Introduction
The genus Porpidia has been the subject of a number of taxonomic studies in recent decades, including Hertel & Knoph (Reference Hertel and Knoph1984) (Central Europe), Gowan (Reference Gowan1989) (North America), Gowan & Ahti (Reference Gowan and Ahti1993) (eastern Fennoscandia) and Fryday (Reference Fryday2005) (N Europe, especially the British Isles). However, problems remain in the delimitation of species, and there are numerous unidentified collections which probably represent undescribed taxa. Buschbom & Mueller (Reference Buschbom and Mueller2004) investigated the phylogeny of the genus using nuclear ribosomal large subunit RNA and nuclear β-tubulin markers. They found that while Porpidia sensu lato formed a strongly supported group, the genus as currently circumscribed was not monophyletic, with Amygdalaria, Cecidonia and Lecidea sensu stricto nested within it. With the exception of studies in the P. flavicunda-P. melinodes group (Buschbom & Mueller Reference Buschbom and Mueller2006, Buschbom & Barker Reference Buschbom and Barker2006), little other molecular work has been carried out to date.
Porpidia contraponenda belongs to Group I of Buschbom & Mueller (Reference Buschbom and Mueller2004), together with Amygdalaria spp., P. cinereoatra, P. lowiana, P. macrocarpa, P. superba, Stenhammarella turgida and others. According to recent authors P. contraponenda is most easily distinguished from related species by the presence of the depside methyl 2′-O-methylmicrophyllinate, which has been considered as almost confined to this species. However, Gowan (Reference Gowan1989) distinguished two chemically similar species, P. contraponenda and P. diversa, on the basis of type of epihymenial pigment, apothecium size, and geographical distribution. Porpidia diversa was said to have a usually aeruginose epihymenial pigment and smaller apothecia than P. contraponenda. The thallus of both species was said to be 100–500 µm thick, continuous or dispersed, and sometimes rimose-areolate, and the apothecia were described as soon becoming sessile. However, Gowan noted that the type of P. contraponenda (from Austria) had “an abnormally thick, rimose thallus”. Gowan & Ahti (Reference Gowan and Ahti1993) described the thallus of P. contraponenda from Finland as thin and verruculose to finely cracked, and the apothecia as sessile. They considered that P. diversa was not present in eastern Fennoscandia, on the basis of apothecial pigmentation. They noted that specimens chemically similar to Finnish P. contraponenda but with a thicker thallus and generally larger apothecia occur in the Alps and western North America. Fryday (Reference Fryday2005) considered that the distinction of P. contraponenda and P. diversa on the basis of epihymenial pigmentation and apothecial size was untenable, and synonymised the two species. He noted that the holotype of P. diversa had a brown epihymenial pigment, and that the apothecia are immature. Fryday reported unidentified sorediate collections which also contained methyl 2′-O-methylmicrophyllinate. Fryday et al. (Reference Fryday, Gilbert, Galloway, Coppins, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) described the apothecia of P. contraponenda as sessile.
In Great Britain, it has been known for at least 10 years that fertile non-sorediate specimens containing methyl 2′-O-methylmicrophyllinate belong to two separate species, differing in morphology and chemistry. More recently, DNA sequencing has supported the separation of the two species, which are described below. One is conspecific with P. contraponenda sensu stricto, but the other is apparently undescribed and is here described as the new species P. irrigua.
Materials and Methods
Thin-layer chromatography was carried out using Solvent Systems A and G, using standard methods (Orange et al. Reference Orange, James and White2010). Fragments of thallus and/or apothecia which were extracted with acetone for TLC were used for subsequent DNA analysis. Ascospore measurements are given as (min–)(
![]() $\bar x$
–SD)–
$\bar x$
–SD)–
![]() $\bar x$
–(
$\bar x$
–(
![]() $\bar x$
+SD) (–max) where min. and max. are extreme values and
$\bar x$
+SD) (–max) where min. and max. are extreme values and
![]() $\bar x$
the arithmetic means and SD the corresponding standard deviation.
$\bar x$
the arithmetic means and SD the corresponding standard deviation.
