INTRODUCTION
Ascaris lumbricoides and Ascaris suum are widespread parasitic nematodes of humans and pigs respectively (O'Lorcain and Holland, Reference O'Lorcain and Holland2000; Crompton, Reference Crompton2001). Predisposition to heavy and light worm burdens has been demonstrated in longitudinal studies for ascariasis in both humans (Elkins et al. Reference Elkins, Haswell-Elkins and Anderson1986; Haswell-Elkins et al. Reference Haswell-Elkins, Elkins and Anderson1987; Thein-Hlaing et al. Reference Thein-Hlaing, Saw and Lwin1987; Holland et al. Reference Holland, Asaolu, Crompton, Stoddart, MacDonald and Torimiro1989; Forrester et al. Reference Forrester, Scott, Bundy and Golden1990; Chan et al. Reference Chan, Kan and Bundy1992; Hall et al. Reference Hall, Anwar and Tomkins1992) and pigs (Boes et al. Reference Boes, Medley, Eriksen, Roepstorff and Nansen1998), and can be detected over multiple rounds of chemotherapy (Holland et al. Reference Holland, Asaolu, Crompton, Stoddart, MacDonald and Torimiro1989; Chan et al. Reference Chan, Kan and Bundy1992). The bases for heterogeneity of infection or predisposition are not yet fully understood yet there are lines of evidence indicating that the underlying mechanism of resistance/susceptibility to Ascaris infection is influenced by host genetics and the host's immune repertoire, which is ultimately under genetic control (Holland et al. Reference Holland, Asaolu, Crompton, Stoddart, MacDonald and Torimiro1989, Reference Holland, Crompton, Asaolu, Crichton, Torimiro and Walters1992; Peng et al. Reference Peng, Xianmin, Xiaomin, Crompton, Whitehead, Jiangqin, Haigeng, Jiyuan, Yang and Weixing1996; Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999, Reference Williams-Blangero, VandeBerg, Subedi, Aivaliotis, Rai, Upadhayay, Jha and Blangero2002, Reference Williams-Blangero, VandeBerg, Subedi, Jha, Correa-Oliveira and Blangero2008).
Convincing evidence for the genetic control of predisposition in humans requires pedigree studies (Quinnell, 2003), which facilitate the separation of the effects of genetic relatedness and the shared household environment. Family-based linkage and association studies coupled with genome scanning have been undertaken on a single Jirel pedigree population in the Jiri region of eastern Nepal (Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999, Reference Williams-Blangero, VandeBerg, Subedi, Aivaliotis, Rai, Upadhayay, Jha and Blangero2002, Reference Williams-Blangero, VandeBerg, Subedi, Jha, Correa-Oliveira and Blangero2008). Williams-Blangero and coworkers demonstrated that 30–50% of variation in worm burdens is attributable to genetic factors (Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999) and later identified 3 significant and 3 suggestive quantitative trait loci (QTL) which influenced infection intensity (Williams-Blangero et al. Reference Williams-Blangero, VandeBerg, Subedi, Aivaliotis, Rai, Upadhayay, Jha and Blangero2002, Reference Williams-Blangero, VandeBerg, Subedi, Jha, Correa-Oliveira and Blangero2008). Recently, Nejsum and coworkers measured heritability for the intensity of A. suum infection in pigs (0·44), and found this to be similar to that reported for A. lumbricoides infection in humans (0·3–0·5) (Williams-Blangero et al. Reference Williams-Blangero, Subedi, Upadhayay, Manral, Rai, Jha, Robinson and Blangero1999; Nejsum et al. Reference Nejsum, Roepstorff, Jørgensen, Fredholm, Göring, Anderson and Thamsborg2009).
Associational studies conducted in the past have demonstrated the HLA 30/31, STAT 6 and β 2-adrenoreceptor genes all play a role in susceptibility to Ascaris infection in humans (Holland et al. Reference Holland, Crompton, Asaolu, Crichton, Torimiro and Walters1992; Ramsay et al. Reference Ramsay, Hayden, Tiller, Burton, Hagel, Palenque, Lynch, Goldblatt and LeSouëf1999; Peisong et al. Reference Peisong, Mao, Enomoto, Feng, Gloria-Bottini, Bottini, Shirakawa, Sun and Hopkin2004), highlighting the multigenic mechanism of resistance. Furthermore, a recent candidate gene study, undertaken in an Ascaris-endemic Columbian population cited 2 genes as being key to resistance to Ascaris infection, one of which is located in one of the QTL detected in the Jirel pedigree population (Williams-Blangero et al. Reference Williams-Blangero, VandeBerg, Subedi, Aivaliotis, Rai, Upadhayay, Jha and Blangero2002, Reference Williams-Blangero, VandeBerg, Subedi, Jha, Correa-Oliveira and Blangero2008; Acevedo et al. Reference Acevedo, Mercado, Vergara, Sanchez, Kennedy, Jiménez, Fernández, Gutierrez, Puerta and Caraballo2009). However, in none of these studies is it clear whether resistance is directed at establishing larvae, tissue-migrating stages or the adult worms.
