Published online by Cambridge University Press: 19 April 2005
Theileria are economically important, intra-cellular protozoa, transmitted by ixodid ticks, which infect wild and domestic ruminants. In the mammalian host, parasites infect leukocytes and erythrocytes. In the arthropod vector they develop in gut epithelial cells and salivary glands. All four intra-cellular stages of Theileria survive free in the cytoplasm. The schizont stages of certain Theileria species induce a unique, cancer-like, phenotype in infected host leukocytes. Theileria undergoes an obligate sexual cycle, involving fusion of gametes in the tick gut, to produce a transiently diploid zygote. The existence of sexual recombination in T. parva has been confirmed in the laboratory, and is presumed to contribute to the extensive polymorphism observed in field isolates. Key parameters in T. parva population dynamics are the relative importance of asymptomatic carrier cattle and animals undergoing severe disease, in transmission of the parasite to ticks, and the extent of transmission by nymphs as compared to adult ticks. Tick populations differ in vector competence for specific T. parva stocks. Recombinant forms of T. parva and T. annulata sporozoite surface antigens induce protection against parasite challenge in cattle. In future, vaccines might be improved by inclusion of tick peptides in multivalent vaccines.
The genus Theileria comprises tick-transmitted sporozoan protozoa that are the causative agents of a variety of disease syndromes in domestic and wild ruminants, and are collectively responsible for economic losses amounting to hundreds of millions of dollars annually in Sub-Saharan Africa and Asia. Theileria are unique among protozoa in that certain species are capable of immortalizing either mammalian lymphocytes or cells of the monocyte/macrophage lineage that they infect. Theileria are currently classified in the class Sporozoa together with human pathogens, including Plasmodium and Toxoplasma. Along with other Sporozoa, Theileria has been included within a sub-phylum designated the Apicomplexa, based on the common possession of an apical complex containing secretory organelles involved in invasion, or establishment, in the cells of their mammalian and invertebrate hosts. However the evolutionary and functional equivalence of the apical complex between different genera and hence the taxonomic validity of the Apicomplexa remains unclear. Analysis of 18s ribosomal RNA gene sequences demonstrates that the genus Theileria is phylogenetically most closely related to Babesia, a genus of tick-borne protozoans infective to the red cells of mammals including domestic livestock, and more distantly to the genus Plasmodium which causes malaria in humans and other species of vertebrates (Allsopp et al. 1994). There are similarities, but also significant differences between features of life cycle, genome organization and mammalian host immune responses to infection when Theileria and Plasmodium are compared.
Economically important Theileria species that infect cattle and small ruminants are transmitted by ixodid ticks of the genera Rhipicephalus, Amblyomma, Hyalomma and Haemaphysalis. Theileria species infective to domestic ruminants are summarized in Table 1. Globally the most important species are Theileria parva, transmitted by Rhipicephalus ticks, that causes a rapidly fatal lympho-proliferative disease known as East Coast fever (ECF). The disease was estimated to have been responsible for 170 million US dollars worth of economic loss in 1989 alone (Mukhebi, Perry & Kruska, 1992) and limits introduction of more productive exotic (Bos taurus) cattle in much of eastern, central and southern Africa. The primary host of T. parva is the African cape buffalo (Syncerus caffer) in which the parasite typically does not cause any disease. Theileria annulata, originating from Asian water buffalo (Bulbulus bubulis), and transmitted by several Hyalomma tick species, is responsible for tropical theileriosis from Southern Europe to China, a vast region in which an estimated 250 million cattle are at risk. Both T. parva and T. annulata induce a transformation-like phenotype in nucleated mammalian host cells, which is the major cause of pathology. Several other non-transforming Theileria are also responsible for disease in domestic ruminants as a result of anaemia induced by the intra-erythrocytic stage. T. sergenti and T. buffeli cause disease and economic loss in cattle in East Asia. T. mutans may be responsible for disease in cattle in sub-Saharan Africa particularly in mixed infections with other tick-borne pathogens, but the economic impact of this species has yet to be accurately assessed using sensitive and specific diagnostic tests. Theileria lestoquardi is the most pathogenic of several small ruminant Theileria species infective to sheep and goats in Northern Africa and widely across Asia. Most wild bovids in Africa are infected by one or more species of Theileria and the epidemiological situation can be complex with East African cattle potentially being infected with up to five Theileria species at one time (Norval, Perry & Young, 1992). Cattle can be infested with several species of tick and more than one species of Theileria can also be transmitted by the same tick. For example, the morphologically very similar sporozoites of T. parva and the normally non-pathogenic T. taurotragi can both occur together in R. appendiculatus salivary glands, although they can be distinguished by molecular methods based on hybridization to rRNA (Bishop et al. 1994). Additional Theileria species that are infective to livestock, and may possibly be pathogenic in specific circumstances, continue to be discovered. The range of T. buffeli has been extended to Africa (Ngumi et al. 1994), and a hitherto undescribed Theileria that is pathogenic to small ruminants has been discovered in North Western China (Schnittger et al. 2000). A Theileria species originally described from schizont-infected lymphocyte culture isolates from Cape buffalo (Conrad et al. 1987; Allsopp et al. 1993) has recently been found in cattle subjected to challenge by ticks that are presumed to have fed on buffalo (Bishop & Musoke, unpublished data). Theileria infections have also been associated with bovine fatalities in the United States, and the presence of Theileria was confirmed in both Amblyomma americanum and Dermacentor variabilis by PCR amplification using primers derived from small subunit rRNA (SSU rRNA) genes sequences (Chae et al. 1999).

