Introduction
The P300 wave is an event-related potential (ERP) that is recorded as a positive deflection on an electroencephalogram (EEG) about 300 ms after a participant receives an attended unusual or task-relevant stimulus. It is typically elicited by infrequent sensory stimuli in a row of repeated stimulus presentations (Polich, Reference Polich1998; Duncan et al. Reference Duncan, Barry, Connolly, Fischer, Michie, Naatanen, Polich, Reinvang and Van Petten2009) and reflects an endogenous cognitive process triggered in analyzing sensory stimuli (Picton, Reference Picton1992; Polich, Reference Polich2007).
The P300 amplitude is interpreted as a correlate of resources allocated to processing the task-relevant stimulus (Curran, Reference Curran2004; Azizian & Polich, Reference Azizian and Polich2007). The origin of the P300 involves the complex summation of activity from multiple brain regions, particularly the various association areas of the cerebral cortex and the limbic system (Picton, Reference Picton1992; Bledowski et al. Reference Bledowski, Prvulovic, Goebel, Zanella and Linden2004; Polich, Reference Polich2007; Mangalathu-Arumana et al. Reference Mangalathu-Arumana, Beardsley and Liebenthal2012). However, little is known about the neurochemical substrates of the P300 and the specific neurotransmitter systems involved in generating this ERP response (Picton, Reference Picton1992; Frodl-Bauch et al. Reference Frodl-Bauch, Bottlender and Hegerl1999; Nieuwenhuis et al. Reference Nieuwenhuis, Aston-Jones and Cohen2005; Polich & Criado, Reference Polich and Criado2006).
Patients with schizophrenia have been reported to have P300 amplitude reduction and latency prolongation compared to healthy controls (Frangou et al. Reference Frangou, Sharma, Alarcon, Sigmudsson, Takei, Binnie and Murray1997; Coburn et al. Reference Coburn, Shillcutt, Tucker, Estes, Brin, Merai and Moore1998; Ozgurdal et al. Reference Ozgurdal, Gudlowski, Witthaus, Kawohl, Uhl, Hauser, Gorynia, Gallinat, Heinze, Heinz and Juckel2008; Shin et al. Reference Shin, Krishnan, Hetrick, Brenner, Shekhar, Malloy and O'Donnell2010; Jahshan et al. Reference Jahshan, Wynn and Green2013). Neurophysiological changes, such as a reduction in P300 amplitude, have been described in populations at ultra-high risk (UHR) of developing schizophrenia (van der Stelt et al. Reference van der Stelt, Lieberman and Belger2005; Bramon et al. Reference Bramon, Shaikh, Broome, Lappin, Berge, Day, Woolley, Tabraham, Madre, Johns, Howes, Valmaggia, Perez, Sham, Murray and McGuire2008; Frommann et al. Reference Frommann, Brinkmeyer, Ruhrmann, Hack, Brockhaus-Dumke, Bechdolf, Wolwer, Klosterkotter, Maier and Wagner2008; van Tricht et al. Reference van Tricht, Nieman, Koelman, van der Meer, Bour, de Haan and Linszen2010) and those changes may run a progressive course, from the prodromal to the chronic phases of schizophrenia (Ozgurdal et al. Reference Ozgurdal, Gudlowski, Witthaus, Kawohl, Uhl, Hauser, Gorynia, Gallinat, Heinze, Heinz and Juckel2008). However, a longitudinal study has shown that these impairments remain stable from the prodrome up to the development of a full psychotic episode (van Tricht et al. Reference van Tricht, Nieman, Koelman, Bour, van der Meer, van Amelsvoort, Linszen and de Haan2011). Two meta-analyses of P300 in auditory oddball paradigms found a large pooled standardized effect size (PSES) of 0.85 and 0.89 respectively for P300 amplitude differences between patients with schizophrenia and controls at central (Cz) and parietal (Pz) midline electrodes (Jeon & Polich, Reference Jeon and Polich2003; Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004). The majority of studies, however, involved patients treated with various antipsychotic drugs and those reports of unmedicated cases were not necessarily antipsychotic naive (Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004).
Although antipsychotic drugs have been found to increase the P300 amplitude in some studies (Mathalon et al. Reference Mathalon, Ford and Pfefferbaum2000a ; Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004; Molina et al. Reference Molina, Munoz, Martin-Loeches, Casado, Hinojosa and Iglesias2004), it is generally not restored to normal levels (Ford et al. Reference Ford, White, Csernansky, Faustman, Roth and Pfefferbaum1994; Hirayasu et al. Reference Hirayasu, Asato, Ohta, Hokama, Arakaki and Ogura1998; Jeon & Polich, Reference Jeon and Polich2003). Family studies suggest that the unaffected and unmedicated relatives of patients may show similar, albeit milder, deficits (Bramon et al. Reference Bramon, McDonald, Croft, Landau, Filbey, Gruzelier, Sham, Frangou and Murray2005). Therefore, P300 measures in auditory oddball paradigms are thought to be vulnerability indicators for schizophrenia. Nevertheless, the influence of antipsychotics on ERP measures is poorly understood and medication confounding remains a possibility.