DNA was extracted from recently collected or frozen specimens, using the Qiagen DNeasy Plant Mini Kit; the manufacturer′s instructions were followed except that warm water was used for the final elution. PCR amplification was carried out using Bioneer AccuPower PCR Premix in 20 µl tubes. The two internal transcribed spacer regions and the 5.8S region (ITS1-5.8S-ITS2) of the nuclear ribosomal genes, and the 5′ end of the nuclear ribosomal large subunit (LSU) were amplified, using the primers ITS1F, LR3, nu-LSU-155-5′ and LR7 (Döring et al. Reference Döring, Clerc, Grube and Wedin2000, Gardes & Bruns Reference Gardes and Bruns1993, Vilgalys & Hester Reference Vilgalys and Hester1990). The PCR thermal cycling parameters were: initial denaturation for 5 min at 94°C, followed by 5 cycles of 30 s at 94°C, 30 s at 55°C, and 1 min at 72°C, then 30 cycles of 30 s at 94°C, 30 s at 52°C and 1 min at 72°C. PCR products were visualized on agarose gels stained with ethidium bromide, and purified using the Sigma GenElute PCR Clean-Up Kit. Sequencing was performed by The Sequencing Service (College of Life Sciences, University of Dundee, www.dnaseq.co.uk) using Applied Biosystems Big-Dye Ver 3.1 chemistry on an Applied Biosystems model 3730 automated capillary DNA sequencer, or by Macrogen Inc.
Sequences were assembled and edited using DNAstar Lasergene software (http://www.dnastar.com/products/lasergene.php). Alignment was carried out using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html); ClustalW was used to create an initial alignment, which was edited manually. The length of the alignments, and number of informative sites, are shown in Table 1.
Table 1. Data for ITS and LSU alignments

Phylogenetic relationships and support values were investigated using a Bayesian approach. Additional support values were obtained using Maximum Likelihood bootstrapping, as implemented in RaxML (Stamatakis Reference Stamatakis2006, Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008), hosted on the CIPRES Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Two datasets were analysed separately: the ITS1-5.8S-ITS2 region (numerous specimens), and the ribosomal LSU (selected specimens only). The few relevant sequences of Porpidia in GenBank were included in the analyses. Models of evolution for the Bayesian analyses were selected using the Akaike Information Criterion (AIC) in MrModeltest 2.2 (Nylander Reference Nylander2004) (Table 1). Gaps were treated as missing data. Using MrBayes 3.1.2 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2005), two analyses of two parallel runs were carried out for 2 000 000 generations, with trees sampled every 100 generations. Stationarity was considered to have been reached when the average standard deviation of split frequencies dropped to <0·01, and the values for the Potential Scale Reduction Factor were close to 1. A burn in sample of 5 000 trees was discarded from each run, respectively. Support values of ≥95% Bayesian posterior probabilities and ≥70% Maximum Likelihood bootstrapping were regarded as significant.
Specimens used in analyses are shown in Table 2.
Table 2. Specimens used in the phylogenetic analyses of Porpidia species. New sequences are in bold

Results
Thin-layer chromatography
The following substances were detected in material of P. contraponenda and P. irrigua (numbered as in Fig. 1):
-
1. Unknown A: spot almost colourless, UV−, UV+ blue-violet; trace amounts in P. contraponenda and P. irrigua, not always present.
-
2. Unknown B: colourless spot, UV−, after heating UV− (greyish); usually a major compound in P. contraponenda, absent or in trace amounts in P. irrigua.
-
3. Methyl 2′-O-methylmicrophyllinate: spot almost colourless, UV−, after heating UV+ blue-violet; a major compound in P. contraponenda and P. irrigua.
-
6. 2′-O-methylmicrophyllinic acid: spot almost colourless, UV−, after heating UV+ blue-violet; trace amounts in P. contraponenda and P. irrigua
-
7. Unknown; trace amounts in P. irrigua.

Fig. 1. Chromatogram of selected species of Porpidia in Solvent system G (diagrammatic). Samples: CON = controls; con = P. contraponenda; cin = P. cinereoatra; irr = P. irrigua; rug = P. rugosa. Substances: A = atranorin, N = norstictic acid, Sa = salazinic acid, St = stictic acid, 1 = inknown A, 2 = unknown B, 3 = methyl 2′-O-methylmicrophyllinate, 4 = confluentic acid, 5 = 2′-O-methylsuperphyllinic acid, 6 = 2′-O-methylmicrophyllinic acid, 7–9 = unidentified.