Alongside these studies in human populations and in pigs, work has been undertaken also in a mouse model, which is particularly useful for dissecting the factors affecting the initial establishment of larvae in the gut and their migration through the liver and lungs. Mouse strains differ markedly in the degree to which they support the migration of larvae to the lung stage of the infection (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006). It is known that there are strong MHC-dependent antibody responses to A. suum which differ between strains, but whether these underpin host-protective immunity is not clear, and indeed doubtful, since the antibody responses in MHC haplotypes were assessed at 14 and 28 days post-infection (p.i.), long after the worms would have disappeared from the lungs and intestines (Kennedy et al. Reference Kennedy, Gordon, Tomlinson and Qureshi1986). Coupled with this Mitchell et al. (Reference Mitchell, Hogarth-Scott, Edwards, Lewers, Cousins and Moore1976) did not detect a link between MHC haplotypes and susceptibility in early A. suum infection in mice. The resistance of strong responder strains is not dependent on T cells since nude, hypothymic BALB/c mice fail to develop intense pulmonary worm burdens (Mitchell et al. Reference Mitchell, Hogarth-Scott, Edwards, Lewers, Cousins and Moore1976), and immunosuppression of CBA/Ca mice with the anti-inflammatory steroid hydrocortisone failed to enhance worm burdens in CBA/Ca mice (Lewis et al. Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007). A particularly marked contrast between strains supporting high and low worm burdens is seen in C57BL/6j and CBA/CA mice, respectively (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006, Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007; Dold et al. Reference Dold, Cassidy, Stafford, Behnke and Holland2010). Following Mitchell et al. (Reference Mitchell, Hogarth-Scott, Edwards, Lewers, Cousins and Moore1976), Lewis et al. (Reference Lewis, Behnke, Stafford and Holland2006) confirmed that C57BL/6j mice are unique among strains in their susceptibility to infection and contrast with all other tested inbred strains which develop much lower worm burdens, similar to those of CBA/Ca. In this context, a particularly interesting recent finding was reported by Pemberton et al. (Reference Pemberton, Knight, Wright and Miller2004b) who showed that, in contrast to most other inbred mouse strains, C57BL mice lack the Intelectin-2 (Itln-2) gene.
Intelectins (Itlns) are a family of galactose-binding lectins with homologues documented in sea squirts, fish, frogs and mammals (Chang et al. Reference Chang, Peavy, Wardrip and Hedrick2004; Chang and Nie, Reference Chang and Nie2007). Differential expression of Itln-2 has been documented in mouse models of Trichinella spiralis and Trichuris muris coupled with heightened expression levels coinciding with expulsion (Pemberton et al. Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a; Datta et al. Reference Datta, Deschoolmeester, Hedeler, Paton, Brass and Else2005; Artis, Reference Artis2006). The dominant expression of Itlns following T. spiralis and T. muris infections in mouse strains resistant to infection led to the suggestion that Itlns may play an anti-nematode role (Pemberton et al. Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a, Reference Pemberton, Knight, Wright and Millerb; Artis, Reference Artis2006). Therefore, given the knowledge on differential expression of Itln-2 in susceptible and resistant mice in other mouse models of helminth infection and the natural deletion of the Itln-2 gene in the susceptible C57BL/6j strain (Pemberton et al. Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a), it was logical to hypothesize that the Itln-2 gene is important in regulating susceptibility and resistance to A. suum infection in the mouse model, and that its lack endowed C57BL/6j mice with their uniquely high levels of susceptibility to the helminth.
This hypothesis was tested in the experiments described in this paper. We predicted that the more copies of the Itln-2 gene a mouse strain possesses the stronger should be its resistance to infection with A. suum and we designed backcross experiments of the progeny of C57BL/6j and CBA/Ca mice to test this hypothesis. Equally, if this single gene makes the dominant contribution to response phenotype we would expect the response phenotypes of the backcrosses to follow simple Mendelian genetics with clear segregation consistent with a single gene hypothesis.
MATERIALS AND METHODS
In order to maximize the efficiency of the breeding colony, both male and female F2 progeny were included in the key experiment reported in this paper. In earlier published work on the A. suum mouse model (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006, Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007; Dold et al. Reference Dold, Cassidy, Stafford, Behnke and Holland2010), only male mice had been used and therefore it was necessary first to investigate whether there is a difference in the phenotypic response to infection between male and female mice of both parental strains. For this reason, a pilot experiment was undertaken in which the effect of sex on infection intensity in both strains was investigated. The protocol detailed for both experiments was approved by the Bioresources ethics committee, Trinity College Dublin and the Department of Health and Children, Ireland.
