In January of 2019, the Food and Drug Administration approved the Amplatzer Piccolo™ Occluder (Abbott, Abbott Park, Illinois, United States of America) for closure of patent ductus arteriosus in premature babies. Studies have demonstrated the safety profile of the device with few patients developing partial obstruction of the branch pulmonary arteries which are generally well tolerated. Reference Sathanandam, Gutfinger and Brien1 We herein present a hitherto unreported complication of the device, that is, late migration of the device into the descending aorta causing significant obstruction.
Case report
A 4-month-old, premature baby born at 23 weeks and 610 g underwent device closure of a large patent ductus arteriosus in the cardiac catheterisation lab with an Amplatzer Piccolo device (Abbott, Abbott Park, Illinois, United States of America) at 2.4 kg. The patent ductus arteriosus measured 2.5 mm at its narrowest dimension and 8.1 mm long and was closed using a 5-2 PICCOLO device based on the proctor’s recommendation in light of then-unpublished data showing increased incidence of embolisation in patients > 2 kg.
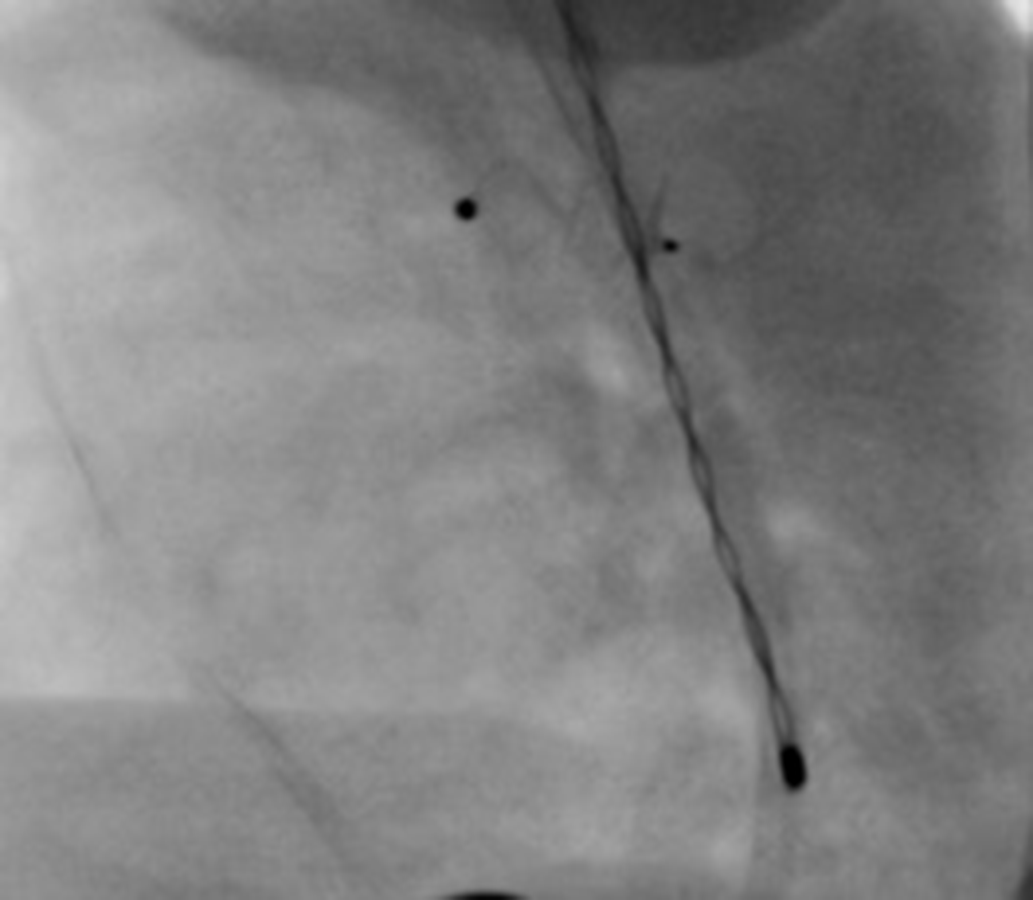
Angiography during the procedure showed a device which sits largely intraductually (Fig 1). Intra-procedural transthoracic echocardiography showed no evidence of obstruction and hence the device was deployed in this position under-reassurance from manufacturer representative and proctor. Following the release of device part of the aortic disc protruded into the aorta while most of the device is still in the ductus (Fig 2). No attempt was made at retrieving or repositioning the device at this point since echographically there appeared to be no obstruction. Post-procedure echocardiogram also showed no evidence of pulmonary or aortic obstruction 6 hours, 24 hours, and 1-week post-procedure (Fig 3).