In addition, the two species share other unidentified compounds in trace amounts (not shown in Fig. 1). Unknown B is usually clearly present in British material of P. contraponenda as a major compound, and is absent or in trace amounts in P. irrigua. It was present in the two syntypes of P. contraponenda examined from BM and M respectively, but only in small quantities. However, only very small samples of these specimens could be used for TLC, so the true concentration in these specimens may be higher than suggested by the TLC plate. Unknown B was present as a major compound in the epitype and topotypes of P. contraponenda, collected in 2012. Unknown A may be overlooked until the plate has been heated, when it is visible by its UV fluorescence.
The compounds above are readily separated in Solvent System G. However, Solvent System A does not separate Unknown B and methyl 2′-O-methylmicrophyllinate, and thus is not suitable for differentiation of the two species.
ITS analysis
The aligned ITS1-5.8S-ITS2 region comprised 543 sites, of which 125 were removed as ambiguous; 140 sites were parsimony-informative (Table 1).
The ITS tree shows (among others) four well-supported clades, one of which (Clade A) consists of two, well-supported subgroups (Fig. 2):
-
A. The Porpidia macrocarpa and P. cinereoatra subgroups, together corresponding to Group I of Buschbom & Mueller (Reference Buschbom and Mueller2004). Porpidia striata is basal to these two subgroups, and they and P. striata together form a well-supported clade.
-
A1: Porpidia macrocarpa, P. flavocruenta, P. crustulata, P. islandica, and at least three well-supported clades representing unidentified or undescribed species. For instance, seven specimens forming a well-supported clade are united by a uniformly rusty-coloured thallus, lack of lichen substances, and occurrence near water in northern Norway.
-
A2: Specimens containing methyl 2′-O-methylmicrophyllinate form three well-supported clades, which correlate with features of the morphology and chemistry of the specimens. The clades are attributed here to Porpidia contraponenda sensu stricto, the new species P. irrigua, and an unidentified taxon from North America, originally named as P. contraponenda. In the voucher specimens of the unidentified North American taxon (Harris 55558 [NY – 01103898] and Harris 52962 [NY – 01518605]) the apothecia are small, 0.50–1.14 mm diameter, and semi-immersed to sessile; the ascospores are 14–18 µm in length, and the presence of methyl 2′-O-methylmicrophyllinate and Unknown A was confirmed by TLC. Four sequences of P. cinereoatra from Wales and Ireland, and one sequence of P. musiva from Turkey also form a clade, but without strong support.
-
-
B. Porpidia hydrophila and P. rugosa; the latter formed part of Group III of Buschbom & Mueller, P. hydrophila was not included in their analyses but Fryday (Reference Fryday2005) considered it to belong to the same infrageneric group as P. rugosa.
-
C. Porpidia flavicunda and P. melinodes, corresponding to Group IV of Buschbom & Mueller.
-
D. Lecidea confluens and L. lapicida, corresponding to Group II of Buschbom & Mueller.

Fig. 2. Phylogenetic relationships amongst Porpidia species, based on a Bayesian analysis of the nuclear ribosomal ITS1-5.8S-ITS2 region. The tree was rooted using Bellemerea alpina. The two support values associated with each branch are posterior probabilities (PP) and maximum likelihood bootstrap (MLb) values, respectively. Branches in bold indicate a support of PP ≥95% and MLb ≥70%. If a node of the Bayesian tree was not recovered by ML bootstrapping, the ML value is replaced by a dash. Chemosyndromes of specimens (where known) are indicated: C=confluentic acid, M=2′-O-methylmicrophyllinate, S=stictic acid, SU=2′-O-methylsuperphyllinic acid, 0=no depside or depsidone secondary products.
The position of P. tuberculosa (Group IV) and Lecidea fuscoatra and L. atrobrunnea (Group II) was not resolved.
LSU analysis
The LSU tree is shown in Fig. 3. Specimens containing methyl 2′-O-methylmicrophyllinate occurred in four clades; two clades represent P. contraponenda and P. irrigua respectively, and the other two comprise a single sequence each, originating from North American specimens. One is basal to P. contraponenda (Fig. 3), the other is basal to P. lowiana (Fig. 3). The voucher specimen (Buschbom 2953; originally identified as P. diversa) is not present in F and could not be traced. The voucher specimen (Cagle 00810-2 [WTU L-19693], originally identified as P. contraponenda) has sessile apothecia 1·1–2·0 mm diameter and ascospores 19–21 µm long, and much resembles P. irrigua in appearance; it contained methyl 2′-O-methylmicrophyllinate and Unknown A by TLC.