Pilot sex experiment
Experimental animals
A total of 60 (30 C57BL/6j and 30 CBA/Ca, 1:1 sex ratio) inbred mice was purchased from Harlan UK Ltd at 7 weeks of age. They were allowed to acclimatize to animal house conditions prior to infection at 8 weeks of age. Mice were housed in an animal maintenance room in the Bioresources Unit, Trinity College Dublin for the duration of the experimental procedure. The room was maintained at approximately 22°C with a daily 12 h light/dark photoperiodicity. Water and commercial pelleted food were supplied ad libitum, and cages were cleaned on a regular basis. The mice were individually weighed on arrival, and randomly assigned, within each strain, to groups of 4 per cage.
Parasite and infection of mice
Approximately 5 000 000 embryonated Ascaris suum ova (batch no. AP 04.04) were supplied by the Danish Centre for Experimental Parasitology (CEP), Copenhagen. Individual doses were adjusted in order to contain 1000 fully embryonated ova in 1 ml of 0·1 m H2SO4 as described previously (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006). At 8 weeks of age, mice were administered an individual dose of 1000 A. suum ova.
Post-mortems, collection of tissues and larval counts
On days 6, 7 and 8 p.i., five female and five male mice of each strain were sacrificed and the lungs were removed. Living larvae were recovered from the lungs by means of the Modified Baermann technique, which has been described previously (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006). A pellet of the isolated viable larvae was suspended in 5 ml of a solution of 0·9% saline and 6% formalin. The solutions were agitated and 2 ml were pipetted into the chamber of a nematode-counting slide (Chalex Corporation). The number of larvae present in the grid area, which represents 1 ml, was counted under ×40 magnification. The number of larvae in a 1 ml solution was multiplied by the total volume in order to estimate the number of larvae in the tissue sample.
Backcross breeding experiment
Mouse strains and breeding
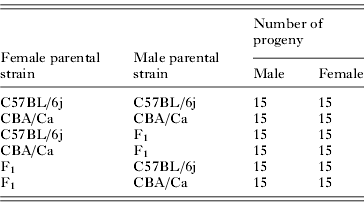
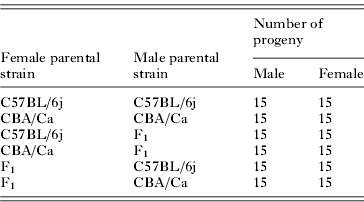
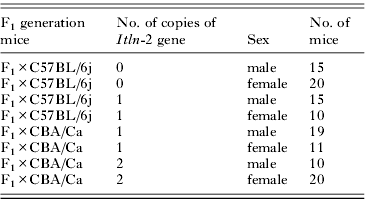
The C57BL/6j (50 female and 15 male) and CBA/Ca inbred strains were used to produce, first F1 and then F2 offspring. Parental strains were purchased from Harlan UK Ltd at 7 weeks of age and were allowed to acclimatize for 1 week prior to breeding. Parental reference strains were bred simultaneously to the F2 progeny. All progeny were weaned at approximately 21 days old and all breeding was undertaken in the Bioresources Unit, Trinity College Dublin.
Phenotyping of mice
At 8 weeks of age, 120 F2 and 60 parental strain mice were infected with 1000 A. suum ova (Table 1). All mice were euthanized on day 7 p.i. The entire lungs from each individual mouse were removed and larvae were isolated using the modified Baermann technique (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006) and enumerated as described above.
Table 1. Number of F2 and parental strain progeny infected with 1000 Ascaris suum ova
Intelectin-2 gene screening
At post-mortem, a tail snip was removed from each individual mouse and placed in 1·5 ml of 20% dimethyl sulfoxide (DMSO) and stored at −80°C. DNA was extracted from tail snips of all F2 mice and 4 reference parental mice (2 C57BL/6j and 2 CBA/Ca mice) using the QIAamp Tissue Kit (Qiagen). Samples were stored at −20°C.
Since the Itln-2 gene is absent in C57BL/6j mice, an Itln-2 specific marker was not used to establish the Itln locus genotype in each F2 mouse. A differential marker and subsequent primers for the Itln locus were previously designed by means of comparing the locus of C57BL/6j, sequenced by Pemberton et al. (Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a) and 129S7 [GenBank: HM370554] mice.
A unique region of the neighbouring gene, SLAMF7 differs in length in C57BL/6j and 129S7 mice. Furthermore, this region was found to be of a similar length in CBA/Ca mice as in 129S7 mice, when SLAMF7 primers were tested on CBA/Ca genomic DNA (Steven Wright, unpublished data) (Fig. 1). Therefore the SLAMF7 gene was considered a suitable marker for the Itln locus in the two mouse strains in the present study and primers were designed for this region.