The T. parva genome, which is approximately 8·5 Mbp in size, is small for a eukaryote with a complex life cycle involving several distinct intracellular stages in both the arthropod vector and the mammalian host. The genome is divided into 4 chromosomes, containing 33 SfiI fragments (Morzaria & Young, 1992). Both the T. parva (http://www.tigr.org/tdb/e2k1/tpa1/) and T. annulata (http://www.sanger.ac.uk/Projects/T_annulata/) genome sequences are currently nearing completion. The current status of knowledge relating to the T. parva genome has been reviewed recently (Nene, Morzaria & Bishop, 1998; Nene et al. 2000, 2002) and will not be discussed in detail in this article. Most research on Theileria to date has been devoted to the life-cycle stages occurring in the mammalian host, particularly the pathogenic, intra-cellular schizont stages of T. parva and T. annulata, which respectively infect cells of the lymphoid and myeloid lineages and activate host signal transduction pathways thereby inducing cancer-like syndromes in infected mammalian lymphocytes, monocytes and macrophages. The only tick-expressed stage of Theileria for which any molecular information is currently available is the sporozoite, which is secreted from the tick salivary glands into the vertebrate host. This article will concentrate on tick vector-associated aspects of Theileria biology and will review the status of vaccine development based on an antigen isolated from the tick-derived sporozoite life cycle stage. The data presented primarily relates to T. parva, the species for which most information is currently available.
Theileria parva life-cycle stages in the tick vector and bovine host have been comprehensively described previously and are summarized in Fig. 1. The three-host brown ear tick Rhiphicephalus appendiculatus is the major vector of the parasite. Theileria parva exhibits a very narrow tick and mammalian host range, and there is no laboratory animal model susceptible to infection with the parasite. Rhipicephalus appendiculatus is classified as a three-host tick because the larvae, nymphs and adults feed on different hosts, which are not necessarily cattle. Transmission is trans-stadial, which means that larval or nymphal instars of the tick acquire infections from a blood-meal, which is then transmitted to a new host, after moulting by nymphs or adults, respectively. Transmission of Theileria has not been shown to be transovarian, unlike the related protozoan Babesia transmitted by the one host tick Boophilus (Norval, Perry & Young, 1992). The Hyalomma vectors of T. annulata are, by contrast, two host ticks, with larval and nymphal instars feeding on the same host. Sporogony takes place in the tick salivary glands in response to a combination of increased temperature and components present within the blood meal. Infective sporozoites are released from about day 3 to day 7 of tick feeding. One infected cell may contain 40–50000 sporozoites, but the mechanics of tick feeding is believed to result in a trickle release of sporozoites from the salivary glands (Shaw, 2002; Shaw & Young, 1994). A tick with a single infected acinar cell can potentially cause severe disease and death. However, the severity of ECF is sporozoite dose dependent and individual animals exhibit different thresholds of susceptibility. Some animals undergo a mild disease reaction and recover due to development of protective cellular immune responses.
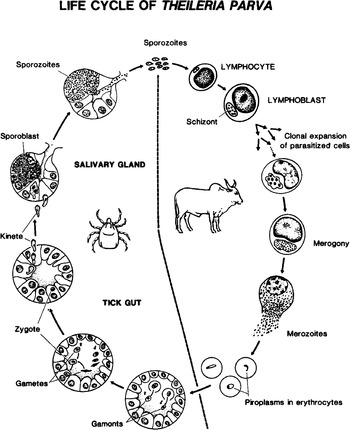
Fig. 1. Life cycle of Theileria parva in cattle and the ixodid tick vector Rhipicephalus appendiculatus.