Fig. 3. Phylogenetic relationships amongst Porpidia species, based on a Bayesian analysis of the nuclear ribosomal LSU region. The tree was rooted using Cecidonia umbonella. The two support values associated with each branch are posterior probabilities (PP) and maximum likelihood bootstrap (MLb) values, respectively. Branches in bold indicate a support of PP ≥95% and MLb ≥70%. If a node of the Bayesian tree was not recovered by ML bootstrapping, the ML value is replaced by a dash. Chemosyndromes of specimens (where known) are indicated: C=confluentic acid, M=2′-O-methylmicrophyllinate, O=no depside or depsidone secondary products.
The Species
Porpidia contraponenda (Arnold) Knoph & Hertel
In Hertel & Knoph, Mitt. Bot. Staatssamml. München 20: 477 (1984).—Lecidea contraponenda Arnold, Verh. K. K. zool.-bot. Gesellsch. Wien 36: 79 (1886); type: [Austria] Auf kleinen Gneissblöcken am Fusswege zwischen Kühtei [Kühtai] und den Finsterthaler Seeen, Tirol, 16 Juli 1884, F.C.G. Arnold (Arnold, Lich. exs. 1055) (BM 764657 – syntype!; M – 0025569 & 0024172 – syntypes!); epitype (selected here): Austria, Tyrol, Kühtai, Finstertal, 47°12.43′N, 11°01.44′E, alt. 2025 m, on stones in flush, 11 August 2012, A. Orange 21126 (NMW – C.2012.002.116).

Fig. 4. Porpidia contraponenda; A, Orange 16228, note thick thallus and mostly immersed apothecia; B, Orange 21126 (epitype), note moderately thick thallus and semi-immersed apothecia. Scales: A & B =1 mm. In colour online.
Prothallus black, usually inconspicuous but sometimes extensive. Thallus white to pale cream, sometimes locally blue-grey, rarely tinged rusty orange; young areoles arising on the prothallus (when visible), soon coalescing and forming a cracked crust 100–800 µm thick; low rugose galls caused by Cecidonia xenophana frequently present.
Apothecia half-immersed to completely immersed when young, when mature usually one quarter to ± completely immersed in thallus, occasionally more or less sessile, at least in thin areas of thallus; up to 1·6 mm diameter; margin 80–160 µm wide, smooth, sometimes flattened when young; disc slightly concave to plane or convex; pruina often present on young disc and on inner edge of young margin, present or absent on mature disc. Exciple at surface with dense pigment which is dull greenish to dull brown, sometimes with bluish tinges, brown within, translucent to opaque in sections 10–20 µm thick, hyphae 3·7–4·1 µm diameter. Hypothecium brown to reddish brown. Hymenium 100–170 µm high. Epihymenium dull brown to dull greenish brown, K−. Paraphyses 1·2–1·6 µm wide in centre of hymenium, widening to 3·7 µm above, tip with colourless to brown wall, but most pigment extracellular. Ascospores simple, colourless, ellipsoid, halonate, (14·0–)15·5–17·0–18·5(–20·5) × (8·0–)8·5–9·4–10·5(–13·0) µm, (1·2–)1·6–1·8–2·1(–2·3) times as long as wide (30 spores measured, from 6 specimens).
Pycnidia occasionally present. Conidia not seen.
Chemistry
Methyl 2′-O-methylmicrophyllinate (major), Unknown B (major), Unknown A (trace, ±), 2′-O-methylmicrophyllinic acid (trace) (Fig. 1). Unknown B is readily seen under short-wave UV on TLC plates, but Unknown A is present only in trace amounts and may be detectable only by its fluorescence in long-wave UV after heating the plate. Thallus K−, PD−, C−; microscopic preparations in K not exuding minute droplets. Solvent System G is recommended for separating the major compounds, which are not separated in Solvent System A.