Fig. 1. Schematic diagram of Itln locus and neighbouring genes (Refbp2, CD244, Ly9, Slamf7) on chromosome 1 of C57BL/6j and CBA/Ca mice.
The sequence of the SLAMF7 primers used were as follows: Forward primer: TGTAGAGGTGGATGGTGCTG; Reverse primer: TGGGGTTCCATTCTGAGTTT. Using SLAMF7 marker primers, C57BL/6j mouse DNA gives a 200 bp product, whereas 129S7 and CBA/Ca mouse DNA gives a 218 bp product. In F2 mice these primers yielded 200 bp and/or 218 bp PCR fragments, depending on the number of copies of the Itln-2 gene in the individual mouse analysed. Since the Itln genes and SLAMF7 are closely linked, the presence of Itln genes from either parental strain can be inferred from the marker sizes following PCR of F2 mouse genomic DNA. F2 mice with a C57BL/6j parent were determined to be homozygous and so have zero copies of the Itln-2 gene, if there is no evidence of the 218 bp SLAMF7 product, or heterozygous, and so have 1 copy of the derived Itln-2 gene, if both 200 bp and 218 bp products are detected. F2 mice with a CBA/Ca parent were determined to be homozygous, so have 2 copies of the Itln-2 gene, if only the higher 218 bp product can be seen, or heterozygous, so to have 1 copy of the Itln-2 gene, if both 200 bp and 218 bp products are detected.
The common forward primer for Itln-1 and Itln-2 gene, MITLNComf1 (5′-GGTTCCTGCCATTACTCAGC-3′), and the Itln-2 specific reverse primer, MITLN2r1-r (5′-TTTATCATGATTGCCACGAGAGT-3′), were used to confirm the results of the SLAMF7 marker analysis (data not shown). These two primers span intron 1, giving a 350 bp product for Itln-2 whereas the paralogous sequence for Itln-1, gives no product.
To confirm the presence or absence of the Itln-2 gene in the genome of the F2 mice, PCR was undertaken on all extracted DNA samples. Samples which were tested using Itln specific primers were loaded on agarose gels, while those tested using SLAMF7 primers were loaded on polyacrylamide gels. Resultant PCR products were imaged using Quantity One® software.
Statistical analysis
Pilot experiment comparing sexes
Larval burdens in the pilot experiment designed to assess the effect of host sex were more variable than had been expected and even after transformations did not conform to the assumptions of parametric statistics. The experiment included 3 main factors (sex, strain and day after infection) but since no three-way non-parametric ANOVA (np ANOVA) is available, we analysed the data in stages by two-way np ANOVA, using bespoke software based on Meddis (Reference Meddis1984) and applied as described by Barnard et al. (Reference Barnard, Gilbert and McGregor2007). Where possible we tested a priori predictions based on earlier published observations (e.g. that larval burdens should be higher in C57BL/6j mice, compared with CBA/Ca and that male mice should harbour more larvae than females) and in these we give the value of z and the corresponding P. Where specific predictions were not possible we applied a general test and give the value of H and its corresponding P. For all tests we considered a P value of 0·05 and lower, as indicating significant difference.
Backcross breeding experiment
Data recorded for the F2 mice and C57BL/6j and CBA/Ca parental strains were analysed at 2 levels. First, we considered any mouse that had more than 45 larvae as susceptible, using the highest burden recorded in the resistant CBA/Ca mice. This allowed F2 hybrid mice to be classified as either resistant or susceptible to A. suum. The percentages of resistant mice in relevant subsets are shown with 95% confidence limits, calculated as described by Rohlf and Sokal (Reference Rohlf and Sokal1995) employing bespoke software. The factors affecting the percentage of resistant mice were analysed by maximum likelihood techniques based on log linear analysis of contingency tables using the software package SPSS (Version 16.0.1.).
An initial full factorial model incorporated the following factors: parental strain with which the F1 were crossed (2 levels, CBA/Ca or C57BL/6j), sex of the F2 hybrid (2 levels, male or female), and number of copies of the Itln-2 gene (3 levels, zero, 1 or 2 copies). Then, beginning with the most complex model involving all possible interactions, those combinations that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest-level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of χ 2 was not significant, indicating that the model was sufficient in explaining the data (these values are given in the legends to the figures as relevant). The importance of each term (i.e. interactions involving resistant/susceptible status and other relevant terms) in the final model was assessed by the probability that its exclusion would alter the model significantly and these values are given in the text. The remaining terms in the final model are summarized for completion, in the legends to appropriate figures, together with the likelihood ratio for the final model.