Figure 1. Initial cardiac catheterisation. ( a ) Angiogram showing a tubular patent ductus arteriosus. ( b ) Angiogram prior to release of device showing device release showing the device intraductully and the aortic disc to the left of the temperature probe.

Figure 2. Immediate post-procedure angiogram showing device placement with the pulmonary artery end in the patent ductus arteriosus and the aortic end is seen just right of the temperature probe.

Figure 3. ( a ) Immediate post-procedure echocardiogram showing intraductal discs of piccolo device and an unobstructed aortic arch by 2D and colour flow mapping. ( b ) A normal descending aorta doppler showing no evidence of coarctation.
A follow-up echocardiogram performed 4 weeks post-procedure showed interval migration of the device into the aorta causing obstruction. The patient was taken back to the cardiac catheterisation lab where significant obstruction with a peak gradient of 50 mmHg in the aortic isthmus was confirmed (Fig 4). Multiple attempts were made to snare the device from the pulmonary end but this was not successful since the pin and the full pulmonary disc were in the ductus. The aortic pin was snared and attempts were made to recapture the device and reposition it but the device could not be moved and instead the entire aorta moved with the device.

Figure 4. Repeat cardiac catheterisation 4 weeks following initial placement. Aortic angiogram showing the device protruding into the aorta. The mid-portion of the device is past the oesophageal probe and the aortic disc is further into the aorta.
The patient was transferred to our hospital for surgical removal of the device in light of the severe obstruction of the aorta. An echocardiogram performed at our institution confirmed severe obstruction of the aorta (Fig 5). The patient underwent removal of ductal device Occluder, closure of the patent ductus arteriosus and repair of the left pulmonary artery and aorta at 18 weeks of life. In view of the concern that the device may have eroded into the aortic wall and danger of aortic disruption during surgical manipulation, a decision was made to perform the operation on cardiopulmonary bypass. The patent ductus arteriosus was dissected along its length and the device could be partially seen and felt from outside. Circulatory arrest was then initiated, and the patent ductus arteriosus was incised opened longitudinally to expose the pulmonary disc. The ductal tissue around the device was freed gently. The body of the Piccolo device and the aortic end of the disc which had migrated well into the aortic lumen were gently freed of all adhesions. The device was then explanted in its entirety by traction on the pulmonary disc. The opening in the aorta was closed primarily using Prolene sutures and the pulmonary end of the ductus was also suture ligated. Cardiopulmonary bypass flows were once again re-established, and rewarming was initiated. Following complete rewarming and restitution of full ventilation, the patient was gradually weaned off cardiopulmonary support in normal sinus rhythm. Completion transesophageal echocardiogram demonstrated good ventricular function with a normal Doppler pattern in the descending thoracic aorta. Following confirmation of haemostasis, the chest was closed. The patient did well in the post-operative period and follow-up echocardiograms continue to demonstrate normal ventricular function with no aortic obstruction.

