INTRODUCTION
Aphasia is very common after a left-hemisphere stroke. It can be described as a “disruption of the linguistic code affecting expression and/or comprehension in oral or written discourse” (Brin, Reference Brin2014). Aphasia has a major impact on patients’ daily life, particularly on their psychoaffective and socioprofessional domains (Aïach & Baumann, Reference Aïach and Baumann2007). Few therapeutic solutions for aphasia recovery exist until now. For now, only speech rehabilitation proved its efficacy on discourse recovery but the effect size of the positive studies is quite low (Brady et al., Reference Brady, Kelly, Godwin, Enderby and Campbell2016; Yu et al., Reference Yu, Jiang, Jia, Xiao and Zhou2017). Nevertheless, the previous research found that the intensity of rehabilitation and the specialization of the therapist were more important than the rehabilitation technique used (Klippi et al., Reference Klippi, Sellman, Heikkinen and Laine2012). Therefore, there is an urgent need to develop new approaches to reduce aphasia in patients with stroke. Improvement of patients with poststroke aphasia seems related to changes in neuronal activity, and it has already been shown that speech-language therapy could modulate neuronal reorganization (de Boissezon et al., Reference de Boissezon, Raboyeau, Simonetta-Moreau, Puel, Démonet and Cardebat2008). But it is also possible to modulate neuronal activity using noninvasive brain stimulation techniques, such as transcranial direct current stimulation (tDCS) (ALHarbi, Armijo-Olivo, & Kim, Reference ALHarbi, Armijo-Olivo and Kim2017; Torres, Drebing, & Hamilton, Reference Torres, Drebing and Hamilton2013). In the context of the existing literature about aphasia and tDCS, potentiation of the effect of speech-language therapy seems possible by combining it with tDCS (Elsner, Kugler, Pohl, & Mehrholz, Reference Elsner, Kugler, Pohl and Mehrholz2015).
Indeed, various studies in the recent literature studied the impact of tDCS on aphasia recovery. Most of them measured an improvement of language parameters after tDCS stimulation combined with speech-language therapy (Baker, Rorden, & Fridriksson, Reference Baker, Rorden and Fridriksson2010; Campana, Caltagirone, & Marangolo, Reference Campana, Caltagirone and Marangolo2015; Cipollari et al., Reference Cipollari, Veniero, Razzano, Caltagirone, Koch and Marangolo2015; Jung et al., Reference Jung, Lim, Kang, Sohn and Paik2011; Marangolo et al., Reference Marangolo, Marinelli, Bonifazi, Fiori, Ceravolo, Provinciali and Tomaiuolo2011, Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014; Monti et al., Reference Monti, Cogiamanian, Marceglia, Ferrucci, Mameli, Mrakic-Sposta, Vergari, Zago and Priori2008; Vestito, Rosellini, Mantero, & Bandini, Reference Vestito, Rosellini, Mantero and Bandini2014; Vines, Norton, & Schlaug, Reference Vines, Norton and Schlaug2011; Wu, Wang, & Yuan, Reference Wu, Wang and Yuan2015; You, Kim, Chun, Jung, & Park, Reference You, Kim, Chun, Jung and Park2011). A recent systematic literature review including five meta-analyses and 48 studies found some evidence in the literature that tDCS could be effective for poststroke aphasia rehabilitation at the chronic stages, but there is still a lot of variability between studies (Biou et al., Reference Biou, Cassoudesalle, Cogne, Sibon, Gabory, Dehail, Aupy and Glize2019). The nature of the task, the stimulation site, the intensity and duration of the stimulation greatly differed from one study to the other (Baker et al., Reference Baker, Rorden and Fridriksson2010; Branscheidt et al., Reference Branscheidt, Hoppe, Zwitserlood and Liuzzi2018; Cherney et al., Reference Cherney, Babbitt, Hurwitz, Rogers, Stinear, Wang, Harvey and Parrish2013; Ewa et al., Reference Ewa, Maciej, Barbara, Wojciech and Anna2013; Jung et al., Reference Jung, Lim, Kang, Sohn and Paik2011; Lee et al., Reference Lee, Cheon, Yoon, Chang and Kim2013; Manenti et al., Reference Manenti, Petesi, Brambilla, Rosini, Miozzo, Padovani, Miniussi and Cotelli2015; Marangolo et al., Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014, Reference Marangolo, Fiori, Shofany, Gili, Caltagirone, Cucuzza and Priori2017; Sebastian et al., Reference Sebastian, Saxena, Tsapkini, Faria, Long, Wright, Davis, Tippett, Mourdoukoutas, Bikson, Celnik and Hillis2016; Vestito et al., Reference Vestito, Rosellini, Mantero and Bandini2014; Vines et al., Reference Vines, Norton and Schlaug2011; Wu et al., Reference Wu, Wang and Yuan2015; You et al., Reference You, Kim, Chun, Jung and Park2011). Nevertheless, there is a consensus in the existing literature on the need of combining stimulation with speech-language therapy. Until now, the two intensities most commonly used in the literature are 1 and 2 mA. Indeed, 19 studies described the use of a stimulation at 1 mA and 22 at 2 mA. A stimulation intensity of 2 mA during 20 min repeated for 10 to 30 sessions has been a frequently used