The dopamine transporter (DAT) is located primarily on the presynaptic membrane of dopaminergic neurons and plays a crucial role in the regulation of dopamine concentration in the synaptic cleft by dopamine reuptake. The involvement of dopaminergic mechanisms in the generation of the P300 is not yet clear (Kenemans & Kahkonen, Reference Kenemans and Kahkonen2011), especially because dopamine modulates the activity of the cortical networks through interaction with other neurotransmitters (Peters et al. Reference Peters, Barnhardt and O'Donnell2004; Ford et al. Reference Ford, Krystal and Mathalon2007). However, the loudness dependence of the auditory evoked potential (LDAEP) was reported to be positively associated with DAT (Lee et al. Reference Lee, Yang, Chen, Huang, Yeh, Lu, Chiu, Yao and Lin2011). D2 antagonists may affect ERP latencies and amplitudes in healthy subjects (Takeshita & Ogura, Reference Takeshita and Ogura1994) and significant correlations of P300 parameters and striatal dopamine D2/D3 receptor availability have been reported (Pogarell et al. Reference Pogarell, Padberg, Karch, Segmiller, Juckel, Mulert, Hegerl, Tatsch and Koch2011).
The aims of our study were to explore ERP differences between drug-naive patients with schizophrenia and healthy controls and to examine if P300 performance is related to DAT availability, both without the confounding effects of medication. This was made possible because a subset of the participants in this study also volunteered to undergo single photon emission computerized tomography (SPECT) with [99mTc]-TRODAT-1. In view of previous studies (Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004; Chen et al. Reference Chen, Yang, Howes, Lee, Landau, Yeh, Chiu, Chen, Lu, David and Bramon2013), we predicted that the P300 amplitude would correlate positively with DAT availability whereas latency would have a negative correlation with DAT availability in patients with schizophrenia.
Method
Sample
All study participants were living in Tainan City, the fifth largest city in Taiwan with a population of 1 873 579. A total of 36 drug-naive first-episode patients with schizophrenia were recruited at the psychiatric out-patient clinic of the National Cheng Kung University Hospital. One hundred and thirty-eight healthy community residents of Tainan City were also recruited through advertisements. These controls were interviewed by senior psychiatrists who had been practicing for more than 10 years, using the Chinese version of the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) to ensure that they were free of any Axis I or Axis II psychiatric diagnoses. Brain magnetic resonance imaging (MRI) scans in the controls and the patients were normal. Among the patients, the mean duration of illness was 34.4 months (s.d. = 66.2, median = 7.9, interquartile range = 21.7).
Before any procedure was performed, written informed consent was obtained from all participants after a complete explanation of the study. The Ethical Committee for Human Research at the National Cheng Kung University Hospital approved the study protocol.
Inclusion criteria were as follows: (1) patients should fulfill DSM-IV criteria for schizophrenia; (2) all participants aged between 18 and 60 years; (3) controls never received any antipsychotic treatment and were free of any psychotropic medication at the time of testing. The patients had never had any psychotropic medications prescribed and our clinic was their first contact with psychiatric services. Exclusion criteria for all participants were: (1) other co-morbid psychiatric illnesses, substance abuse/dependence or neurological illnesses; (2) physical illness or unstable vital signs, or history of substance abuse/dependence as assessed during the clinical interview with the research psychiatrist, at the time of enrollment; (3) mental retardation; (4) all female participants of child-bearing age had to take an acceptable form of contraceptive throughout the duration of the study to be included; they also underwent an instant urine pregnancy test prior to starting the experiment; (5) all patients who were deemed at risk of acute suicide/self-harm were excluded from the study for their safety.
ERP data acquisition and analysis
A two-tone auditory P300 auditory oddball task was used for collecting EEG data. Stimuli were 80-dB tones with a 2-s interstimulus interval presented through bilateral intra-aural earphones; 80% of the tones were ‘non-targets’ of 1000 Hz and 20% were ‘targets’ of 2000 Hz in a random sequence. Subjects were instructed to press a button in response to targets only (Frangou et al. Reference Frangou, Sharma, Alarcon, Sigmudsson, Takei, Binnie and Murray1997). EEG data were collected from three midline scalp sites [frontal (Fz), central (Cz) and parietal (Pz)] according to the 10/20 International System (Jasper, Reference Jasper1958) using a 32-channel Quik-Cap with silver/silver-chloride sintered electrodes (Compumedics Neuroscan, USA). FPz was the ground, linked bilateral mastoids were the reference and a vertical bipolar channel with electrodes placed above and below the left eye was used to monitor eye blinks and eye movements. Data were digitized continuously at 500 Hz with a 0.1–40-Hz bandpass filter (24 dB/octave roll-off). Impedances were kept below 5 kΩ. Epochs from 100 to 600 ms pre- and post-stimulus respectively were averaged separately for target and non-target tones. Only epochs with correctly detected targets and correctly ignored non-targets were included in the averages. Epochs with evidence of eye blinks (±50 μV) were automatically rejected, as were those showing movement or other artifacts in any of the 32 channels. The P300 was defined as a positive waveform generated by the target tones and peaking between 200 and 400 ms post-stimulus. Its peak amplitude (measured with respect to the baseline) and its peak latency (measured from time zero) were both calculated using a computer algorithm that made the process blind to clinical group status (Bramon et al. Reference Bramon, Dempster, Frangou, McDonald, Schoenberg, MacCabe, Walshe, Sham, Collier and Murray2006, Reference Bramon, Shaikh, Broome, Lappin, Berge, Day, Woolley, Tabraham, Madre, Johns, Howes, Valmaggia, Perez, Sham, Murray and McGuire2008).