Ecology and distribution
In Great Britain the species is known from damp siliceous rock, including flushed rock faces, boulders, fine scree, and stones in snow beds, at altitudes of 100–1140 m. Numerous associated species are recorded, including the lichens Amygdalaria consentiens, Ionaspis lacustris, I. odora, Pilophorus strumaticus, Porpidia macrocarpa, P. tuberculosa, Rhizocarpon hochstetteri, R. lavatum and R. sublavatum, and the bryophytes Andreaea alpina, A. rothii subsp. falcata, A. rupestris, Kiaeria falcata, Marsupella adusta, M. emarginata and Racomitrium sudeticum. Two collections were also associated with Porpidia irrigua. Examples of relevés containing P. contraponenda are shown in Table 3. At the type locality, visited in 2012, the species occurred on stones on damp ground in flushes. It is known from North Wales, the English Lake District, western Scotland, and from Austria. Other records are uncertain due to confusion with P. irrigua.
Table 3. Community ecology of Porpidia contraponenda. Examples of relevés from Great Britain.
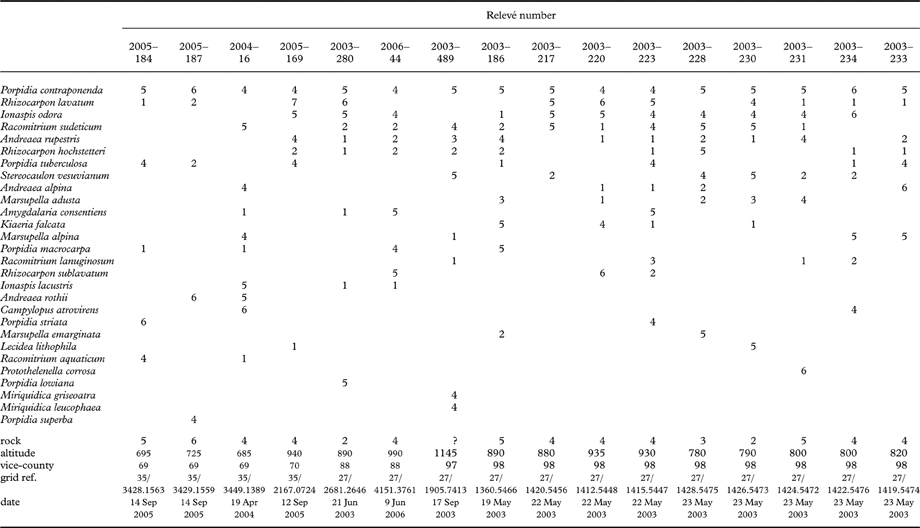
Relevés recorded on stands homogeneous in terms of vegetation. Cover expressed by the Domin scale. Species listed in order of frequency and cover; those recorded only as Domin 1, 2 or 3 omitted from table.
Typification
There are two specimens of Arnold, Lichenes exsiccati 1055 in F.C.G. Arnold's herbarium in M. The specimen numbered M0025569 was annotated as ‘holotype’ by H. Hertel in 1969, who was probably unaware of the second specimen. The specimen might be better regarded as a lectotype, but this is not proposed here as there is no evidence that the exsiccata is heterogeneous. M0025569 has a well-developed whitish to pale grey thallus; the young apothecia are at most half-immersed, later immersed only at base, and no more than one-quarter immersed when mature. M0024172 comprises one fragment with a thinner thallus than M0025569 with more black prothallus visible, and a second fragment with a well-developed thallus. The syntype in BM has the same details as the exsiccati specimens, but the label is handwritten. This specimen and M0025569 contain the unknown substance above methyl 2′-O-methylmicrophyllinate (unknown B) on TLC plates. Several specimens (topotypes) collected from the type locality in 2012 agree with the syntypes in morphology and chemistry, and one is proposed here as an epitype.
Notes:
There is considerable variation in the thallus thickness; specimens from apparently more extreme environments tend to have a thinner thallus and consequently more prominent apothecia, also a more extensive prothallus and more blue-grey thallus pigment. In some British specimens the apothecia are more or less immersed in the thallus (Fig. 4A) and thus strikingly different to P. irrigua, but in others they are more prominent and more closely resemble the type of P. contraponenda, for instance Orange 14499 (Fig. 4B). Although Cecidonia galls are frequent, they tend to be lower and less conspicuous than in P. irrigua.