The larval burden data were highly overdispersed, and therefore non-parametric procedures were employed throughout. Non-parametric statistics cannot deal with 3 explanatory factors but a two-way non-parametric ANOVA based on Meddis (Reference Meddis1984), has been described by Barnard et al. (Reference Barnard, Gilbert and McGregor2007) and was employed using bespoke software. We analysed the F2 hybrids separately in 2 steps, the first being the F1 to C57BL/6j hybrid offspring, and the second the F1 to CBA/Ca hybrid offspring. This approach is justified because the former hybrids can only have zero or 1 copy of the gene, and the latter can only have 1 or 2 copies, and unless a model can be fitted that controls for the parental strain (C57BL/6j or CBA/Ca), the outcome is likely to be heavily influenced by copy number in the parents. In each case we tested 2 a priori predictions. The first was that worm burdens should be higher in male mice and, crucially, the second that more copies of the Itln-2 gene should be associated with lower worm burdens.
RESULTS
Pilot sex experiment
The changes in lung worm burdens in both sexes of both strains are shown in Fig. 2. Overall mean worm burdens were higher in C57BL/6j mice compared with CBA/Ca mice and this difference between strains was significant on day 6 and day 7, and showed borderline significance on day 8 (2-way np ANOVA, for the specific prediction C57BL/6j > CBA, z=2·23 [P=0·0129], 3·41 [P=0·0003] and 1·63 [0·0511], respectively) at which time larval numbers had declined in both male and female C57BL/6j mice and were only marginally higher than those in CBA/Ca mice.

Fig. 2. Changes in mean larval burden (±s.e.m.) in the lungs of male and female C57BL/6j and CBA/Ca mice, following inoculation with 1000 Ascaris suum ova.
Larval burdens recorded at individual time-points were highly variable in C57BL/6j mice (on day 6 in males the range was 5–420, and in females 0–500). On day 7 p.i. slightly lower larval numbers were recorded in male and female C57BL/6j mice but by day 8 p.i., worm burdens in both strains had dropped considerably, as expected from earlier studies (Lewis et al. Reference Lewis, Behnke, Stafford and Holland2006, Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007; Dold et al. Reference Dold, Cassidy, Stafford, Behnke and Holland2010).
In the CBA/Ca mouse strain larval burdens were similar in male and female individuals, throughout the experiment; whereas in C57BL/6j those in male mice appeared to be higher especially on days 6 and 7 p.i. (Fig. 2). However, no significant sex-effect was detected in the two-way np ANOVA (with sex and strain as main effects) at any of the post-mortem time-points. A further test, confined to C57BL/6j mice, but incorporating all 3 post-mortem time-points gave borderline significance for the comparison between the sexes (2-way np ANOVA, days post-infection and sex of mice as main effects, for the specific prediction that males > females z=1·56, P=0·06), and despite the drop in the mean larval recovery between days 7 and 8 p.i., a general test of the effect of time on larval burdens was not significant (H 2=4·8, P=0·09).
Backcross breeding experiment
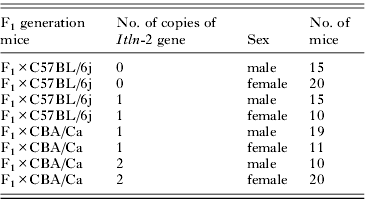
Pulmonary larval burdens were recorded on day 7 p.i. in male and female parental reference strains, C57BL/6j and CBA/Ca, and F2 mice. Since CBA/Ca mice have 2 copies of the Itln locus and C57BL/6j mice none, it was expected that F2 mice generated from crossing F1 to C57BL/6j mice would have either 1 copy or none, and F2 from the cross of F1 to CBA/Ca mice would have either 1 or 2 copies. As shown in Table 2, this expectation was proved correct.
Table 2. The number of male and female F2 hybrid offspring with zero, 1 and 2 copies of the Itln-2 gene
Analysis based on percentage of responders in each F2 subset
As in earlier sections of this paper, responder mice were considered to be those animals that carried 45 or fewer pulmonary larvae at autopsy. Log linear analysis revealed, as expected, that the number of copies of the Itln-2 gene carried by F2 hybrids was dependent on the strain to which the F1 were backcrossed (so either C57BL/6j or CBA/Ca; Table 2; χ 22=91·7, P<0·001).
Resistant/susceptible status was dependent on the strain to which the F1 were backcrossed (χ 21=14·7, P<0·001). Thus comparing the data in Fig. 3 (A) with Fig. 3 (B), the percentage of resistant mice among the F1 backcross to C57BL/6j mice (Fig. 3A) was 46·7% (range: 36·29 to 57·50), and among F2 backcrossed to CBA/Ca (Fig. 3B) was 80·0% (70·09 to 87·57).