[99mTc]-TRODAT-1 SPECT and MRI
Each subject received a bolus intravenous injection of 740 MBq (20 mCi) of [99mTc]-TRODAT-1 (Institute of Nuclear Energy Research, Taiwan) in a quiet environment approximately 10 min after the intravenous line was set up. The brain SPECT images were acquired 4 h after the injection. To avoid tilt and misalignment of the participant's head, we carefully positioned participants and monitored them during scanning, and used a head holder to further reduce movement artifacts. Before image acquisition, the participant was informed of the necessity to avoid head movement. Sinograms were reviewed blind to diagnosis to determine whether post-acquisition correction for head movements was needed. Movement correction was conducted with the motion correction software ICON version 8.5 KB21 (Siemens, USA).
We used a triple-headed rotating gamma camera (Multispect 3; Siemens, USA) with ultra high-resolution fan-beam collimators, which yields an image resolution of approximately 8.5 mm for the full-width at half-maximum (FWHM). The SPECT images were acquired over a circular 360° rotation, with 120 steps, at a rate of 50 s/step, in a 128 × 128 × 16 matrix. The images were then reconstructed using Butterworth and Ramp filters (Friston et al. Reference Friston, Frith, Liddle, Dolan, Lammertsma and Frackowiak1990) (cut-off frequency = 0.3 Nyquist, power factor = 8), with attenuation according to Chang (Reference Chang1978). The reconstructed transverse images were realigned parallel to the canthomeatal line. The slice thickness of each transverse image was 2.89 mm. For semiquantitative analyses, six consecutive transverse slices on which the highest striatum uptake was best visualized were combined to obtain a slice of thickness 17.34 mm. Then regions of interest (ROIs) were placed over the striatum and the occipital cortex. The ROIs were drawn directly on the SPECT images by an experienced nuclear medicine physician who was blind to the participants' clinical data. The participants' MRI scans (GE, SIGNA CV-I, 1.5 T, USA), obtained within 2 weeks after SPECT examination, were used as a visual reference to determine the areas of the ROIs. The sizes of all ROIs were at least twice that of the FWHM. The specific striatal [99mTc]-TRODAT-1 binding (which represents striatal DAT availability) was calculated as the mean count of ROIs in the striatum divided by the mean count of ROIs in the occipital region (St/Oc) (Hwang et al. Reference Hwang, Yao, Wey and Ting2004).
Psychopathology ratings
On the day of recruitment, standardized psychopathology ratings were collected for all patients using the Clinical Global Impression Severity of Illness (CGI-S; Guy, Reference Guy1976) and the Structured Clinical Interview for the Positive and Negative Syndrome Scale (SCI-PANSS; range 30–210 from least to most symptomatic) (Kay et al. Reference Kay, Fiszbein and Opler1987).
Statistical analyses
We performed a sample size calculation by a power analysis, which indicated that 40 patients and 160 controls would yield an 80% chance (power = 0.8) of detecting an effect size (ES) of 0.5 between two groups using an independent-sample t test (two-tailed α = 0.05). Demographic differences between patients and controls were examined with χ 2 tests for categorical variables or with the Student t test for continuous variables. For the latter, Levene's test was used to assess the assumption of equality of variances. Diagnostic plots and one-sample Kolmogorov–Smirnov tests were used to test for normality.
Local study
The first aim of our study was to assess the differences between antipsychotic-naive patients with schizophrenia and healthy controls in the P300 ERP. Mixed modeling was used to compare P300 amplitude or latency at three midline scalp sites (Fz, Cz and Pz) between the two clinical groups. The models included subject-varying intercepts to acknowledge the correlations between the three repeated measures for each participant. To assess whether group differences varied between the three sites, we tested whether a group × electrode interaction was evident. Based on the previous literature we considered age and gender to be potential confounders of the group effect on P300 measures and therefore adjusted our analyses by including age and gender as covariates in our analysis model (Jeon & Polich, Reference Jeon and Polich2003; Wang et al. Reference Wang, Hirayasu, Hiramatsu, Hokama, Miyazato and Ogura2003; Yu et al. Reference Yu, Chen, Chen, Tsai and Lee2005). To evaluate the effects of aging and gender on the P300 amplitude and latency, we present the regression coefficients obtained from the above-described model. We used residual diagnostics to determine the shape of the relationship between age and P300 latency or amplitude, comparing a model for a quadratic relationship with the simpler linear relationship model. We further expanded this model to check whether group effects varied with age or gender by testing respective interaction terms. Where interaction terms were not significant, they were dropped from the final model.
The second aim of this study was to investigate a possible relationship between P300 amplitude or latency and DAT availability measured by SPECT with [99mTc]-TRODAT-1. Thus, for the subset of participants who provided DAT availability measures, we expanded the final model described above, adding the mean TRODAT binding ratio as an explanatory variable whose effect is of interest. As in our recent study and others (Hwang et al. Reference Hwang, Yao, Wey and Ting2004; Chen et al. Reference Chen, Yang, Howes, Lee, Landau, Yeh, Chiu, Chen, Lu, David and Bramon2013), the specific striatal [99mTc]-TRODAT-1 binding was calculated as St/Oc. The expanded analysis assumes that missingness of the TRODAT binding ratio was driven only by explanatory variables of the model (age, gender, and group).