Selected specimens examined. Great Britain: Wales: V.C. 48, Merioneth, Cader Idris, near Llyn y Gafr [23/71.14], 1960, Wade (NMW – 60.494.107); Cadair Idris, near Llyn Arran, 23/7424.1420, 2003, Orange 14611 (NMW – C.2005.001.413). V.C. 49, Caernarvonshire, Nantgwynant, Afon Cwm Llan, 23/6267.5142, 2011, Orange 20447 (NMW – C.2011.014.9). V.C. 50, Denbighshire, Llanarmon, above Swch-cae-rhiw, [33/13.35], 1938, W. Watson (NMW – 38.859.15). England: V.C. 69, Westmorland, Helvellyn, Brown Cove, 35/3428.1563, 2005, Orange 16224 (NMW – C.2005.001.305). V.C. 70, Cumberland, rocks by Stickle Beck below the Tarn, [35/28.07], 1916, Wheldon (NMW – 25.146.4776); near Eskdale, Lingcove Beck, 35/2356.0453, 2005, Orange 16217 (NMW – C.2005.001.301); near Eskdale, Lingcove Beck. 35/2340.0437, 2005, Orange 16220 (NMW – C.2005.001.304); Hopegill Head, Hobcarton Crags, 35/1886.2209, 2004, Orange 15490 (NMW – C.2005.001.632). Scotland: V.C. 88, Mid-Perthshire, Beinn Heasgarnich, NW of Stob an Fhir-Bhogha, 27/4151.3761, 2006, Orange 16545 (NMW – C.2005.001.583). V.C. 97, Westerness, Ben Nevis, Coire na h-Urchaire, 27/1509.7159, 2003, Orange 14829 (NMW – C.2004.002.373); Ben Nevis, Coire Leis, 27/1719.7167, 2003, Orange 15323 (NMW – C.2004.002.389). V.C. 98, Main Argyll, Glen Coe, Coire nam Beitheach, 27/1360.5466, 2003, Orange 14499 (NMW – C.2004.002.162).—Austria: Tyrol: Kühtai, Finstertal, 47°12.48′N, 11°01.43′E, alt. 2025 m, 11 August 2012, Orange 21123 (NMW – C.2012.002.115); same locality and date, 47°12.43′N, 11°01.44′E, alt. 2025 m, Orange 21127 (NMW – C.2012.002.117); same locality and date, Orange 21129 (NMW – C.2012.002.118).
Porpidia irrigua Orange sp. nov.
MycoBank no: MB807961
Thallus white or pale grey, apothecia sessile, to 2 mm diam., ascospores 15–21·5 µm long, containing 2′-O-methylmicrophyllinate as the only major compound.
Type: Great Britain, Wales, Breconshire, Glyntawe, Nant y Llyn, national grid reference 22/8452.2068, 51°52.34′N, 3°40.65′W, altitude 495 m, 8 November 2011, on Old Red Sandstone, on unshaded, flushed, gently sloping bedrock in Nardus-rich grassland, with Rhizocarpon lavatum, Andreaea rothii subsp. falcata, A. Orange 20712 (NMW – C.2013.001.218 – holotype, MSC – isotype; GenBank accession no: KJ162300).

Fig. 5. Porpidia irrigua; A, holotype, note sessile apothecia and rugose galls of Cecidonia; B, Orange 17372. Scales: A & B=1 mm. In colour online.

Fig. 6. Porpidia species, sections of thalli and apothecia (semi-diagrammatic); A – E, P. contraponenda (A, Orange 15490; B & C, Orange 20447; D & E, Orange 14499); F – I, P. irrigua (F, Orange 16494; G, Orange 12542; H, Orange 17372; I, Orange 15772) Scale=1 mm.
Prothallus blue-black to black. Thallus white or pale grey, occasionally light blue-grey, areoles arising on prothallus when visible, soon coalescing and cracking; thallus more or less plane, or gently convex between cracks, 100–400 µm thick; very frequently with raised rugose galls caused by Cecidonia xenophana.
Apothecia sessile when mature, becoming sessile as soon as young disc begins to expand; up to 2·0 mm diameter, margin smooth, rarely faintly striate, 60–220 µm wide, eventually ± excluded in old apothecia; disc plane to gently to strongly convex when mature; pruina sometimes present on inner edge of young apothecial margin, present or absent on mature disc. Exciple densely pigmented at surface, brown to dull green-brown or dark dull greenish blue, within paler, brown throughout; hyphae 3·5–8·0(–12) µm wide. Hypothecium brown to red-brown. Hymenium 100–140 µm high. Epihymenium dull green-brown or brown, K−. Paraphyses c. 1·5–2·0 µm wide in centre of hymenium, tips 2·9–3·3 µm wide, walls colourless or dilute brown, surrounded by pigment (epihymenial pigment mostly extracellular). Asci clavate, 8-spored, tholus with an I+ blue tube-structure. Ascospores ellipsoid, simple, colourless, halonate, (15·0–)16·5–18·3-20·5(–21·5) × (7·5–)8·0–9·3–10·5(–12·5) µm, (1·4–)1·7–2·0–2·2(–2·5) times as long as wide (33 spores measured, from 7 specimens).