Fig. 3. Percentage of responder mice among F2 backcross hybrids, by number of copies of the Itln-2 gene and by sex. (A) F1 backcrosses to C57BL/6j mice (B) F1 backcrosses to CBA/Ca mice.
Resistant/susceptible status was also independently affected by the sex of the hybrid offspring (χ 21=5·2, P=0·023). Among male F2 the percentage of responders was 53·3% (42·50 to 63·71) whereas among females it was 73·3% (62·99 to 81·92). This is best seen among the offspring of F1 to C57BL/6j crosses (Fig. 3A) where the discrepancy between the sexes was relatively large, but there is also a trend in favour of females among the F1 to CBA/Ca crosses carrying 1 copy of the Itln-2 gene although not among those with 2 copies.
In addition to the 3 terms given in the text, there was significant interaction between number of copies of the Itln-2 gene and host sex reflecting simply the differences in numbers of mice in the subsets given in Table 2. The goodness-of-fit of the overall model was given by the likelihood ratio χ 212=6·3 (P=0·9).
Crucially, there was no significant link between number of copies of the Itln-2 gene carried and resistant/susceptible status (Fig. 3).
Analysis based on pulmonary larval burdens in each subset
Among F1 to C57BL/6j hybrids, male mice carried significantly higher larval burdens than females (z=2·87, P=0·002), as can be seen in Fig. 4 (A). However, there was no significant effect of sex for the F1 to CBA/Ca hybrids (z=0·7, P=0·24, Fig. 4B). In neither case did copy number of the Itln-2 gene affect the larval burden (for the F1 to C57BL/6j hybrids z=1·16, P=0·12; for the F1 to CBA/Ca hybrids z=0·63, P=0·26, Fig. 4).

Fig. 4. Mean pulmonary larval burdens among F2 backcross hybrids, by number of copies of the Itln-2 gene and by sex. (A) F1 backcrosses to C57BL/6j mice (B) F1 backcrosses to CBA/Ca mice.
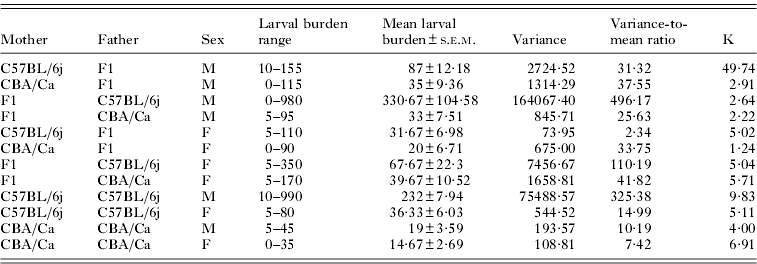
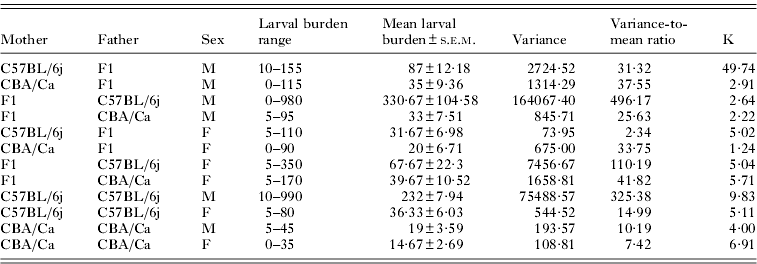
A high degree of variability in the larval burdens was recorded in individual mice, which was particularly evident in male C57BL/6j mice (variance-to-mean ratio: 325·38) and male F2 mice with male C57BL/6j parental strains (variance-to-mean ratio: 496·17) (Table 3). The dispersion of larval burdens in female mice of these two strains was comparatively uniform (variance-to-mean-ratio in C57BL/6j: 14·99, F1×C57BL/6j: 110·19). Individual larval burdens were most consistent in CBA/Ca parental reference strains (variance-to-mean ratio in males: 10·19, females: 7·42) and female F2 mice with C57BL/6j mothers (variance-to-mean ratio: 2·34) (Table 3).
Table. 3. The distribution of Ascaris suum larvae in F2 and parental reference strain mice
DISCUSSION
The unique susceptibility of the C57BL/6j mouse to developing intense lung burdens with the larvae of A. suum, and the observation that this strain in particular has a deletion in the Itln-2 gene cassette on chromosome 1, suggested to us that these may be causally linked. Equally if just a single gene is responsible we would expect backcross progeny to segregate in their response phenotypes along Mendelian predictions, and in this case in line with resistance being dominant. The results reported in this paper are clearly not consistent with either prediction. Our data show convincingly that the Itln-2 gene does not underlie the differing response phenotypes of C57BL/6j mice and that susceptibility in this system is controlled by more than a single gene. Unexpectedly our results also emphasize the confounding effect of host sex, particularly when both sexes are housed in separate cages but in close proximity.