stimulation paradigm (Branscheidt et al., Reference Branscheidt, Hoppe, Zwitserlood and Liuzzi2018; Marangolo et al., Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b; Meinzer et al., Reference Meinzer, Darkow, Lindenberg and Flöel2016; Sebastian et al., Reference Sebastian, Saxena, Tsapkini, Faria, Long, Wright, Davis, Tippett, Mourdoukoutas, Bikson, Celnik and Hillis2016; Volpato et al., Reference Volpato, Cavinato, Piccione, Garzon, Meneghello and Birbaumer2013; Wu et al., Reference Wu, Wang and Yuan2015). The electrode’s placement differs from one study to another. Language recovery depends on a neural reorganization based on two major phenomena: at first, the right hemisphere takes charge of the usual left-hemispheric language abilities before its reactivation; then, the left perilesional regions restore gradually their previous activation level if possible and new neural pathways are created into the left hemisphere (Hartwigsen & Saur, Reference Hartwigsen and Saur2017; Saur & Hartwigsen, Reference Saur and Hartwigsen2012). This reorganization depends partly on the control of the interhemispheric inhibition balance. More precisely, if the right hemisphere inhibits the activation of the left one, language improvement will be weaker because it depends on the left hemispheric activations. On the contrary, the reactivation of the language areas in the left hemisphere correlates with an improvement of the speech function (de Boissezon et al., Reference de Boissezon, Raboyeau, Simonetta-Moreau, Puel, Démonet and Cardebat2008; Naeser et al., Reference Naeser, Martin, Theoret, Kobayashi, Fregni, Nicholas, Tormos, Steven, Baker and Pascual-Leone2011). Therefore, it seems appropriate to set up a bihemispheric stimulation with a neuronal inhibition on the right hemisphere near Broca’s homologous area and a neuronal activation on Broca’s area in the left hemisphere.
Although bihemispheric stimulation has often been considered promising, there has been little empirical research on its effect on aphasia recovery (Fiori et al., Reference Fiori, Nitsche, Iasevoli, Cucuzza, Caltagirone and Marangolo2017; Lee et al., Reference Lee, Cheon, Yoon, Chang and Kim2013; Marangolo et al., Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014; Santos et al., Reference Santos, Cavenaghi, Mac-Kay, Serafim, Venturi, Truong, Huang, Boggio, Fregni, Bikson and Gagliardi2017). Lee et al. (Reference Lee, Cheon, Yoon, Chang and Kim2013) compared the effect of bi and monohemispheric tDCS on naming and verbal fluency ability (the number of syllables produced in 1 min during an image description) in 11 patients with poststroke aphasia and found a significant improvement of naming in both conditions but a significant shorter naming response time for the group with bihemispheric stimulation.
In the recent literature, naming has been the most frequently used outcome measure for assessing aphasia recovery. The main value of this task is the ease of the evaluation, but its major disadvantage is that it does not reflect the patient’s ability to communicate (Elsner, Kugler, Pohl, & Mehrholz, Reference Elsner, Kugler, Pohl and Mehrholz2013; Elsner et al., Reference Elsner, Kugler, Pohl and Mehrholz2015; Otal et al., Reference Otal, Olma, Flöel and Wellwood2015; Shah-Basak et al., Reference Shah-Basak, Wurzman, Purcell, Gervits and Hamilton2016). The two main naming tasks found in the literature are naming of nouns and naming of verbs. The share is larger for nouns naming because of its use when authors use standardized language evaluation batteries (Baker et al., Reference Baker, Rorden and Fridriksson2010; Branscheidt et al., Reference Branscheidt, Hoppe, Zwitserlood and Liuzzi2018; Campana et al., Reference Campana, Caltagirone and Marangolo2015; Cherney et al., Reference Cherney, Babbitt, Hurwitz, Rogers, Stinear, Wang, Harvey and Parrish2013; Fiori et al., Reference Fiori, Coccia, Marinelli, Vecchi, Bonifazi, Ceravolo, Provinciali, Tomaiuolo and Marangolo2011; Flöel et al., Reference Flöel, Meinzer, Kirstein, Nijhof, Deppe, Knecht and Breitenstein2011; Fridriksson et al., Reference Fridriksson, Rorden, Elm, Sen, George and Bonilha2018; Galletta et al., Reference Galletta and Vogel-Eyny2015; Kang et al., Reference Kang, Kim, Sohn, Cohen and Paik2011; Lee et al., Reference Lee and Thompson2011; Marangolo et al., Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014, Reference Marangolo, Fiori, Sabatini, De Pasquale, Razzano, Caltagirone and Gili2016, Reference Marangolo, Fiori, Shofany, Gili, Caltagirone, Cucuzza and Priori2017, Reference Marangolo, Fiori, Caltagirone, Pisano and Priori2018; Meinzer et al., Reference Meinzer, Darkow, Lindenberg and Flöel2016; Monti et al., Reference Monti, Cogiamanian, Marceglia, Ferrucci, Mameli, Mrakic-Sposta, Vergari, Zago and Priori2008; Pestalozzi et al., Reference Pestalozzi, Di Pietro, Martins Gaytanidis, Spierer, Schnider, Chouiter, Colombo, Annoni and Jost2018; Polanowska et