Statistical significance was established at p < 0.05. SPSS version 16 (SPSS Inc., USA) was used for all analyses.
Meta-analysis
We conducted a random effects meta-analysis combining the published literature and the local study presented here. We searched the Institute for Scientific Information Web of Knowledge, Scopus and PubMed (U.S. National Library of Medicine, NLM) using the following key words: ‘drug-naïve’, ‘schizophrenia’, ‘event-related potential’ and ‘P300’. The search covered the period between 1950 and the beginning of 2012 and yielded a total of 16 articles with English abstracts. Of these, we excluded one review, four studies with visual ERP, including one with stroboscopic stimuli, two studies with mixed medicated and drug-naive patients compared to controls, one pharmaceutical study without patient recruitment, one study with correlations only reported between P300 and brain CT imaging, and two conference abstracts. Thus, the meta-analysis included five articles published between 1998 and 2010 that focused on drug-naive schizophrenia patients using the auditory oddball paradigm P300 ERP. Two of the five articles (Hirayasu et al. Reference Hirayasu, Asato, Ohta, Hokama, Arakaki and Ogura1998; Wang et al. Reference Wang, Hirayasu, Hokama, Tanaka, Kondo, Zhang and Xiao2005) did not offer the mean and standard deviation values of the latency and amplitude, so finally only three articles (Wang et al. Reference Wang, Hirayasu, Hiramatsu, Hokama, Miyazato and Ogura2003, Reference Wang, Tang, Li, Mecklinger, Xiao, Zhang, Hirayasu, Hokama and Li2010; Xiong et al. Reference Xiong, Zeng, Zhu, Tan, Xu, Lu, Wan and Ma2010) were included in our meta-analysis. The standardized mean difference between controls and patients was computed for each primary study as Cohen's d (Cohen, Reference Cohen1988). The meta-analysis was conducted using the metan routine available in Stata version 10 (Stata Corporation, USA).
Results
Local study
The sample included 36 drug-naive patients with a DSM-IV diagnosis of schizophrenia or schizophreniform psychosis along with 138 healthy controls. All the patients were presenting to services for the first time. The demographic characteristics of the patient and control groups are summarized in Table 1. Patients and controls had a similar gender distribution and smoking habits, with only a minority of participants, 10% of patients and 23% of controls, being smokers. However, compared to the controls, the patients were significantly younger (t = 4.56, df = 95.4, p < 0.001), had spent significantly less time in education (t = 3.32, df = 171, p = 0.001) and were less likely to live with a partner (t = 7.38, df = 1, p = 0.007). Of the 36 patients included, 33 completed the PANSS and 25 completed the CGI-S (Table 1).
Table 1. Demographic characteristics of patients with schizophrenia (n = 36) and controls (n = 138)

DAT, Dopamine transporter; PANSS, Positive and Negative Syndrome Scale; CGI, Clinical Global Impression Severity of Illness; n.a., not applicable; df, degrees of freedom.
a M includes married and living with a partner; S includes single, divorced, married but separated.
b The specific striatal [99mTc]-TRODAT-1 binding as the measure of DAT availability was calculated as the mean count of regions of interest (ROIs) in the striatum divided by the mean count of ROIs in the occipital region (St/Oc).
Values given as number or mean (standard deviation) range.
Group comparisons on P300 performance
The P300 ERP latency and amplitude of both patients and controls were normally distributed (non-significant Kolmogorov–Smirnov test and diagnostic plots showed no departure from normality). The group averages are shown in Fig. 1. The group × electrode interactions were not significant (latency: F = 0.46, df = 2,343, p = 0.63; amplitude: F = 1.97, df = 2,343, p = 0.14) and were therefore dropped from the model.