Pycnidia frequent; up to 560 µm diam. in surface view, multilocular, with several ostioles, apex uneven, black. Conidia simple, colourless, straight, 8–11×0·8 µm.
Chemistry
Methyl 2′-O-methylmicrophyllinate (major), Unknown A (±, trace), 2′-O-methylmicrophyllinic acid (trace), unknown (trace) (Fig. 1). Thallus K−, PD−, C−; microscopic preparations in K not exuding minute droplets.
Ecology and distribution
On damp siliceous rock, frequently where seasonally flushed, on bedrock and boulders, occasionally on fine scree but usually only in small quantities, at altitudes of 215–950 m. Associated species include Cladonia diversa, C. subcervicornis, Ephebe lanata, Pilophorus strumaticus, Porpidia tuberculosa, Rhizocarpon hochstetteri, R. lavatum and the bryophytes Andreaea alpina, A. rothii, A. rupestris and Racomitrium sudeticum. Andreaea rothii ssp. falcata is a particularly frequent associate on seasonally flushed surfaces. The species is apparently more tolerant of seasonal drought than P. contraponenda. Examples of relevés containing P. irrigua are shown in Table 4. Although the known distribution of the two species is quite similar, P. irrigua is the more frequent in southerly and lowland sites. The two can be found growing together in the same community. Recorded from North and South Wales, North-west England, West and Central Scotland, and Norway. Distribution outside Britain poorly known due to confusion with P. contraponenda.
Table 4. Community ecology of Porpidia irrigua. Examples of relevés from Great Britain.
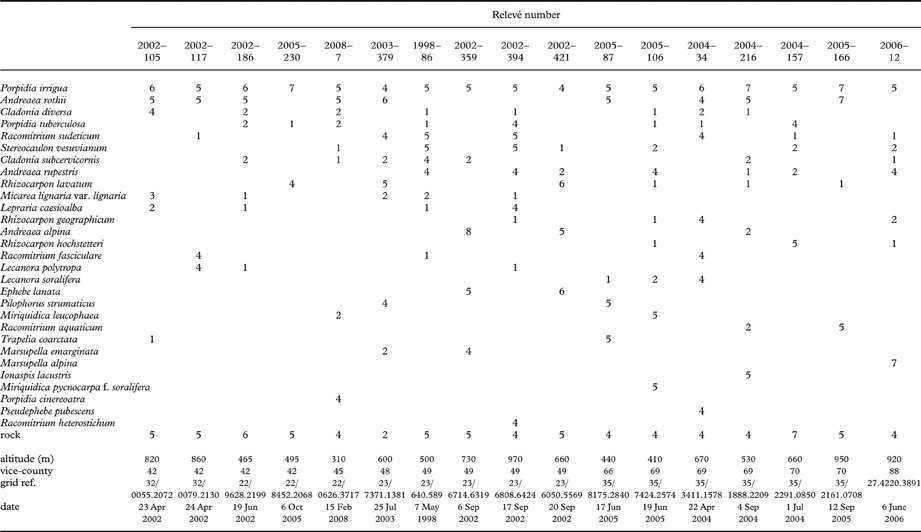
Relevés recorded on stands homogeneous in terms of vegetation. Cover expressed by the Domin scale. Species listed in order of frequency and cover; those recorded only as Domin 1, 2 or 3 omitted from table.
Notes
Porpidia irrigua differs from P. contraponenda in the generally thinner thallus (although the ranges of thickness overlap greatly), the apothecia which are sessile from a very early stage, and methyl 2′-O-methylmicrophyllinate as the only major compound. In contrast, the apothecia in P. contraponenda are at least partly immersed in the thallus, and in some specimens they are completely immersed; in addition, there is a second unidentified depside as a major compound in addition to methyl 2′-O-methylmicrophyllinate.