al., Reference Polanowska, Leśniak, Seniów and Członkowska2013; Sandars et al., Reference Sandars, Cloutman and Woollams2017; Santos et al., Reference Santos, Gagliardi, Mac-Kay, Boggio, Lianza and Fregni2013, Reference Santos, Cavenaghi, Mac-Kay, Serafim, Venturi, Truong, Huang, Boggio, Fregni, Bikson and Gagliardi2017; Shah-Basak et al., Reference Shah-Basak, Norise, Garcia, Torres, Faseyitan and Hamilton2015; Spielmann et al., Reference Spielmann, van de Sandt-Koenderman, Heijenbrok-Kal and Ribbers2018; Vestito et al., Reference Vestito, Rosellini, Mantero and Bandini2014; Vines et al., Reference Vines, Norton and Schlaug2011; Volpato et al., Reference Volpato, Cavinato, Piccione, Garzon, Meneghello and Birbaumer2013; You et al., Reference You, Kim, Chun, Jung and Park2011). Furthermore, generalization has been measured in several studies: some of them assessed the transfer of competence on an untrained task (Lee et al., Reference Lee and Thompson2011; Marangolo et al., Reference Marangolo, Marinelli, Bonifazi, Fiori, Ceravolo, Provinciali and Tomaiuolo2011, Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014, Reference Marangolo, Fiori, Sabatini, De Pasquale, Razzano, Caltagirone and Gili2016, Reference Marangolo, Fiori, Shofany, Gili, Caltagirone, Cucuzza and Priori2017; Manenti et al., Reference Manenti, Petesi, Brambilla, Rosini, Miozzo, Padovani, Miniussi and Cotelli2015; Sebastian et al., Reference Sebastian, Saxena, Tsapkini, Faria, Long, Wright, Davis, Tippett, Mourdoukoutas, Bikson, Celnik and Hillis2016; Vestito et al., Reference Vestito, Rosellini, Mantero and Bandini2014; Woodhead et al., Reference Woodhead, Kerry, Aguilar, Ong, Hogan, Pappa, Leff and Crinion2018; Wu et al., Reference Wu, Wang and Yuan2015); some others showed the improvement of untrained items in addition of trained items (Baker et al., Reference Baker, Rorden and Fridriksson2010; De Aguiar et al., Reference de Aguiar, Bastiaanse, Capasso, Gandolfi, Smania, Rossi and Miceli2015; Manenti et al., Reference Manenti, Petesi, Brambilla, Rosini, Miozzo, Padovani, Miniussi and Cotelli2015; Meinzer et al., Reference Meinzer, Darkow, Lindenberg and Flöel2016; Marangolo et al., Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014, Reference Marangolo, Fiori, Sabatini, De Pasquale, Razzano, Caltagirone and Gili2016; Sebastian et al., Reference Sebastian, Saxena, Tsapkini, Faria, Long, Wright, Davis, Tippett, Mourdoukoutas, Bikson, Celnik and Hillis2016; Vestito et al., Reference Vestito, Rosellini, Mantero and Bandini2014; Woodhead et al., Reference Woodhead, Kerry, Aguilar, Ong, Hogan, Pappa, Leff and Crinion2018); and some others found an improvement in certain language tasks following nonspecific training of the tested tasks (Campana et al., Reference Campana, Caltagirone and Marangolo2015; Cipollari et al., Reference Cipollari, Veniero, Razzano, Caltagirone, Koch and Marangolo2015; Galletta et al., Reference Galletta and Vogel-Eyny2015; Vines et al., Reference Vines, Norton and Schlaug2011). Some studies did not find a generalization effect in all patients (Marangolo et al., Reference Marangolo, Marinelli, Bonifazi, Fiori, Ceravolo, Provinciali and Tomaiuolo2011; Vestito et al., Reference Vestito, Rosellini, Mantero and Bandini2014). Only Sandars et al. (Reference Sandars, Cloutman and Woollams2017) did not find a generalization effect for untreated items.
Kintsch et al. (Reference Kintsch1994) made the distinction between microlinguistics and macrolinguistics. More precisely, discourse microanalyses focused on the study of lexical, syntactic, word finding, grammatical units, and characteristics of spoken language (pauses, paraphasias, and fluidity), while macroanalyses focused on studying the coherence of discourse (local coherence at sentence level and global coherence at discourse level). The measures of main concepts and single word information in the form of C-Units (Correct Information Units) (Brookshire & Nicholas, Reference Brookshire and Nicholas1994) emerged as the best measures for discourses analysis in the review of Pritchard, Hilari, Cocks, and Dipper (Reference Pritchard, Hilari, Cocks and Dipper2017). According to Davis et al. (Reference Davis, O’Neil-Pirozzi and Coon1997) and Bastiaanse and Jonkers (Reference Bastiaanse and Jonkers1998), microlinguistic and macrolinguistic components can be analyzed separately, but it seems useful to combine structural and functional analyses (Sherratt, Reference Sherratt2007). All language levels come into play in the spontaneous discourse of the patients with poststroke aphasia (Chapman & Ulatowska, Reference Chapman and Ulatowska1989), which is why current studies tend towards a mixed analysis that combines both sides (Falconer & Antonucci, Reference Falconer and Antonucci2012; Marini, Andreetta, del Tin, & Carlomagno, Reference Marini, Andreetta, del Tin and Carlomagno2011; Pritchard, Hilari, Cocks, & Dipper, Reference Pritchard, Hilari, Cocks and Dipper2018).