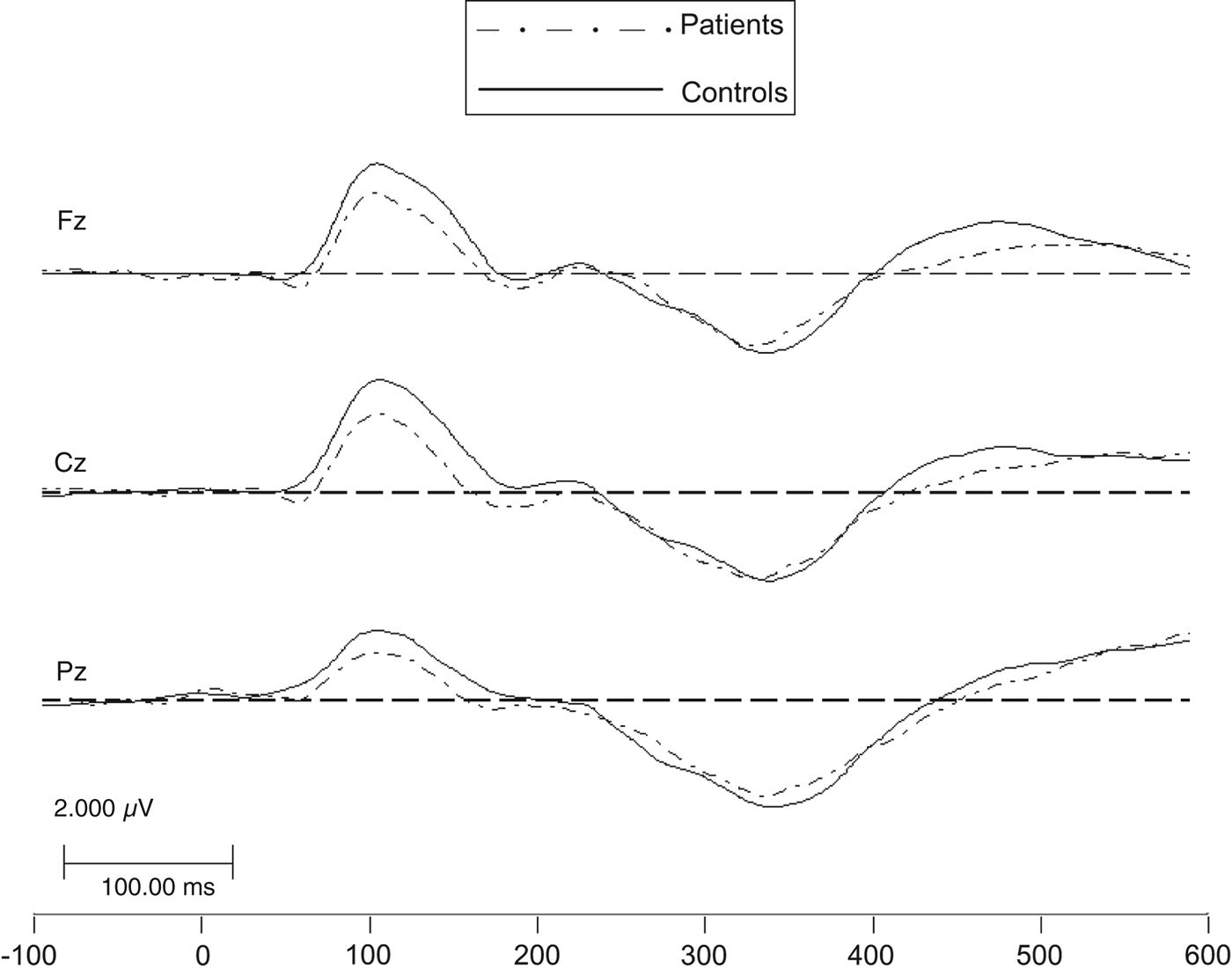
Fig. 1. P300 event-related potential (ERP) group averages. An eliciting event is presented repeatedly and the resulting P300 ERPs (aligned at the time point of the eliciting event) are averaged to yield the subject's average P300 ERP on different sites (Fz, Cz, Pz), in which activity not time-locked to the eliciting event averages out (i.e. to zero) and what remains is the time-locked wave series. P300 is a positive-going wave with a scalp amplitude distribution in which it is largest parietally (Pz) and smallest frontally (Fz), taking intermediate values centrally (Cz).
After controlling for age and gender, the P300 ERP showed no significant difference between patients and controls in latency [estimated group difference = −3.85 ms, 95% confidence interval (CI) −15.60 to 7.91 ms; F = 0.42, df = 1,170, p = 0.52] or in amplitude (estimated group difference = 0.27 μV, 95% CI −1.14 to 1.68 μV; F = 0.14, df = 1,170, p = 0.71), as in Table 2.
Table 2. Latency/amplitude of patients with schizophrenia (n = 36) and controls (n = 138)

Age, sex and electrode site controlled for statistical analyses.
Values given as mean (standard deviation).
In the same model, there was no effect of gender on P300 latency (estimated difference = 1.34 ms, 95% CI –7.93 to 10.60 ms; F = 0.81, df = 1,170, p = 0.78). However, there was a significant effect on P300 amplitude, which was lower in males than in females (estimated difference = −1.49 μV, 95% CI −2.60 to −3.78 μV; F = 6.99, df = 1,170, p = 0.01). Similarly, we did not observe any significant effects of age on P300 latency (estimated difference = −0.19 ms/year, 95% CI −0.69 to 0.31 ms/year; F = 0.56, df = 1,170, p = 0.46) or on P300 amplitude (estimated difference = −0.04 μV/year, 95% CI –0.098 to 0.02 μV/year; F = 1.58, df = 1,170, p = 0.21). In addition, with regard to the effects of age, the residual diagnostics showed no evidence of departure from a linear effect and the quadratic effect of age was not significant (latency: p = 0.39; amplitude: p = 0.43), thus the simpler linear age model was used.
The interactions between group and sex on both P300 measures were not significant and were removed from the final model (latency: F = 1.01, df = 1,169, p = 0.32; amplitude: F = 0.04, df = 1,169, p = 0.95). Similarly, the effect of group × age interaction on P300 amplitude was not significant (F = 2.70, df = 1,169, p = 0.10). Of note, there was a significant effect of age × group interaction on P300 latency (estimated difference = 1.51 ms, 95% CI −0.24 to 3.27 ms; F = 3.99, df = 1,169, p = 0.048), with the difference between patients and controls in P300 latency became more pronounced with aging, and with latency increasing faster with age in the patient group (Fig. 2).

Fig. 2. A significant effect of age × group interaction was seen on parietal P300 latency, with the difference between patients and controls in P300 latency becoming more pronounced with aging and with latency increasing faster in the patients. The relationship between latency and age was similar at the other two sites (central and frontal).