Besides P. contraponenda, the only previously described species in the genus which contains 2′-O-methylmicrophyllinate is P. diversa (Lowe) Gowan, described from eastern North America. This species was regarded as a synonym of P. contraponenda by Fryday (Reference Fryday2005). Specimens with a similar morphology and chemistry to P. irrigua occur in North America, but cannot currently be identified (see discussion).
Selected specimens examined. Great Britain: Wales: V.C. 41, Glamorgan, Rhondda Fach, Ynyshir, Mynydd Troed-y-rhiw, 31/012.934, 2006, Orange 16494 (NMW – C.2005.001.542). V.C. 42, Breconshire, Brecon Beacons, Corn Du, alt. 860 m, 32/0079.2130, 2002, Orange 13737 (NMW – C.2001.024.570); Glyntawe, Nant y Llyn, 22/8452.2068, alt. 495 m, 2005, Orange 16321 (NMW – C.2005.001.311; topotype). V.C. 43, Radnorshire, near Llanelwedd, Carneddau, Caer Fawr, [32/05.53], 1959, Wade (NMW – 60.139.122). V.C. 45, Pembrokeshire, near Fishguard, Newport, Mynydd Carn Ingli, Carn Ingli, 22/0644.3733, 2007, Orange 17372 (NMW – C.2007.001.143). V.C. 48, Merioneth, north-west of Dinas Mawddwy, Nant y Graig-wen, 23/828.175, 1998, Orange 12144 (NMW – C1999.011.72); Ganllwyd, Gwynfynydd, 23/7336.2802, 2009, Orange 18014 (NMW – C.2010.001.22).. V.C. 49, Caernarvonshire, Nantgwynant, Afon Cwm Llan, 23/6235.5159, 2011, Orange 20448 (NMW – C.2011.014.10). England: V.C. 69, Westmorland, Teesdale, Falcon Clints, 35/8175.2840, 2005, Orange 16166 (NMW – C.2005.001.201). V.C. 70, Cumberland, Derwent Fells, north of Dale Head, 35/2293.1663, 2004, Orange 15486 (NMW – C.2005.001.628). Scotland: V.C. 88, Mid Perthshire, Ben Lui, [27/26.26], 1911, Wilson & Wheldon (NMW – 25.146.4613). Ireland: V.C. H1, South Kerry, 13 km SE of Kenmare, Slaheny river, 10/028.659, 1998, Orange 11947 (NMW – C98.8.78). Norway: Sogn og Fjordane, Førde, N of lake Åsvatnet, Nordbø, 61°26.3′N, 6°01.7′E, 32V LP 415 152, 1998, Ekman 3183 (BG – L-38157).
Discussion
Porpidia contraponenda has been interpreted broadly in Great Britain, with the name encompassing two species differing in subtle morphological characters, chemistry, and ITS sequence. One of these has been shown to correspond to the type of P. contraponenda, and the other is described here as new.
Although P. contraponenda has been reported from North America (Gowan Reference Gowan1989, Fryday Reference Fryday2005), further work is necessary to confirm whether or not the species occurs there. In the present study, the ITS and LSU analyses distinguished either two or three species in North America which contain 2'-O-methylmicrophyllinate (the possibility that the taxon from which the ITS sequences were derived is conspecific with one of the taxa from which the LSU sequences were derived cannot be ruled out), and none of these are conspecific with sequenced specimens from Europe. Examination of three North American voucher specimens (the fourth was not found) for the ITS and LSU sequences suggests that these taxa resemble either P. contraponenda or P. irrigua in morphology. A resolution of these, and confirmation of the identity of P. diversa, will require a wide-ranging molecular study of Porpidia in North America. Thus, it is not possible at present to identify P. irrigua with any described or undescribed American taxon, and it is consequently described as a new species.
The ITS analysis demonstrates that there is a great deal of undescribed diversity in species related to P. macrocarpa. Fryday (Reference Fryday2005) described P. flavocruenta and P. islandica as new taxa within the P. macrocarpa group; these are supported by the present analyses of ITS data, but further undescribed taxa appear to be present. Some of the well-supported clades in the ITS analysis (Fig. 2) may correspond to described species, but more detailed studies are needed.
I would like to thank James Lendemer (NY) for kindly collecting material of Porpidia, the curators of F, M, NY and WTU for loan of material, and Jutta Buschbom and an anonymous referee for their helpful comments.