Until now, only few studies using tDCS explored more functional activities such as spontaneous speech, but the selected tasks remained mostly semidirected tasks of image or video clip descriptions (Campana et al., Reference Campana, Caltagirone and Marangolo2015; Galletta & Vogel-Eyny, Reference Galletta and Vogel-Eyny2015; Marangolo et al., Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014; Norise, Sacchetti, & Hamilton, Reference Norise, Sacchetti and Hamilton2017). Marangolo et al. (Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014), in a double-blind randomized controlled study including eight patients receiving 10 days of rehabilitation with a monohemispheric tDCS stimulation of 1 mA during 20 min, studied the effects of a conversational therapy on a video clip description task. They found an improvement of the average number of C-Units following a stimulation of the Broca’s area significantly higher than after stimulation of the Wernicke’s area or after sham stimulation. To the best of our knowledge, no study evaluated the effect of tDCS on spontaneous speech in response to an open question, yet neither used a bihemispheric stimulation to assess its effects on a speech task.
The aim of the present study was also to assess the impact of a bihemispheric tDCS stimulation coupled with speech-language therapy on spontaneous language performances of patients with poststroke aphasia answering to an open question.
MATERIALS AND METHODS
This study was a randomized, double-blind, multicentric interventional trial, comparing a bihemispheric tDCS stimulation to a sham stimulation in patients with a left-hemispheric stroke, using a cross-over design. Three neurorehabilitation units located in the Parisian area participated in the study by including patients.
Patients
Between November 2015 and January 2018, 14 left-hemispheric stroke patients were included in the present study. Inclusion criteria were age ≥ 18 years old (y.o.), French language proficiency, and a first and unique stroke located in the left hemisphere of the brain (confirmed by magnetic resonance imaging or computerized tomography scan), regardless of the delay since stroke onset. The French adaptation of the Boston Diagnostic Aphasia Examination (HDAE, Mazaux and Orgogozo, Reference Mazaux and Orgogozo1982) score had to be one or more at the inclusion time. Indeed, we excluded patients with an aphasia severity rating scale=0, which corresponds to “no intelligible expression and no oral comprehension,” because we needed to transcribe a minimum number of corpus for the speech analysis. Exclusion criteria were previous neurological disorders and the usual contraindications for tDCS (metal material or epileptic seizures occurring in 2 months before the inclusion).
Patients were in- or out-patients from three different neurorehabilitation units at the inclusion time. Each patient hospitalized in one of these three units and suffering from aphasia was screened by a speech and language therapist of this rehabilitation unit. If one patient fulfilled the inclusion criteria and did not present any of the exclusion criteria, one of the study investigators asked them to participate. An oral and written information was given to the patients and their relatives to explain the protocol. After a 1-week period of reflection, the patients and their relatives signed an informed consent form.
Transcranial Direct Current Stimulation
tDCS was applied with a battery-driven Eldith (NeuroConn GmbH, Ilmenau, Germany) programmable with a pair of surface-soaked sponge’s electrodes (5 × 7 cm). Bihemispheric stimulation was administrated. Real stimulation consisted of 20 min of 2-mA direct current with the anode placed over the IFG (F7 international 10–20 system for electroencephalography (EEG)) and the cathode over the contralesional IFG (F8 international 10–20 system for EEG). The duration of fade-in and fade-out was 8 s with an increment every second. For sham stimulation, the same position was used but the current was turned off after 30 s (Gandiga, Hummel, & Cohen, Reference Gandiga, Hummel and Cohen2006).
Study Design
A cross-over design was used to compare the active tDCS stimulation and the sham stimulation (Sham). The administration order of active-tDCS and sham was randomized by the Raymond Poincaré Hospital Clinical Investigation Center (CIC1429), using a stratified randomization by center. After signing informed consent, patients were included in the protocol for a period of 10 weeks.
Outcome measures were completed 6 times during the protocol: first, patients achieved as baselines three evaluations (1–2–3), one per week during 3 weeks (baseline period = P0). Just after that, began the first 3-week period of speech-language therapy combined with stimulation (active-tDCS or sham) (P1). Outcome measures’ assessments were done after P1 (evaluation 4) and just before P2 started (evaluation 5). Between the two periods of stimulation, patients had 1 week of washout without any stimulation and during which the speech-language therapy trained exclusively other cognitive processes than language. After the washout period began a second 3-week period of speech-language therapy combined with stimulation (active-tDCS or sham depending on the randomization order) (P2). At the end of the second period, outcome measures assessments were performed again (evaluation 6), in addition to the Likert scales to assess the tolerance, feasibility, and satisfaction of the patients and therapists, and then a last time (evaluation 7) after 1 week of washout (Figure 1).

Fig. 1. Overview of the study design.