Relationships between P300 measures and DAT availability
The mean striatal DAT availability measures as determined by SPECT with [99mTc]-TRODAT-1 are shown in Table 1 for patients and controls. Having adjusted for age and gender in the same model, we found no effect of DAT availability on P300 latency (F = 1.30, df = 1,111, p = 0.26) or amplitude (F = 1.28, df = 1,111, p = 0.26).
Meta-analysis
We conducted a random effects meta-analysis of the available literature of drug-naive patients with the auditory oddball paradigm P300 ERP between 1950 and 2012, including three previously published articles and our current data. The combined sample included 105 drug-naive patients with schizophrenia and 214 healthy controls. The latency PSES computed as Cohen's d was −0.13 (95% CI −0.37 to 0.12, p = 0.31), indicating that patients and controls did not differ significantly. There was no significant between-study heterogeneity in ES (coefficient = −0.13, p = 0.38). The amplitude PSES size, again computed as Cohen's d, was 0.48 (95% CI −0.002 to 0.97, p = 0.05), with only patients showing a significant reduction in amplitude. There was no significant between-study heterogeneity in ES (coefficient = 0.48, p = 0.14). Fig. 3 shows the forest plots for the latency and amplitude meta-analyses.
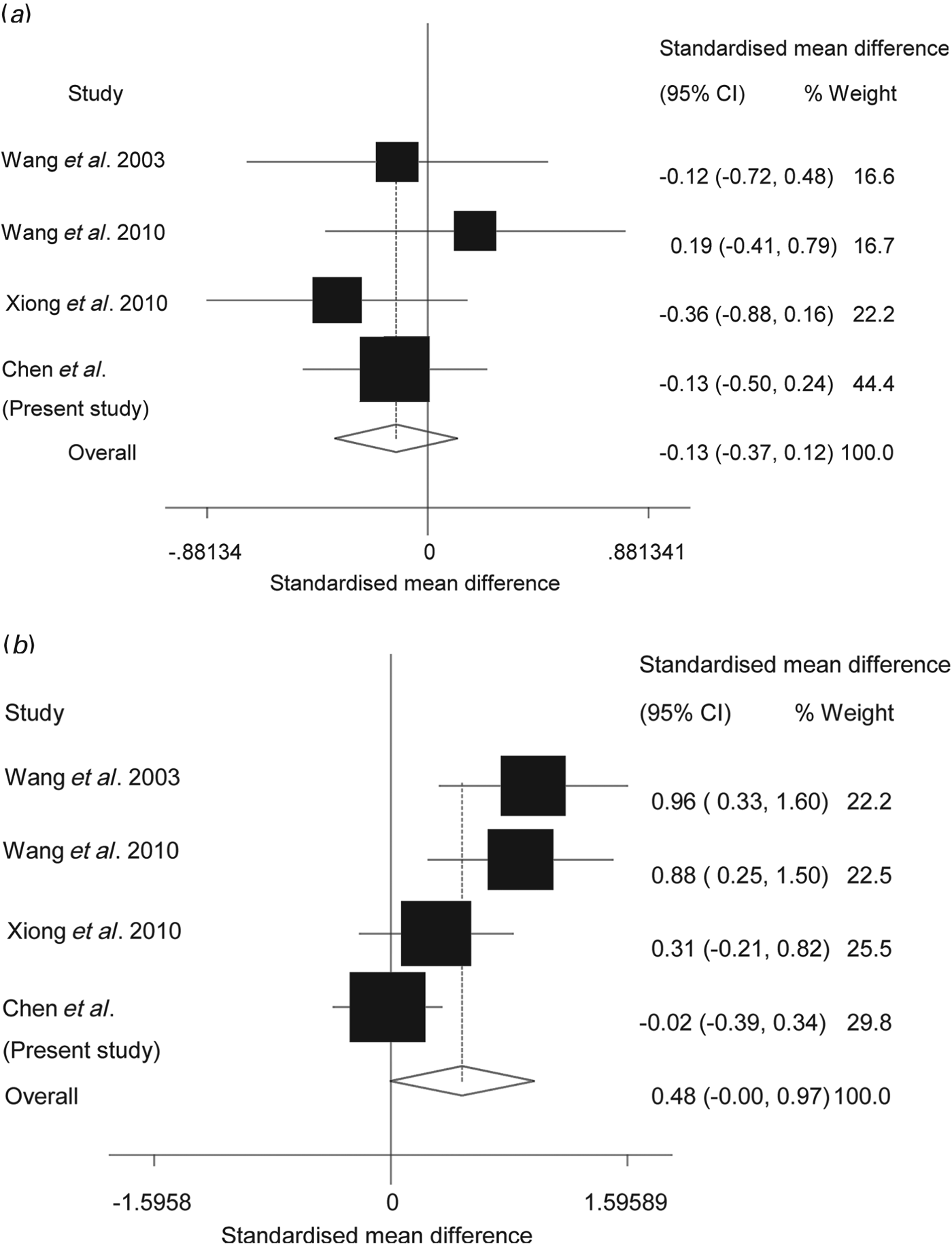
Fig. 3. Meta-analysis forest plots of (a) P300 latency and (b) P300 amplitude, showing the standardized effect sizes (SES) for each study. The horizontal lines represent 95% confidence intervals (CIs) for the SES in each study. The size of the squares represents the weights given to the studies. The diamond shows the pooled standardized effect size (PSES) of all studies using meta-analysis tools in Stata 10.
