-gradient variation in these traits or simply a lack of community-wide trait variation. Although more detailed community-wide trait data are required to resolve this issue, this result is in contrast with the commonly held expectation that plants at higher altitudes exhibit traits consistent with a more conservative growth strategy (Read et al. Reference READ, MOORHEAD, SWENSON, BAILEY and SANDERS2014). In addition, our data highlight the potential for community-wide trait variation patterns to obscure the responses of individual clades to environmental gradients.
These results suggest that lowland tropical forests should be a strong conservation priority, as in addition to being taxonomically diverse, they harbour a great deal of the functional variation of the melastomes. However, there is a pressing need for more community-wide and clade-based studies of plant function and physiology across altitudinal gradients in the tropics, where climate change is already impacting the composition of forest communities and may be impacting clades in distinct ways (Feeley et al. Reference FEELEY, HURTADO, SAATCHI, SILMAN and CLARK2013). We propose that the considerable range in functional traits, varied plant growth forms, and wide altitudinal range throughout the tropics make the melastomes an ideal group in which to investigate the ecology and evolution of plant response to environmental pressures in the tropics.
ACKNOWLEDGEMENTS
We thank the Organization for Tropical Studies for logistic support, J. G. Huertas for field assistance, M. Cruz for measuring seed dimensions, M. Gavrutenko for assistance with DNA sequencing, J. H. Astaiza and D. Clark for helpful advice. C. Fortunel, I. McFadden, L. Chen and two anonymous reviewers helped to improve the manuscript. NSF grant (OISE-CNIC-1444192) funded this research fund. GSK was supported by a Graduate Research Fellowship from NSF (2014, grant DGE-1650604) and MCV was supported by a CAPES doctoral fellowship (2014, BEX:10079-13-0).