Once introduced into the host, T. parva sporozoites invade a restricted range of cattle and buffalo cells. In vitro they enter purified B cells or T cells which express receptors which are either CD4+, CD8+, or CD4−/CD8− (Baldwin et al. 1988), and also afferent lymph veiled cells and monocytes (Shaw, Tilney & Mckeever, 1993), although the latter cannot subsequently be immortalised. The entry process is probably energy dependent and is likely to involve specific receptors located on the host cells (Shaw, 1997, 2002). T. annulata infects cells of the monocyte/macrophage lineage, although it can also enter B cells. Other species such as T. taurotragi, originally isolated from eland (Taurotragus oryx), appear to have a much wider host range, at least in vitro (Stagg et al. 1983). Like viruses transmitted by R. appendiculatus (see chapter by Nuttall & Labuda in this Supplement) components of tick saliva appear to promote T. parva pathogen transmission by facilitating sporozoite entry into host leukocytes (Shaw, Tilney & McKeever, 1993), although the mechanism may be an indirect one involving non-specific activation of host lymphocytes by saliva components. Unlike other sporozoans, T. parva sporozoites are not motile. They do not possess all of the components of a typical apical complex as described for other Apicomplexa, and also by contrast with Plasmodium entry into the host cells is not orientation specific, and the rhoptries and microspheres are involved in establishment in the host cell subsequent to entry rather than in the entry process itself (Shaw, Tilney & Musoke, 1991). Unlike other Apicomplexa, T. parva sporozoites also appear to lack morphologically distinguishable micronemes (Shaw, Tilney & Musoke, 1991). The newly internalized parasite is surrounded by a host cell membrane which is rapidly (<15 min) removed by the parasite, thereby evading intracellular host cell defense mechanisms. In this respect, Theileria is similar to a phylogenetically diverse range of intracellular pathogens including Listeria, Shigella and Trypanosoma cruzi (reviewed by Andrews & Webster, 1991), presumably as a result of independent evolution of this solution to the problem of intracellular survival. Rapid dissolution of the host cell membrane by T. parva is closely correlated with the discharge of the contents of the rhoptries and microspheres. The major surface molecule of T. parva sporozoites, p67, and MHC class I molecules on the host cell are implicated in the entry process as monoclonal antibodies (mAbs) to these proteins inhibit sporozoite entry (Musoke et al. 1984; Shaw et al. 1991). Because of the restricted host cell specificity it seems unlikely that MHC class I molecules constitute the sole recognition site of sporozoites. Mobilization of calcium stores within sporozoites, parasite and host cell proteases, and signal transduction via host cell and parasite protein kinases and G proteins have all been demonstrated to play a role in the entry process (Shaw, 1997). Sporozoite organelles that discharge their contents after entry are likely to contain a microtubule nucleating factor as the free intracellular sporozoites are rapidly surrounded by a network of microtubules.
The schizont is a multinucleated, syncytial body. Association of the schizont with the host cell nuclear spindle ensures that daughter host cells remain infected during cytokinesis (Carrington et al. 1995). Although the parasite and host cells divide in synchrony, schizont DNA synthesis occurs as the host cell enters mitosis and is immediately followed by division when the host cell is in metaphase (Irvin, Ocama & Spooner, 1982). The molecular basis of T. parva schizont-induced B and T cell transformation is not yet fully understood, but there is evidence for increased bovine casein kinase II activity (Ole-MoiYoi et al. 1993). Existing data indicate subversion of lymphocyte signal transduction pathways resulting in activation of the JNK mitogen-activated protein kinases, phosphatase PP2A and constitutive expression of nuclear factor kappa beta (NF-kB) and also IL2 and its receptor in the infected cells (reviewed by Heussler, 2002). Recently it has been demonstrated that constitutive activation of NF-kB may be effected by the recruitment of the IKB regulatory kinase complex onto the surface of the schizont (Heussler et al. 2002). In testing a disease model for transformation induced by T. parva, it was demonstrated that dysregulation of casein kinase II mRNA expression, along with myc oncogene activation, results in the development of fulminant leukemia in transgenic mice (Ole-MoiYoi, 1995; Seldin & Leder, 1995). When injected sub-cutaneously into nude or SCID mice infected T. parva and T. annulata lymphoblastoid cells form a huge tumour mass (Irvin et al. 1975; Fell, Preston & Ansell, 1990). Despite these insights into parasite modulation of host cell phenotype, nothing is yet known about the parasite molecules involved in this process. The schizont stages of both T. parva and T. annulata are the major cause of pathology and disease. Merogony occurs within infected lymphocytes (Shaw & Tilney, 1992) and merozoites are released by host cell rupture. The merozoites invade red blood cells (RBCs) in a manner similar to that described for sporozoite entry into lymphocytes, where they develop into piroplasms, which, like the intra-lymphocytic schizont, lie free in the erythrocyte cytoplasm. There is very little multiplication of the piroplasm stage of T. parva, although intra-erythrocytic multiplication of the piroplasm does occur in T. annulata infections and this stage contributes to the pathology of tropical theileriosis. Other species of Theileria, especially the non-transforming ones, such as T. mutans, T. buffeli and T. sergenti, exhibit high levels of intra-erythrocytic merogony resulting in anaemia and pathology due to RBC lysis. Ticks become infected after feeding on blood containing piroplasm-infected RBCs which lyse in the tick gut. Morphologically distinct forms of the parasite are found in the tick gut which is the likely site of gametogenesis (Norval, Perry & Young, 1992). Gametes fuse to form a zygote which invades the cells of the gut epithelium. Kinetes are released into haemolymph and invade cells of the salivary gland, where they reside until suitable environmental conditions are presented. Full sporogony is initiated on stimuli received when the tick attaches to the mammalian host and appears to be closely linked with the moulting cycle of the tick. This completes the life cycle. All stages of the life cycle are haploid except for zygote in the tick gut.