Discussion
Our study shows that the medication-naive patients with schizophrenia did not differ significantly from the healthy controls in measures of the P300 ERP. Furthermore, in our sample we found no evidence that DAT availability influenced P300 amplitude or latency. The strength of our study is that we recruited a relatively large and clinically homogeneous sample, all the subjects were drug naive and patients were at a uniform and early stage of their illness.
We suggest that our failure to find P300 amplitude or latency differences between drug-naive patients and normal controls might be related to the relatively short duration of psychotic illness of our patients. Indeed, previous studies have identified a correlation whereby the P300 amplitude was more reduced and latency more delayed with longer illness duration (Mathalon et al. Reference Mathalon, Ford, Rosenbloom and Pfefferbaum2000b ). The P300 ERP involves the complex summation of activity from multiple brain regions, including the various association areas of the cerebral cortex and the limbic system (Picton, Reference Picton1992; Bledowski et al. Reference Bledowski, Prvulovic, Goebel, Zanella and Linden2004; Polich, Reference Polich2007; Mangalathu-Arumana et al. Reference Mangalathu-Arumana, Beardsley and Liebenthal2012), which in turn are thought to originate from deeper brain sources such as the striatum (Kellendonk et al. Reference Kellendonk, Simpson, Polan, Malleret, Vronskaya, Winiger, Moore and Kandel2006; Howes & Kapur, Reference Howes and Kapur2009). The lack of correlation between P300 performance and striatal DAT availability in the current study is in line with our previous findings that DAT availability is not impaired in drug-naive patients with schizophrenia (Chen et al. Reference Chen, Yang, Howes, Lee, Landau, Yeh, Chiu, Chen, Lu, David and Bramon2013) and with the progressive brain pathophysiological process correlating with duration of illness as mentioned (Mathalon et al. Reference Mathalon, Ford, Rosenbloom and Pfefferbaum2000b ).
There was a significant gender effect in our combined sample, with the P300 amplitude being lower in males than females, as reported in a previous study on healthy individuals (Schiff et al. Reference Schiff, Valenti, Andrea, Lot, Bisiacchi, Gatta and Amodio2008). This is consistent with the later age of onset and lower incidence of schizophrenia in females (Faraone et al. Reference Faraone, Chen, Goldstein and Tsuang1994).
Previous studies have reported that the unaffected relatives of patients and other populations at risk for psychosis have similar, but milder, P300 amplitude and latency deficits, suggesting that the P300 amplitude reductions and latency delays may be conceptualized as biomarkers of genetic predisposition to psychosis (van Beijsterveldt & van Baal, Reference van Beijsterveldt and van Baal2002; Bramon et al. Reference Bramon, McDonald, Croft, Landau, Filbey, Gruzelier, Sham, Frangou and Murray2005, Reference Bramon, Shaikh, Broome, Lappin, Berge, Day, Woolley, Tabraham, Madre, Johns, Howes, Valmaggia, Perez, Sham, Murray and McGuire2008). Our data challenge this notion and indicate that the P300 could instead be a marker of disease chronicity/progression.
Some studies have shown a P300 amplitude reduction in populations at UHR for psychosis (Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005) either prior to the onset of psychosis compared to controls (Bramon et al. Reference Bramon, Shaikh, Broome, Lappin, Berge, Day, Woolley, Tabraham, Madre, Johns, Howes, Valmaggia, Perez, Sham, Murray and McGuire2008; Frommann et al. Reference Frommann, Brinkmeyer, Ruhrmann, Hack, Brockhaus-Dumke, Bechdolf, Wolwer, Klosterkotter, Maier and Wagner2008) or after transition to psychosis compared to the state before transition (van Tricht et al. Reference van Tricht, Nieman, Koelman, van der Meer, Bour, de Haan and Linszen2010). However, the basis of the vulnerability to psychosis is not fully understood (Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Bechdolf, Addington, Riecher-Rossler, Schultze-Lutter, Keshavan, Wood, Ruhrmann, Seidman, Valmaggia, Cannon, Velthorst, De Haan, Cornblatt, Bonoldi, Birchwood, McGlashan, Carpenter, McGorry, Klosterkotter, McGuire and Yung2013a ,Reference Fusar-Poli, Yung, McGorry and van Os b ). Hence, such patients could have a different underlying pathophysiology, compared with established schizophrenia, that is separate from a genetic vulnerability to schizophrenia and may relate to early states of mixed psychopathology. It is also possible that some ERP components, such as P300 amplitude abnormalities, could be present before a psychotic episode but do not show further progression immediately following the psychotic onset (van Tricht et al. Reference van Tricht, Nieman, Koelman, Bour, van der Meer, van Amelsvoort, Linszen and de Haan2011), assuming of course that such individuals are not on antipsychotic medication, which cannot always be guaranteed. Therefore, our data suggest the P300 could instead be a marker of disease chronicity/progression in patients with schizophrenia but not in the UHR population. Nevertheless, the discrepancy between our data and results from UHR population ERP studies needs further exploration.