The stimulation was applied 2–5 times per week during two periods of 3 weeks. Patients were blinded with respect to the administration of active-tDCS or sham stimulation. To control the blindness at the end of each session, all participants were asked about their awareness of the received stimulation, by answering the following question: “Do you think that the stimulation was on during the present session?” (The attempted answers were “yes”, “no” or “I don’t know”). The session’s number and the duration of each session were recorded. The speech therapists set the stimulation at the beginning of each speech-language therapy’s session, but the evaluators were also blinded regarding the stimulation. During both conditions (active-tDCS or sham stimulation), patients simultaneously underwent speech-language therapy. The tDCS electrodes were removed at the end of the session.
Speech-language Therapy
There is yet no consensus about the prevalence of a language rehabilitation technique over another (Brady et al., Reference Brady, Kelly, Godwin, Enderby and Campbell2016), and no consensus exists in France either according to the HAS (Higher Health Authority of the country), to determine the better strategy for an optimal speech-language therapy. In the present study, speech rehabilitation was performed in accordance to the preferences of each center at the frequency of 2–5 therapy sessions per week during the entire inclusion period. The number of sessions could vary depending on the poststroke delay, according to the French recommendations (Biga, Reference Biga2017).
Assessment
The main judgment criterion was the number of different nouns used during the HDAE subtest question “Describe your Job” (Goodglass & Kaplan, Reference Goodglass and Kaplan2007). Patients’ oral answers were recorded during 2 min and analyzed by two different blinded speech therapists. The answers’ analyses started with the exclusion of inadequate answers (unrelated to the task). For the flow measures, the number of nouns, verbs, adjectives, adverbs, and pronouns within the patients’ answers were recorded. Regarding the quality measures, we recorded the number of repetitions, blank ideas (utterance without novel idea), ideas (utterances related to the question), utterances with grammatical errors, and paraphasias.
The secondary judgment criteria were the verbal fluency tests assessed during 2 min each [categorical (animals) and lexical (words starting with a P)] (Godefroy & Groupe de réflexion sur l’évaluation des fonctions exécutives, Reference Godefroy2008), the time and the number of omissions on the Bells test as a measure of attention (Gauthier, Dehaut, & Joanette, Reference Gauthier, Dehaut and Joanette1989), and the verbal working memory span (Godefroy & Groupe de réflexion sur l’évaluation des fonctions exécutives, Reference Godefroy2008). The description of outcome measures is available in Appendix 1 in Supplementary Material.
Furthermore, at the end of the treatment, various Likert’s scales were completed by the patients, their relatives, and the speech therapist in order to evaluate their satisfaction and the tolerability of the tDCS sessions. Likert’s scales included five levels: strongly disagree, disagree, neither agree nor disagree, agree, and strongly agree.
We expected the improvement to be better when the stimulation was active (A) compared to the sham stimulation (B).
Ethics
The study was promoted by the Garches Foundation and funded by a grant from the Bennetot Foundation (N° AP-FPB-14-002). It was authorized by the French Ethics Committee N° 15025 “Ile de France Paris XI” on 11 June 2015 and by the French National Agency for the Safety of Medicines and Health Products (ANSM, N°2014-A01773-44). Prior to inclusion, all participants received an information note. After a minimum period of 24 hr, written consents were obtained for all participants. The Clinical Trials registration number of the study is N° NCT02612753.
Statistics
Power calculation: According to data from Marangolo (2014), we estimated the difference in the number of words when describing a video between rehabilitation alone and rehabilitation with stimulation as 17 words with a standard deviation (SD) of 10 and an effect size of 1.7. A number of six subjects per center highlight a size effect of 1.5 with a power of 80% and an α risk of 5%. In order to take into account the risk related to dropouts of around 20%, we decided to recruit eight patients per center.
The statistical analyses were performed using the SPSS (IBM corporation, USA) software version 22.
The characteristics of the population (sociodemographic, speech, characteristics of the brain damage, etc) were described using means (SD) for continuous variables and frequency for qualitative variables. We calculated the difference between the raw scores of the first poststimulation evaluation (evaluation 4) and of the last baseline measure (evaluation 3) to obtain the improvement score during the first stimulation period (P1). We also calculated a difference between the raw scores of the second poststimulation (evaluation 6) and of the post-washout (evaluation 5) to obtain the improvement score during the second stimulation period (P2). Because of the low number of participants in our study, we used nonparametric tests, that is, matched Wilcoxon tests. For Wilcoxon Signed-Rank tests, effect sizes were calculated by hand by using the following formula: r = Z/√N as proposed by Tomczak and Tomczak (Reference Tomczak and Tomczak2014). A p-value <.05 was considered as statistically significant.
RESULTS
Description of the Study Population
We included 14 participants (4 women). The mean age was 53.8 (min = 24; max = 69; SD = 13.1). Ten of them had more than 12 years of education, four had 9–12 years of education (mean education duration = 2.7; min = 2; max = 3; SD = .45). Four patients (three in group 1) did not complete every major evaluation (two because of missing visits (one because of holidays), two because of unexpected medical events unrelated to the protocol (fracture and surgery) and could therefore not be included in the statistical analyses. All participants had a left-hemispheric stroke (ischemic for 12 and hemorrhagic for 2 of them). The delay since stroke onset ranged from 1 month to 5 years (mean = 18 months; min = 1 month; max = 68 months; SD = 21.3 months). The mean aphasia score on the HDAE was 2.64 (min = 1.0; max = 4.0; SD = 1.1).