In the present study we assessed the contribution of a candidate gene, Itln-2, to host variation in the A. suum mouse model. Our genomic PCR data are consistent with the presence of Itln-2 in the Itln locus on chromosome 1 of the CBA/Ca genome, but since we did not have the resources to fully characterize the Itln locus in the CBA/Ca mouse, which may indeed contain multiple Itln genes as compared to a single Itln gene (Itln-1) in C57BL/6j mice, the results of the Itln screening were effectively the number of copies of the CBA Itln locus, rather than the Itln-2 gene itself.
In the backcross experiment undertaken, F2 mice with varying numbers of copies of the Itln-2 gene were derived from the parental strains C57BL/6j and CBA/Ca. The single gene hypothesis, based on resistance being dominant, predicts that the outcome of F2 breeding experiments resulting from 2 parental reference mouse strains, which represent the extremes of resistance and susceptibility, should yield all resistant mice in the backcross to the resistant parent and 50% resistant offspring when backcrossed to the susceptible parent. When mice were separated into subsets based on the cut-off pulmonary burden of 45 larvae, a 50–50 split in responders and non-responders was not evident among F1 to C57BL/6j hybrid offspring, and some of the F1 mice backcrossed to CBA/Ca were susceptible. On this basis, it is likely that more than one gene is involved in resistance to A. suum infection in the mouse, and the quantitative analysis based on worm burdens concluded that there was no association between the Itln-2 gene and resistance to A. suum infection.
The lack of an association between the candidate gene of interest and resistance to A. suum infection contrasts with studies in which expulsion of T. spiralis and T. muris coincides with upregulation of Itln-2 expression (Pemberton et al. Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a, Reference Pemberton, Knight, Wright and Millerb; Datta et al. Reference Datta, Deschoolmeester, Hedeler, Paton, Brass and Else2005; Artis, Reference Artis2006). Immunolocalization of Itln-2 protein in Paneth and goblet cells (Pemberton et al. Reference Pemberton, Knight, Wright and Miller2004b; Artis, Reference Artis2006) may explain the common role of Itln-2 in resistance against T. spiralis and T. muris as both parasites inhabit the intestinal environment at the time of the host immune response, which results in expulsion. However, even though expression of Itln-2 and another Itln gene, Itln-1 in the small intestine was evident during Nippostrongylus brasiliensis infection (Voehringer et al. Reference Voehringer, Stanley, Cox, Completo, Lowary and Locksley2007), Itlns cannot be the sole mechanism of worm expulsion because C57BL/6j mice expel the parasite with the same kinetics as BALB/c mice (Ishiwata et al. Reference Ishiwata, Nakao, Nakamura-Uchiyama and Nawa2002). Similarly, T. muris parasites are expelled from C57BL/6j mice before adult worms develop (Faulkner et al. Reference Faulkner, Renauld, Van Snick and Grencis1998) despite lacking the Itln-2 gene (Pemberton et al. Reference Pemberton, Knight, Gamble, Colledge, Lee, Pierce and Miller2004a). Furthermore, Voehringer et al. (Reference Voehringer, Stanley, Cox, Completo, Lowary and Locksley2007) demonstrated that high Itln mRNA transcript levels in the lung of transgenic mice did not enhance parasite clearance. It is not yet known whether expulsion of T. spiralis and T. muris is influenced by Itln locus genotype, but we have shown clearly here that resistance to A. suum is not. The mechanism of resistance to A. suum infection appears to manifest itself in the liver of CBA/Ca mice (Dold et al. Reference Dold, Cassidy, Stafford, Behnke and Holland2010) and Itln-2 has not yet been documented to contribute to an immune response against a murine liver infection.
Analysis of the larval burdens observed in the pilot experiment indicated that mouse sex did not significantly affect worm burdens, although there was a clear tendency towards male mice carrying heavier larval worm burdens (Dold et al. Reference Dold, Cassidy, Stafford, Behnke and Holland2010). In addition, the heightened larval burdens in the susceptible strain were not considered to be a concern because they merely enhanced the divergence between the pulmonary burdens of the two mouse strains of the model in the direction that we expected (i.e. higher worm burdens in C57BL6j mice). Coupled with this, it was more practical to work with both male and female progeny in the much larger and logistically more complicated backcross experiment, not the least for ethical reasons.
The variability in larval burdens among susceptible individuals was magnified in the larger F2 study and among the parental reference strain mice. Despite this, divergence of the susceptible C57BL/6j and resistant CBA/Ca mice strains was still clearly evident. The mechanism behind the larval burden intra-strain variability, particularly of the susceptible male individuals, is not fully understood and requires further investigation, but we hypothesize that an important factor may be that, in contrast to earlier work based on only one sex, here mice of both sexes were present in close proximity in the animal house, albeit in separate cages.