The effect of psychotropic medication on evoked potentials is controversial. P300 amplitude and latency in patients with schizophrenia may tend to normalize after pharmacological intervention (Coburn et al. Reference Coburn, Shillcutt, Tucker, Estes, Brin, Merai and Moore1998; Gonul et al. Reference Gonul, Suer, Coburn, Ozesmi, Oguz and Yilmaz2003), but the absence of such drug effects has also been reported (de Wilde et al. Reference de Wilde, Bour, Dingemans, Koelman, Boeree and Linszen2008). Several studies conducted on unmedicated patients who were not drug naive suggest that they also show amplitude reductions and latency delays (Bramon et al. Reference Bramon, Rabe-Hesketh, Sham, Murray and Frangou2004). Our findings of normal P300 performance in medication-naive patients would support an enduring confounding effect of medication; however, longitudinal studies before and after introducing medication are required to clarify this matter.
The meta-analysis including our data found that drug-naive patients with schizophrenia do not have significant impairments in P300 latency compared with controls. Considering the pooled ES of −0.13 and the range of ESs included in the 95% CI, the SES is unlikely to exceed −0.37, which is conventionally considered to be a moderate effect (Cohen, Reference Cohen1988). Therefore, our data and the meta-analysis of the literature show fairly convincingly that, in medication-naive patients, the P300 latency is unlikely to be impaired.
The meta-analysis of P300 amplitude yielded a much larger PSES of 0.48 with a trend for significance. Considering all the evidence, we are inclined to conclude that there are probably no clinically relevant impairments in amplitude in drug-naive patients in the early stages of the illness. We excluded studies of drug-free previously treated patients that were reported as showing P300 differences between patients and controls; hence their inclusion may have changed the outcome of our meta-analysis. Unfortunately, there were too few studies eligible for inclusion for us to examine publication bias and heterogeneity in a meaningful way.
We found a significant effect of age × group interaction on P300 latency in our data, whereby the patients showed greater latency delays with aging; thus the difference between patients and controls in P300 latency became more pronounced with increasing age (Gilmore, Reference Gilmore1995; O'Donnell et al. Reference O'Donnell, Faux, McCarley, Kimble, Salisbury, Nestor, Kikinis, Jolesz and Shenton1995; Wang et al. Reference Wang, Hirayasu, Hiramatsu, Hokama, Miyazato and Ogura2003; Araki et al. Reference Araki, Kasai, Kirihara, Yamasue, Kato, Kudo, Nakagome and Iwanami2006). This finding is not surprising and could reflect a progressive neurodegenerative change, a faster age-related decline in the speed of neural transmission among patients with schizophrenia.
A potential limitation is that patients recruited into our study had to be relatively stable and well enough to complete an extensive battery of clinical, cognitive, EEG and neuroimaging tests, which may potentially reduce the generalizability of our findings to a wider population including more severely ill and/or less cooperative patients. Furthermore, a study of only non-smokers would not be representative of most clinical populations, and we included participants regardless of their smoking habit. Our patient and control groups were not significantly different in smoking status; hence, smoking is unlikely to have confounded our results. Our controls were recruited from the local community through research advertisements and, compared to the patients, they were significantly older, had spent more time in education and were more likely to be living with a partner; these demographic differences were comparable to previous studies (Loughland et al. Reference Loughland, Draganic, McCabe, Richards, Nasir, Allen, Catts, Jablensky, Henskens, Michie, Mowry, Pantelis, Schall, Scott, Tooney and Carr2010). We adjusted all our analyses by age and gender; thus we consider that our controls provided a suitable comparison group and were representative of the local healthy population. Family studies suggest that the unaffected and thus unmedicated relatives of patients may show similar, albeit milder, deficits in the P300 as schizophrenia patients. As our controls were not screened against having a family history of psychosis, this might potentially confound the results and contribute to a smaller difference between patient and control groups.
Our findings suggest that the P300 ERP is not altered in the early stages of schizophrenia before medication is introduced, and that the DAT availability does not influence the P300 ERP amplitude or latency. The P300 ERP could be an indicator of the progression of illness and chronicity. We identified gender differences and aging effects that could have clinical significance and may be taken into account in future studies. As there are only four P300 ERP studies, including this one, of drug-naive patients with schizophrenia, all of them cross-sectional, further longitudinal studies are needed to explore the effects of medication and duration of illness on the P300 ERP.
Acknowledgments
This research was funded by the National Science Council of Taiwan (NSC 91-2314-B-006-074, NSC 93-2314-B-006-107, NSC 97-2314-B-006-006-MY3, NSC 99-2314-B-006-019-MY3, and NSC 101-2314-B-006-065) and the Atomic Energy Council of Taiwan (NSC 91-NU-7-006-002). This research also received funding from the Headquarters of University Advancement at the National Cheng Kung University, which is sponsored by the Ministry of Education, Taiwan. A.S.D. and S.L. acknowledge support from the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at the South London and Maudsley National Health Service (NHS) Foundation Trust and the Institute of Psychiatry, King's College London. E.B. holds a Medical Research Council (MRC) New Investigator Award. Further support to E.B. was provided by the NIHR UK (post-doctoral fellowship), the Psychiatry Research Trust, the Schizophrenia Research Fund and the Brain and Behavior Research Foundation [National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award].
We thank C. L. Chu, T. H. Chang, Y. T. Tseng and C. T. Lin for their administrative support. Special thanks go to all the study participants.
Declaration of interest
None.