Regarding the 10 patients who completed the whole study, 5 received active tDCS during the first period (P1) and 5 during the second period (P2). The average frequency of speech-language therapy sessions was 3.85 sessions per week (min = 2; max = 5; SD = 1.1). The mean duration of speech-language therapy sessions was 72.8 min per session (min = 45 min; max = 120 min; SD = 33.2 min).
Table 1 shows the sociodemographic and clinical data of the 14 participants.
Table 1. Sociodemographic and clinical data at baseline of the 14 patients included in the study

Note: The first column shows the number of the participants’ inclusion. The second column shows group distribution (Group 1 = active t-DCS first (P1); Group 2 = active t-DCS during period 2 (P2)). The next columns show, respectively, gender, age, frequency of the speech-language therapy sessions, time since stroke onset, type of stroke, type of aphasia, pretreatment HDAE score, and educational level (3 = more than 12 years, 2 = 9–12 years; 1 = 8 years or less).
There was no significant difference at baseline between the patients receiving active-tDCS first (group 1) or sham first (group 2) regarding age (p = .35), gender (Khi2 = .4, p = .53), education (p = 1.00), time since stroke onset (p = .42), and pretreatment aphasia severity measured using the HDAE (p = .79). There was no significant difference concerning the main judgment criterion between the patients receiving active tDCS first or active tDCS second (z = −.67, partial η 2 = −.30, p = .50).
Effect on the Primary Outcome Measure
There was no significant difference between active-tDCS and sham tDCS regarding the raw improvement scores of the primary outcome measure (the number of different nouns used during the HDAE job description subtest) (z = −.71, partial η 2 = −.22, p = .48) (Figure 2).

Fig. 2. Raw improvement scores for the number of nouns of the 10 participants who completed the study.
The figure’s axis represents each patient included in the study. The improvement score at the end of the active tDCS is represented in dark grey, and the improvement score at the end of the sham tDCS is represented in light grey. Patients on the left part of the figure received active tDCS first, and those on the right part received sham stimulation first.
Effect on the Secondary Outcome Measures
There was no significant difference between active-tDCS and sham tDCS conditions concerning the secondary outcome measures (i.e., for verbs, adjectives, adverbs, pronouns, words, repetitions, blank ideas, ideas, utterances with grammatical errors, paraphasias, verbal working memory span direct, verbal working memory span reverse, number of omissions during the Bells test, total duration of the Bells test in seconds, and verbal fluency tests; Table 2).
Table 2. Main and secondary outcome measures between the active tDCS group and the sham-tDCS group
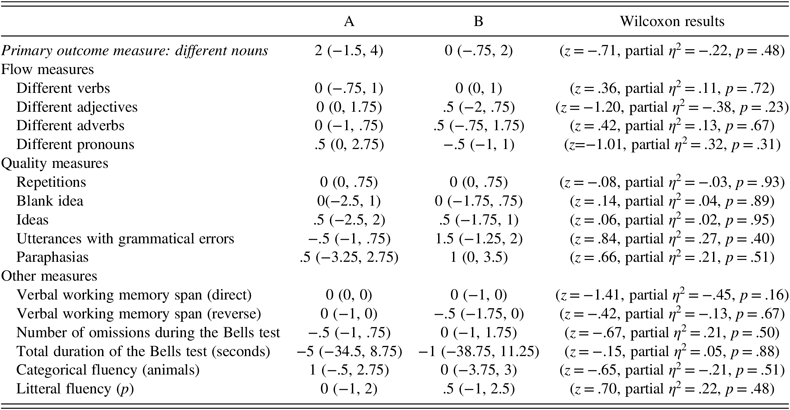
Note: The first column shows the primary and secondary outcome measures; the second column shows the raw improvement score after the active tDCS condition [median (Quartile 1, Quartile 3)]; the third column shows the raw improvement score after the sham tDCS condition [median (Quartile 1, Quartile 3)]; the fourth column presents the Wilcoxon test. p < .05 was considered as statistically significant.
Patient’s Acceptability and Satisfaction
We asked participants about their acceptability of the tDCS stimulation, using a five-level Lickert scale. Regarding the following statement: “there are no concerns about the use of this technique”, 3/9 (33%) of patients strongly agreed, 5/9 (56%) agreed, and 1/9 (11%) were undecided. In response to this same statement, 1/5 (20%) of relatives strongly agreed, 1/5 (20%) agreed, 1/5 (20%) were undecided, and 2/5 (40%) disagreed. Regarding therapists’, 1/9 (90%) of them strongly agreed and 1/10 (10%) of them agreed. Afterwards, participants were asked to give their opinion concerning the following statement: “I am satisfied with this new therapeutic approach,” 4/9 (44%) of patients strongly agreed, 3/9 (33%) agreed, 1/9 (11 %) were undecided, and 1/9 (11%) disagreed. In response to this same statement, 1/5 (20%) of relatives strongly agreed, 3/5 (60%) agreed, and 1/5 (20%) were undecided. Regarding the therapists, 9/10 (90%) of them strongly agreed with the statement and 1/10 (10%) of them agreed. And finally, 9/10 (90%) of therapists strongly agreed and 1/10 (10%) agreed that the technique is easily used in rehabilitation (Table 3).