Figure 5. ( a ) The arrow points to the piccolo device which is seen at the aortic isthmus obstructing antegrade flow across the aortic arch. Colour flow mapping shows aliasing of flow in this region. ( b ) Continuous wave spectral doppler showing a peak systolic gradient of 54 mmHg and mean of 26 mmHg across the aortic arch.
Discussion
Patent ductus arteriosus device closure in smaller children, including premature infants, has been an area of research, as traditional devices were too large for these patients. In January 2019, the Food and Drug Administration approved the Amplatzer Piccolo™ Occluder (Abbott, Abbott Park, Illinois, United States of America). This device was designed for closure of patent ductus arteriosus in premature infants weighing more than 700 g and over 3 days of age. Although traditional surgical ligation of the patent ductus arteriosus via a left thoracotomy has a very high success rate, it is associated with potential complications such as pneumothorax, bleeding, vocal cord palsy, and chylothorax. Long-term sequelae such as scoliosis and restrictive lung disease have been reported. Percutaneous patent ductus arteriosus closure is not only associated with a more rapid improvement in respiratory status compared to surgical ligation but is also less likely to develop post-ligation syndrome. Reference Ogando, Asensio, Sanchez de la Blanca and Martı2 Percutaneous device placement would seem to be a favourable alternative to surgical ligation in light of these potential risks. Reference Sathanandam, Dalduf and Chilakala3
A recently published prospective multicenter study of 200 patients, including 100 patients <2 kg showed that the Piccolo device could be used safely in infants >700 g. Reference Sathanandam, Gutfinger and Brien1 Left pulmonary artery stenosis has been described in patients immediately after implantation. A prospective review of patients who had undergone device closure of patent ductus arteriosus with the Amplatzer Piccolo Occluder showed that device deformation and left pulmonary artery obstruction was a common complication occurring in 8 of the 14 patients. Three infants developed obstruction (“coarctation”) of the aorta all of which resolved gradually with time. These were believed to be due to oversizing of the device. Reference Chien, Wang and Lin4 The cases of mild “coarctation” did not translate into clinical significance. Recently there have been reports of neo-coarctations following placement of this device, most are self-resolving but there has been a report of at least one case requiring surgical intervention. Reference Malekzadeh-milani, Akhavi, Douchin and Dauphin5,Reference Tomasulo, Munson and Dori6
The Piccolo is designed for both the pulmonary and aortic ends of the device to be positioned in the ductus itself for patients below 2 kg; in patients above 2 kg this is not the case. The Piccolo device is made of Nitinol which is a Nickel−Titanium alloy with elasticity and memory. The elasticity allows the device to fit through a small calibre long sheath and the memory allows it to reform in its original shape when it comes out of the long sheath. On review of the angiograms, it seems like the device was under more compression than usual, and over time, the device tried to regain its native shape. Since the patent ductus arteriosus was closed, the only place for it to expand was into the aorta. The authors hereby acknowledge that the device used was oversized for the patient. It is important to note that in the current era, many operators deviate from the Amplatzer size charts (Fig 6) based on personal experience with these devices. In this case, the interventionalist’s choice of device was guided by manufacturer representative and visiting proctor.

Figure 6. Amplatzer Piccolo sizing chart (Year 2020).
Had a smaller device been used as predicted by either the <2 kg or the >2 kg manufacturer sizing charts, it seems this complication would have been unlikely to happen. There are some important lessons learnt from this case. Embolisation of Piccolo devices occurs more frequently in patients > 2 kg. Reference Sathanandam, Gutfinger and Morray7 Careful selection of device based on patient’s dry weight is important. The decision of intra or extra ductal placement of the device depends on the patient’s weight. A smaller, longer device was indicated in this case and oversizing does not prevent complications. A combination of high quality transthoracic echocardiography and angiographic appearance and lie of device during placement should guide decision to release device. In patients above 2 kg an aortogram should be performed if there is any doubt about device position. Early retrieval of device should be undertaken if the appearance of the device is not favourable.
The Piccolo device has revolutionised management of the small infant with patent ductus arteriosus but it is not without risk. This case is intended to draw the attention of cardiologists to the risk of aortic migration. Should the device be oversized for the patient.
Conclusion
Percutaneous closure of patent ductus arteriosus in premature infants using the Piccolo Amplatzer Occluder device is being increasingly practiced. Appropriate selection of device size is crucial for good outcomes. Aortic migration and obstruction are potentially serious complications of this device. Close and extended monitoring is required to diagnose this complication. Surgical intervention may be needed to address this issue.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
The authors have no conflicts of interest to disclose regarding this manuscript.
Ethical standards
No specific ethical approval from Institutional Review Boards is necessary for this type of publication. The authors assure that all patient data provided in this case report are anonymised.