Sex differences in immune function are mediated, in part, by steroid hormones (Grossman, Reference Grossman1985; Klein, Reference Klein2000, Reference Klein2004). The androgen testosterone is known to have suppressive effects on the immune system (Ahmed et al. Reference Ahmed, Dauphinee and Talal1985; Alexander and Stimson, Reference Alexander and Stimson1988; Hillgarth and Wingfield, Reference Hillgarth, Wingfield and Beckage1997). Host gender is an important factor in determining immunity to Trichuris muris (Hayes et al. Reference Hayes, Bancroft and Grencis2007; Hepworth and Grencis, Reference Hepworth and Grencis2009) and a recent study conducted by Hepworth et al. (Reference Hepworth, Hardman and Grencis2010) demonstrated that endogenous sex steroid hormones have a direct effect on the generation of a Th2 response to the intestinal roundworm. Increased host testosterone levels have led to higher levels of Strongyloides ratti (Kiyota et al. Reference Kiyota, Korenaga, Nawa and Kotani1984), N. brasiliensis (Solomon, Reference Solomon1966; Bone and Bottjer, Reference Bone and Bottjer1986), Nippostrongylus muris (Haley, Reference Haley1958) and T. spiralis (Charniga et al. Reference Charniga, Stewart, Kramar and Stanfield1981) and Heterakis spumosa (Harder et al. Reference Harder, Wunderlich and Marinovski1992), demonstrating that immunosuppression by testosterone is responsible for increased susceptibility to infection. Moreover, as discussed by Bohus and Koolhaas (Reference Bohus, Koolhaas, Adler, Felten and Cohen1991), exposure to environmental and social stressors can lead to immunosuppression and therefore increased susceptibility to infection. In captivity, aggression in male rodents can be triggered by female scent alone (Barnard et al. Reference Barnard, Behnke, Gage, Brown and Smithurst1997). Therefore, it is possible that even though the male mice in this study were not housed in cages with females, the scent of the females in close proximity may have increased aggression and investment in courtship behaviours among the male mice. Hence, the stressors associated with the presence of female mice may have also led to increases in gonadal steroids in males. The observed intra-strain variation in larval burdens may be a result of differing responses to an environmental challenge, influenced by social organization (Laudenslager and Kennedy, Reference Laudenslager, Kennedy and Ader2008). Infection in dominance hierarchies has been documented previously in response to the protozoan Babesia microti (Barnard et al. Reference Barnard, Behnke and Sewell1994), but data on dominance were not collected in the current work. Genetic variation and differences in expression of sex steroid-related immunosuppression may account for the varied and increased susceptibility in C57BL/6j but not CBA/Ca individuals. Mice with different MHC haplotypes have significantly different responses to Heterakis spumosa infection, which only become apparent after treatment with testosterone (Harder et al. Reference Harder, Danneschewski and Wunderlich1994).
The dramatically increased larval burdens in a number of male C57BL/6j mice and male F2 offspring bred from male C57BL/6j parents is of particular interest as previous attempts at immunosuppression, using the steroid hydrocortisone, did not enhance A. suum larval burdens in the susceptible mouse strain (Lewis et al. Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007). Therefore, it is possible that the stressor resulting from the presence of females in close proximity induced heightened testosterone levels in male individuals, which lead to some form of potent immunosuppression. The contrasting results between work conducted by Lewis et al. (Reference Lewis, Behnke, Cassidy, Stafford, Murray and Holland2007) and the current study may indicate that a single pulse administration of hydrocortisone does not have as great an influence on the mechanism of resistance as a continuous stressor. The target of this effect, however, remains intriguing since resistance to A. suum in the mouse is known to be independent of T-cells (Mitchell et al. Reference Mitchell, Hogarth-Scott, Edwards, Lewers, Cousins and Moore1976).
While larval burdens were confounded by an unexpected effect of co-habitation of male and female mice, this study found no evidence that Itln-2 plays a significant role in preventing the accumulation of A. suum larvae in the lungs of resistant strains of mice.
Acknowledgements
The authors would like to thank the technical staff in the Bioresources Unit, Trinity College Dublin and H. Ward for providing assistance in maintaining breeding colonies, A. Boyce and H. Ward for help with tissue processing and Baermanns, and S. Wright and J. Pate in Easter Bush Veterinary Centre for help with Intelectin screening. We would also like to thank S. Wright for undertaking preliminary experiments using the SLAMF7 primers on the parental mouse strains. S.W. was funded by the Biotechnology and Biological Sciences Research Council (BBE009069/1). This project was funded by Science Foundation Ireland Research Frontiers Programme (06/RFP/EEB009).