The life cycle stages of Theileria species in their ixodid vectors have been relatively little studied due to the difficulty of culturing them. The available information has been reviewed previously (Mehlhorn & Schein, 1984; Norval, Perry & Young, 1992; Shaw & Young, 1994; Shaw, 2002). This section draws heavily on these earlier accounts.
The life cycle of Theileria in the tick begins with the ingestion of piroplasm-infected erythrocytes within the blood meal, and at repletion millions of infected erythrocytes are ingested by adult ticks, even from an animal with a low piroplasm parasitaemia. The volumes of blood ingested by larvae and nymphs are much smaller and hence the numbers of parasites surviving and developing in the midgut will be correspondingly smaller. Due to the inability to culture the red blood cell stages of T. parva it is unknown whether gametocytes (stages that are predetermined to differentiate into gametes in the tick gut) occur in the circulating blood as they do in Plasmodium infections (reviewed by Sinden et al. 1996). Since there is currently no evidence for sexual differentiation within the mammalian erythrocytic stages, and there is also a marked drop in temperature after the tick completes repletion and falls to the ground, it is possible that the processes controlling sexual differentiation in Theileria and Babesia differ markedly from other sporozoan protozoa. It is believed that the vast majority of the ingested piroplasms are rapidly destroyed in the gut lumen possibly by acid phosphatases secreted by gut epithelial cells (Shaw & Young, 1994). Soon after the completion of feeding, structures designated ‘Strahlenkorper’, or ray bodies, can be observed in smear preparations by light microscopy. The significance of these as sexual structures, originally thought to represent microgametes, was initially recognized by Schein and colleagues (Mehlhorn & Schein, 1984). The process of gametogenesis appears similar in those species that have been studied, including T. parva and T. annulata, but there is no evidence regarding the factors required for triggering gamete development. Recent studies suggest that in Babesia (Gough, Jorgensen & Kemp, 1998) there is no clear indication of morphologically distinguishable micro- and macro-gametes and the zygote is formed by the fusion of two similarly sized and structured ray bodies (gametes) that differ only in that one is more electron dense than the other. The recently reported formation of the zygote from two structurally similar gametes in Theileria and Babesia differs significantly from most other sporozoans, including Plasmodium (Shaw, 2002). Subsequently the spherical zygote invades a gut epithelial cell and differentiates into a motile kinete. It is currently not known what type of tick gut cell the zygotes enter, or what the mechanism of the entry process is. However it is possible that a stiletto-like apical arrowhead structure observed on the ray body is involved in this process, since a similar structure is present in the gut stages of Babesia and appears to be important in invasion of tick gut cells (Rudzinska et al. 1983). While the mechanism of entry of the zygote into gut epithelial cells is uncertain, the developing intracellular parasite is not enclosed in a parasitophorous vacuole and lies free in the cytoplasm. Therefore, a common feature of all four intracellular stages of Theileria, the kinete in the tick gut, the sporozoite in the tick salivary gland and the intra-lymphoytic and erythrocytic stages in the mammalian host, is that they rapidly escape any enclosing host membrane and lie free in the cytoplasm. Theileria kinetes develop only in the tick gut cells and are typically uni-nucleate.
The motile kinetes subsequently invade salivary glands and this process is very specific in that kinetes have not so far been recorded in other tick organs, including reproductive tissues. Development of kinetes and their appearance in the tick haemocoel is associated with the tick moulting cycle (Shaw, 2002). To reach the salivary gland, the kinete must cross several obstacles, the basal membrane and lamina to allow exit from the gut cells in which it is originally located, followed by other barriers, to allow entry into the salivary gland acinar cell in which sporogony occurs. Ixodid salivary glands contain four types of acini, I, II and III are present in both males and females, while type IV is in males only (see chapter by Bowman & Sauer, in this Supplement). Type I acini are non-granular and thought to be involved primarily in off-host osmoregulation, whereas types II, III and IV contain a variety of secretory cell types designated by lower case letters from a–h. It appears that the motile kinetes probably have the ability to recognize specifically the type III salivary gland acinar cells within the salivary glands and may also be able to invade selectively e cells within the acinus. It has been suggested that the carbohydrate composition of the type III acinus determines the susceptibility of this cell type to parasite infection (reviewed by Shaw, 2002). Theileria develop almost exclusively within the e cells of the type III acinus, although it has been demonstrated that in ticks very highly infected with T. annulata the d cells and type II acini can also be infected (Mehlhorn & Schein, 1984).