Table 3. Acceptability and satisfaction results

Note: The first column specifies the statement and the respondents (9 patients, 5 relatives, and 10 Speech and Language Therapists). The next columns present the number of answers by level on the Lickert scales with the corresponding percentage into brackets.
Side Effects
No side effects appeared during the treatment, which was well tolerated by each participant.
DISCUSSION
The aim of our study was to determine if tDCS combined with speech-language therapy in routine clinical practice improved spontaneous speech in response to an open question in patients with poststroke aphasia. No significant difference was found between the two conditions (active-tDCS and sham-tDCS) regarding the primary outcome measure, that is, the number of different nouns used during the HDAE subtest question “Describe your Job,” and regarding the secondary outcome measures (flow measures, quality measures, verbal working memory, Bells test, and fluency tests). No adverse effects occurred and patients and speech therapists were satisfied with the use of the tDCS device.
Comparison with Previous Studies
Some studies compared the effect of mono- and bihemispheric tDCS on a learning task in healthy controls. For example, Lindenberg et al. (Reference Lindenberg, Renga, Zhu, Nair and Schlaug2010) found a larger improvement on a choice reaction time task when using bihemispheric stimulation in elderly subjects, just like Fiori et al. (Reference Fiori, Nitsche, Iasevoli, Cucuzza, Caltagirone and Marangolo2017) using a verbal learning task. But only few studies explored the impact of bihemispheric tDCS on language performances in patients with poststroke aphasia (Lee et al., Reference Lee, Cheon, Yoon, Chang and Kim2013; Marangolo et al., Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b, Reference Marangolo, Fiori, Sabatini, De Pasquale, Razzano, Caltagirone and Gili2016). Yet Lee et al. (Reference Lee, Cheon, Yoon, Chang and Kim2013) found a larger improvement of reaction time in naming pictures, using a bi- rather than a monohemispheric stimulation in 11 patients with poststroke aphasia. Furthermore, Marangolo et al. (Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b) found in nine patients an increase of accuracy and speed during an articulatory task but also an improvement in other language tasks. More recently, a systematic review (Biou et al., Reference Biou, Cassoudesalle, Cogne, Sibon, Gabory, Dehail, Aupy and Glize2019) concluded on the effectiveness of tDCS on poststroke aphasia rehabilitation at the chronic stages. Faced with these promising results, we chose to evaluate the impact of a bihemispheric stimulation on spontaneous speech in patients with poststroke aphasia. Our results are in accordance with Norise et al. (Reference Norise, Sacchetti and Hamilton2017) who recently evaluated in a randomized study in nine patients with poststroke aphasia, the effect of monohemispheric tDCS in a patient-dependent optimal response site (2 mA, 20 min) on verbal fluency. They did not find any significant improvement of the number of nouns generated, the sentence length, the proportion of well-formed sentences, and the proportion of pronouns during the Cookie Theft Picture Description Task after active stimulation. But the task used was semidirected (which means that the discourse is guided by the question asked and that the expected elements are explicit (e.g., the characters or the scenario in the image) and also not similar to ours which used an open question (where the theme (professional activity) is guided, but the form and content are different and specific to each patient). But Marangolo et al. (Reference Marangolo, Fiori, Cipollari, Campana, Razzano, Di Paola and Caltagirone2013b, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014), in two double-blind randomized studies, found a significantly higher number of C-units, verbs, mean percentage of correct, and number of sentences for trained items after a Broca’s area stimulation than a Wernicke’s area or a sham stimulation. Concerning untrained items, the results were more heterogeneous depending on the clip for C-Units (Content Units are clusters of elements and/or isolated phrases not always accompanied by a verb but with high communicative value (Loban, Reference Loban1963), numbers of verbs and sentences). The mean percentage of endophoric’s references was also significantly higher (endophoric’s references are words referring to concepts that have been previously mentioned in the flow of discourse, anaphoras, conjunctions, ellipses, word repetition, etc. Halliday & Hasan, Reference Halliday and Hasan2009). But these studies did not evaluate the effect of tDCS stimulation on the number of nouns and the outcome measures were trained specifically during the speech-language therapy sessions, which differs from our study. Lastly, the stimulation parameters used in these studies (a monohemispheric stimulation with an intensity of 1 mA) differed from ours. Campana et al. (Reference Campana, Caltagirone and Marangolo2015) assessed in a randomized controlled study the impact of tDCS combined with speech conversational therapy on various language abilities (picture description, nouns, and verbs naming) in 20 nonfluent chronic patients with poststroke aphasia. The main assessment criterion was the difference of percentage of correct answers before and after treatment (percentage of correct nouns and verbs in naming and of correct sentences in picture description). They found a significant improvement of the three outcome measures but only after anodic tDCS. The stimulation modality was similar to ours but with a monohemispheric configuration. However, 9/20 patients did not improve on the picture description task. Moreover, the percentage of improvement for a picture description task is a questionable measure as it depends on the total number of sentences.