Sporogony, which is the only multiplicative stage within the tick, occurs within the salivary gland and is typically triggered by feeding, although it has been shown that sporogony can also be completed at high ambient temperatures in a proportion of acini (Young & Leitch, 1982). The number of sporozoites produced during sporogony has been estimated at between 30000–50000 per infected acinar cell for female R. appendiculatus and up to 140000 for T. taurotragi (Norval, Perry & Young, 1992; Shaw & Young, 1994). There are indications that T. parva sporozoite numbers are lower in male R. appendiculatus than in females and it has also been demonstrated that numbers of sporozoites are lower in the e cells of nymphal compared to adult ticks, which has potential consequences for the relative importance of different instars in T. parva transmission (Ochanda et al. 1996). Theileria mutans in the salivary glands of Amblyomma variegatum typically produces fewer (approximately 5000) but larger sporozoites. The ultrastructure of the mature sporozoites has been described in detail (Fawcett, Doxsey & Buscher, 1982). Unlike other genera of the Apicomplexa, there is no clearly defined apical complex. A spherical body enclosed by three or more membranes is located close to the nucleus and may correspond to the apicoplast described from Plasmodium and other genera of Sporozoa (reviewed in Wilson & Williamson, 1997). As in other life cycle stages of Theileria (Shaw & Tilney, 1992; Ebel et al. 1997) structures corresponding to the endoplasmic reticulum and Golgi apparatus of higher eukaryotes are not visible although proteins associated with the classical secretory pathway are present in Theileria (Janoo et al. 1999). There is a number of peripherally located membrane-bound bodies termed microspheres and also a group of up to six larger, membrane-bound rhoptries. Both these secretory organelles discharge after entry into the host cell in association with establishment of the parasite (Shaw et al. 1991). Specific proteins encoded by cloned genes have been localised to these organelles. These include the p104 antigen located in the rhoptries (Iams et al. 1990) and the p150 and PIM antigens in the microspheres (Skilton et al. 1998). There is some evidence to suggest that sporozoites are released gradually from an infected cell in a manner similar to the release of secretory granules by apocrine secretion (summarized in Shaw & Young, 1994). This will have implications for the nature of the parasite challenge to the mammalian host and hence for the viability of control strategies using novel recombinant vaccines as agents for control of Theileria.
As in Plasmodium species, T. parva undergoes an obligatory sexual cycle in the tick vector. While the mammalian life cycle stages are haploid, there is a transient diploid phase in the tick gut after fusion of gametes (Gauer et al. 1995). In T. parva, according to direct measurements of the DNA content of single cells by fluorescence microscopy, a post-zygotic meiosis appears to occur in the gut prior to differentiation of the kinete, rapidly restoring the haploid condition. In T. annulata, by contrast, the kinete appears to remain diploid and meiotic division may occur at a later stage during sporogony in the salivary glands (Gauer et al. 1995). Laboratory crosses have been performed between T. parva Muguga and either of two other parasite stocks, T. parva Uganda and T. parva Marikebuni by feeding ticks on cattle co-infected with these stocks (Morzaria et al. 1993). Hybridisation of ‘stock specific’ oligonucleotides derived from T. parva Muguga and Uganda (Allsopp et al. 1989; Bishop et al. 1993) revealed that 38% of single-infected acini derived from progeny of the Muguga/Uganda cross appeared to be recombinant. Subsequent analysis of a cloned parasite derived from one of the putatively recombinant acini revealed mixed profiles using several probes in Southern blot assays. The data suggested that at least four independent, physical crossover events had occurred on the two smallest chromosomes. In the case of the T. parva Muguga/Marikebuni cross, three independent clonal progeny were isolated and in all three progeny isolated from this cross a T. parva Muguga-type TpR locus appeared to have been introgressed into a genome background in which the majority of other polymorphic loci were of Marikebuni type. These probably represented independent occurrences of similar recombination events, since the Muguga-type Tpr genotypes differed from one another and were different from that of the previously characterized T. parva Marikebuni TpR RFLP pattern (Bishop et al. 2002).
To what extent recombination occurs in T. parva in field populations is currently unknown, although as in Plasmodium (Paul & Day, 1998; Awadalla et al. 2001) there is considerable genetic heterogeneity between individuals and populations in the field, and it seems reasonable to assume that this is attributable at least partially to a consequence of the obligate sexual cycle. This issue of the extent of recombination between different parasite clones can only be resolved by direct examination of kinetes using PCR analysis of single copy allelic markers, as has been done for Plasmodium oocytes from mosquito populations in the field. In a recent study, a high percentage of apparently recombinant P. falciparum zygotes were found at a Tanzanian study site (Babiker et al. 1994). However, such studies will be technically more difficult for Theileria infections in ticks. According to research on Plasmodium, levels of out-crossing may vary according to the force of infection in different epidemiological situations, and even quite high levels of inbreeding may still result in linkage equilibrium between different alleles in Plasmodium populations (Paul et al. 1995; reviewed by Paul & Day, 1998). It is possible that factors such as host and vector movements may be more important in genetic substructuring of T. parva populations than the extent of meiotic recombination, and that this may differ substantially between cattle and the wildlife reservoir host buffalo (S. caffer). For example, the major determinant of genetic substructuring among nematode parasites of sheep, cattle and a wildlife reservoir host, white tailed deer, was host movement, with genetic differentiation of parasite populations being greater in the white tailed deer due to more extensive movement of the livestock species between different localities (Blouin et al. 1995). Another issue that has yet to be addressed for Theileria or other parasites, including Plasmodium (Day et al. 1992), is the extent to which additional processes of diversification, such as somatic mutation, genome rearrangements, gene deletions and gene conversion during the asexual multiplication phases, in addition to classical recombination during the obligate sexual cycle, are important in generation of diversity in T. parva parasite populations.
The transmission dynamics and epidemiology of theilerosis vary in different areas of eastern, central and southern Africa according to a complex interplay of factors, including the level of tick control, cattle genotype and management regime, proximity of a wildlife reservoir of disease, and the interaction of tick and parasite populations with differing genetic composition. Initial transmission dynamic modelling of T. parva infections (Medley, Perry & Young, 1993) focused on a particular epidemiological situation described by the term ‘endemic stability’ (Norval, Perry & Young, 1992) in which African Zebu (Bos indicus) cattle, previously exposed to T. parva over several generations and subjected to little or no tick control, are continuously challenged by infected ticks, and either become immune or die by day 150 of exposure. In endemically stable situations the majority of ticks are believed to exhibit relatively low levels of infection in terms of both prevalence (percentage of ticks infected) and abundance (mean number of infected acini/tick), both of which are frequently low in these epidemiological circumstances (Young et al. 1986). Laboratory data on experimental tick infections (Buscher & Otim, 1986), and also field data (S. Morzaria, unpublished data) indicate that the abundance of T. parva infections can frequently be described by a negative binomial distribution. Therefore the abundance of mature T. parva infections in salivary glands is typically over-dispersed, with a small proportion of ticks having many infected acini, while a majority may contain only a single infected acinus. The key conclusion from preliminary transmission dynamic modelling work (Medley, Perry & Young, 1993) is that the degree to which animals that have recovered from theileriosis, but remain infected (known as ‘the carrier state’), are able to transmit the infection to tick larvae or nymphs is a crucial determinant of the dynamics of infection in a herd. Currently there is no comparative experimental data on the relative tick transmissibility of T. parva parasites from carrier animals relative to those undergoing acute infections. It is known that the level of piroplasm parasitaemia is the most important parameter influencing levels of infection in R. appendiculatus ticks experimentally fed on cattle (Young et al. 1996). However, no correlation has been found between the number of zygotes and kinetes present in the tick gut and haemolymph, respectively, and the number of sporoblasts in the salivary glands (Buscher & Otim, 1986). This implies that there is some degree of parasite destruction occurring either with the gut cells or during kinete migration through the haemolymph. Although the mechanisms are not yet fully understood there may be an element of Theileria specificity, since R. appendiculatus haemolymph exhibited no apparent effect on bacteria in vitro (Watt et al. 2001). The issue of arthropod vector immune responses to parasites is an emerging area of research that has been most extensively researched in Drosophila and Anopheles mosquitoes (reviewed by Dimopoulos, 2003). Recent experimental work suggests that certain stocks may not induce a long-term carrier state, and that even when experimentally infected or vaccinated cattle are persistently infected with T. parva, as judged by positivity using a PCR-based assay with T. parva-specific primers, the infection may not always be experimentally tick-transmissible (Skilton et al. 2002). Data generated using PCR assays also indicate that animals from endemic areas that are often close to 100% serologically positive for Theileria infection, according to application of immunofluorescence or ELISA tests (Young et al. 1986), may not necessarily be carriers of T. parva parasites based on the failure of PCR assays to detect parasite DNA in these animals (R. Bishop, unpublished data).