Discursive Language Measurement and Task Training
Two studies made by Marangolo et al. (Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014) moved towards a discursive approach. The first one showed an improvement of verb denomination, nouns, and image description after active-tDCS stimulation. The rehabilitation used was pragmatic, with a dialogue between the patient and the therapist about cartoons. The purpose of this rehabilitation was to make the patient more and more informative. In this study, the evaluated task was not specifically trained. However, the outcome measures included paraphasias, which may be misleading. In opposition, in our study, we removed all language and speech deviations, therefore paraphasias were not considered as correct responses. The second study found an improvement of the number of endophoric references after active-tDCS stimulation. In this latter study, conversational improvement was analyzed through the description of video clips. However, such a task could be considered as a semidirected and not a spontaneous discourse, such as the open-ended question used in our study. In addition, it should be emphasized that three video clips were used during training sessions, and language improvement was significant on these trained “T-video clips,” while there was no significant improvement on the two untrained clips, which were used only for evaluations. This suggests that the beneficial effect could be due, at least in part, to task learning.
In most other studies that reported a naming improvement after tDCS stimulation, the patients were trained with the outcome assessment task.
Strengths and Limitations of the Present Study
To the best of our knowledge, no previous study addressed the effect of tDCS on a spontaneous language task such as the answer to an open question. Previous research relied mainly on semidirected speech tasks through video clips (Marangolo et al., Reference Marangolo, Fiori, Calpagnano, Campana, Razzano, Caltagirone and Marini2013a, Reference Marangolo, Fiori, Campana, Calpagnano, Razzano, Caltagirone and Marini2014) or picture’s description (Campana et al., Reference Campana, Caltagirone and Marangolo2015; Norise et al., Reference Norise, Sacchetti and Hamilton2017). Ecological tasks such as answering an open question could be assumed to reflect more accurately the daily life functioning of patients with poststroke aphasia. In our study, we had to deal with both methodological and clinical practice constraints. One of the strength of the present study is its multicentric, randomized, double-blind cross-over controlled design. Each cross-over period lasted 3 weeks which corresponded to the longer duration found in the previous research with tDCS (Cipollari et al., Reference Cipollari, Veniero, Razzano, Caltagirone, Koch and Marangolo2015; Manenti et al., Reference Manenti, Petesi, Brambilla, Rosini, Miozzo, Padovani, Miniussi and Cotelli2015; Marangolo et al., Reference Marangolo, Fiori, Sabatini, De Pasquale, Razzano, Caltagirone and Gili2016). However, in our current practice, progress observed clinically appears mainly more often after months than after weeks. Unfortunately, the administrative constraints of our protocol did not allow us to follow our population longer, which is a limitation of our study. Contrary to previous studies, we used three baseline evaluations in order to control the intraindividual variability of speech production, which is widely known in patients with poststroke aphasia (Duncan, Schmah, & Small, Reference Duncan, Schmah and Small2016; Villard & Kiran, Reference Villard and Kiran2018). Nevertheless, in order for multiple baselines to be relevant and meaningful, the Single-Case Experimental Design (SCED) methodology would have required optimally five baselines at least. Furthermore, two assessors translated and analyzed the audio records until reaching a consensus in order to avoid an evaluation bias. Finally, to facilitate reproducibility, we tried to stay as close as possible to the number, duration, and content of speech-language therapy sessions usually done in rehabilitation centers in our country. Hospitalization times, budget allocations for speech and language rehabilitation, number of sessions that could be performed taking into account the number of speech and language therapists, and patient attendance days could not be completely objectively adapted to the specific requirements of a clinical study (i.e., a precise homogeneity of the duration and frequency of sessions between subjects for example).
But our study has some limitations. Although the sample size is similar to most of the previously published studies in the field, our small number of patients limited the statistical power. We included only 14 patients, while we had planned to include 24 participants. French rehabilitations centers receive very heterogeneous patients, and it is often difficult to find patients respecting inclusions criteria and moreover the duration of hospitalization is often insufficient to follow a study of 10 weeks. Additionally, our sample was quite heterogeneous in terms of poststroke delay, type of aphasia, and age, but it was representative of the population of most of the French rehabilitation centers. We observe that there is a factual limit between the need for homogeneous and specific samples for a specific clinical study, and the varied profiles actually present in our services, all the more so within a defined time range.
CONCLUSION
Our study evaluated the impact of bihemispheric tDCS combined with speech-language therapy on an ecological language production task and did not find any significant improvement in 10 patients with poststroke aphasia. The small number of patients, even if similar to most previous studies, and the limited time of therapy, should be taken into account in the interpretation of our results. Further studies using a SCED methodology may increase statistical power and reduce intra- and intervariability of patients with poststroke aphasia assessment and may help obtaining a better understanding of tDCS effectiveness in clinical practice to facilitate learning or to improve speech function in patients with poststroke aphasia.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719001036
ACKNOWLEDGEMENTS
The authors thank the Foundation Garches, which promoted the present study. They also thank the Foundation Bennetot which funded the study (AP-FPB-14-002). The Foundation Bennetot did not participate in the study design, collection, analysis, interpretation of data, and decision to submit the article for publication. They thank Anna Moraïtis who copyedited the manuscript. The authors thank the Raymond Poincaré Hospital Clinical Investigation Center (CIC1429), particularly Sandra Pottier, who helped with the methodological aspects and the randomization management.
CONFLICTS OF INTEREST
The authors have nothing to disclose.