Two important parameters in tick-borne disease transmission dynamics for which most currently available data relates to transmission of T. parva by R. appendiculatus have potential relevance to transmission of other livestock and human pathogens by ixodid tick species: (1) differences in vector competence for pathogens between different tick stocks: (2) the importance of nymphal/larval transmission of pathogens relative to larval/nymphal transmission. Distinct R. appendiculatus tick stocks have varying effectiveness in their ability to transmit two different T. parva stocks (from Kenya and Zimbabwe) to cattle in an experimental context in the laboratory (Ochanda et al. 1998). These authors also demonstrated interaction between parasite and tick in terms of transmission efficiency. Specific tick stocks transmitted certain parasites efficiently and other parasite stocks less well. This effect was dependent on the particular combination of tick and parasite genotpye (Ochanda et al. 1998). Furthermore, the offspring of individual ticks within families derived from two different R. appendiculatus stocks (Kiambu and Muguga) have been demonstrated to have heritabilities between approximately 0·4 and 0·6 for susceptibility to infection with T. parva (Young et al. 1995), indicating that these differences are, at least in part, genetically determined. The basis of such differences has not yet been investigated for ticks but, by analogy with insects, may be related to factors such as levels of expression of nitric oxide in tick gut tissues or antimicrobial peptides, such as cecropins and serpins, in a variety of tissues. This suggests that selection of tick lines that are refractory or susceptible to T. parva infection may be possible. The first study to attempt to make an objective comparison of transmission of T. parva between different instars is that of Ochanda et al. (1998). The conclusion of this research was that larval/nymphal transmission is less efficient than nympal/adult transmission. One factor probably underlying this result is that that mean numbers of type III acini are 1736 in females, 1346 in males and only 87 in nymphae (Ochanda et al. 1996). However, since numbers of nymphal and larval ticks infecting livestock are higher than that of adults, Norval et al. (1992) suggested a ratio of 1[ratio ]100[ratio ]1000 for adult/nymphal and larval instars and, in addition, the numbers of the nymphal and larval immature stages are technically much more difficult to quantify than adults, the issue of the relative quantitative importance of nymphal versus larval transmission of T. parva is not yet fully resolved (Ochanda et al. 1996). It is interesting to note that, due to being infected by markedly lower numbers of parasites, nymphal transmission may typically result in milder T. parva infections and hence be important in induction of immunity in cattle that maintain the carrier state.
Vector biology and population dynamics have obvious relevance to Theileria transmission. This topic has been reviewed previously (Norval, Perry & Young, 1992; Shaw & Young, 1994) and is only covered briefly in this article. The ixodid ticks that are most important for transmission of economically important Theileria species, namely, R. appendiculatus, H. anatolicum and A. variegatum, have a wide variety of hosts but all have a preference for feeding on large mammals, including wild as well as domestic species as adults. However, the larvae and nymphs also frequently infest small mammals, mainly carnivores and lagomorphs in the case of Rhipicephalus, but additional vertebrate taxa including reptiles and birds for Hyalomma and Ambylomma (see Norval, Perry & Young, 1992 for further details). There are typically several tick species capable of transmitting the same Theileria species. For example in southern Africa, R. appendiculatus and A. variegatum are replaced by R. zambeziensis and A. hebraeum as major agents in the transmission of T. parva and T. mutans, respectively. Another issue is geographical variation in the behaviour of tick populations which can affect transmission. For example in Southern Africa, R. appendiculatus undergoes diapause during the cold dry season resulting in a distinct seasonality of transmission which is different from the situation in Eastern Africa (Norval, Perry & Young, 1992; Shaw & Young, 1994). The existence of mildly pathogenic, or non-pathogenic, Theileria that can also be transmitted to domestic livestock and co-infect the same ticks complicates the epidemiological picture. Another issue in relation to vector population dynamics is the extent to which infection with Theileria may impact on the survival of the vector. Although, as mentioned previously, most T. parva infections in the field are at low levels and probably do not impact significantly on vector survival, Hyalomma ticks have been found in the Sudan with sufficiently high infections of T. annulata to reduce their feeding efficiency and survival (Walker et al. 1983).
Currently management of ECF is primarily effected through control of the tick vector using acaricides although this is unsustainable in the medium term (Tatchell, 1987). An ‘infection and treatment’ live vaccine based on the injection of a potentially lethal dose of sporozoites together with a long-acting dose of tetracycline (Radley et al. 1975) has been developed. However, the live vaccine has not been widely deployed due to problems related to use of potentially lethal live parasites, the parasite strain-specificity of the immunity induced in a proportion of vaccinated cattle and, most importantly, the requirement for a cold chain and skilled veterinarians for effective delivery.
Research has shown that humoral immune responses directed against surface epitopes on the sporozoite stage can also be protective by neutralising infectivity of sporozoites for bovine lymphocytes (Dobbelaere et al. 1984; Musoke et al. 1984). The gene encoding p67, the sporozoite surface protein that is a major target of this neutralizing activity, has been cloned (Nene et al. 1992) and demonstrated to induce protection in cattle against experimental needle challenge with a lethal dose of sporozoites (Musoke et al. 1992). Both a bacterially-expressed recombinant (Musoke et al. 1992) and a form expressed in insect cells (Nene et al. 1995) have consistently been shown to induce approximately 70% protection against lethal needle challenge with sporozoite stabilates using susceptible taurine cattle in the laboratory. Furthermore, nucleotide sequence analysis of the p67 gene reveals absolute conservation among cattle-derived parasites (Nene et al. 1996) indicating that the vaccine should be robust against heterologous parasite challenge, a hypothesis which has since been experimentally confirmed. In order to promote further development and optimisation of the p67 recombinant vaccine, neutralising epitopes with the protein have recently been identified using synthetic peptides (Nene et al. 1999). In addition, an 80 amino acid section located near the C-terminus of the protein, which is easier to produce in recombinant form, has recently been demonstrated to provide equivalent levels of protection to the full-length molecule (Bishop et al. 2003). Several p67 vaccine field trials have been performed in Kenya in order to determine protective efficacy against tick challenge within different production systems and epidemiological situations. These indicated reduction in severe disease of 47–50% relative to un-immunised cattle (Musoke, unpublished data). Since there is no demonstrable heterogeneity in the p67 gene among cattle-derived parasites (Nene et al. 1996), factors that contribute to the lower levels of protection against T. parva sporozoite challenge observed under field conditions may well include differences in the immune responses to vaccination, differences in the individual threshold of infectivity and immuno-modulatory molecules secreted by ticks during feeding. These p67 field trial results suggest that the T. parva p67 experimental vaccine would be a useful model system to evaluate whether incorporation of tick salivary gland antigen components into a multivalent vaccine might improve the efficacy of a recombinant anti-pathogen vaccine when subjected to a field tick challenge.
The major surface antigen of T. annulata, known as SPAG1, exhibits significant sequence identity (40%) with p67. SPAG1 shares cross-reacting neutralizing determinants that inhibit invasion of leukocytes in an in vitro assay and it has been demonstrated that recombinant forms of these proteins exhibit a level of mutual cross-protection on heterologous challenge (Knight et al. 1996; reviewed by Boulter & Hall, 1999). By contrast with p67, several T. annulata SPAG1 isolates from cattle are quite polymorphic in deduced amino acid sequence (Katzer, Carrington & Knight, 1994). An orthologous gene has also been cloned from sporozoite RNA of the pathogenic small ruminant parasite T. lestoquardi (Skilton et al. 2000). This molecule has been expressed in a recombinant form but not yet evaluated against sporozoite challenge in sheep or goats. The molecule (designated SLAG1) is closer to the SPAG1 (58% identity) than to p67 (40% identity) in amino acid sequence.
Although significant information has been accumulated regarding the tick-infective stages of Theileria over the last 25 years, much of this currently relates to the sporozoite stage. Knowledge of the tick gut and haemolymph stages, including molecular processes involved in infection of ticks by the intra-erythrocytic stages of Theileria, factors triggering differentiation of gametes, and the invasion of the gut epithelia and subsequent development of the motile kinete, is still rudimentary. This may remain true in the near future due to the difficulty of isolating and culturing these life cycle stages. The completion of two Theileria genome sequences, combined with comprehensive genome-wide micro array studies using RNA from different life cycle stages, and generation of extensive Expressed Sequence Tag (EST) data from T. parva-infected R. appendiculatus salivary glands (V. Nene, M. Gardner & R. Bishop, unpublished data), will generate new information relating to T. parva genes that are specifically expressed in sporozoites, and the tick-infective intra-erythrocytic piroplasm stages. As EST and proteomics data become available from proteins expressed in tick salivary glands, understanding of host–parasite interaction at the molecular level will increase. This should include mechanisms of action of tick molecules involved in modulation of mammalian immune and inflammatory systems and insight into how tick-secreted salivary gland molecules promote Theileria transmission. Another potential research area is on the interaction of Theileria spp. and their ixodid vectors in relation to differences in vector competence. Molecular markers for genotyping the parasite have recently been developed and are now available to for application in such studies. Field work on Theileria population genetics using new molecular markers and immunological assays will allow data be collected on how the immune response of the host impacts on parasite transmission as a result of re-infection by Theileria parasites with differing genoptypes. This information, combined with data on the relative infectivity to ticks resulting from different types of host infection, should result in the generation of more realistic models of transmission dynamics. This will improve understanding of parasite transmission and prediction of the impact of control measures. The possibility of combining the p67 sporozoite recombinant vaccine with one or more tick saliva components in order to enhance protection against tick challenge is an attractive future option for the control of East Coast fever.
We are grateful to Dr Paul Spooner for critical proof reading of the manuscript. This is ILRI publication 200302.

Table 1. Theileria species infective to domestic ruminants, their ixodid tick vectors and geograpical distribution. * Indicates Theileria species infective to small ruminants. Other species are infective to cattle

Fig. 1. Life cycle of Theileria parva in cattle and the ixodid tick vector Rhipicephalus appendiculatus.