Mastitis is the most common milk production disease in modern dairy farming. Despite mastitis control programmes, it is estimated to affect up to 30% of dairy cattle in the EU and cost the EU dairy industry approximately €1·55 billion in 2005 (SABRE, Reference Faraday2006). This economic loss is due to increases in veterinary and treatment costs and a decrease in the quality and quantity of milk produced by infected animals. The ability of any individual animal to overcome mastitis is dependent on treatment and that animal's innate immune response. This response begins with the host recognizing the presence of foreign pathogens and is followed by responses at cellular, tissue and organismal level, leading to the eradication of the pathogen. The differential inflammatory responses elicited during intramammary infection correlate with the outcome of the infection, and variations in cytokine production have been described for different pathogens (Bannerman et al. Reference Bannerman, Paape, Lee, Zhao, Hope and Rainard2004b; Strandberg et al. Reference Strandberg, Gray, Vuocolo, Donaldson, Broadway and Tellam2005; Yang et al. Reference Yang, Zerbe, Petzl, Brunner, Gunther, Draing, von Aulock, Schuberth and Seyfert2008).
Current control methods rely heavily on antibiotics for both therapeutic and prophylactic purposes. This method is not only costly, but is frequently ineffective in chronic subclinical infections, with cure rates for Staphylococcus aureus mastitis cases ranging widely from 4 to 92% (Barkema et al. Reference Barkema, Schukken and Zadoks2006). There are also increasing concerns regarding the overuse of antibiotics in veterinary medicine and the emergence of antimicrobial resistant pathogens (Barkema et al. Reference Barkema, Schukken and Zadoks2006). This has led to an increased interest in the development of alternative treatments for mastitis (Diarra et al. Reference Diarra, Petitclerc, Deschenes, Lessard, Grondin, Talbot and Lacasse2003; Alluwaimi, Reference Alluwaimi2004; Gill et al. Reference Gill, Pacan, Carson, Leslie, Griffiths and Sabour2006a; Kauf et al. Reference Kauf, Vinyard and Bannerman2007). Recently the application of live bacteria as a potential mastitis therapeutic has gained interest. Probiotic bacteria can be used to control several infectious inflammatory and immunologic conditions through antagonism and immunomodulation (Cross, Reference Cross2002). Commensal bacteria, with a broad spectrum of antimicrobial activity, have previously been isolated from healthy bovine udders and suggested as potential anti-mastitis agents (Al-Qumber & Tagg, Reference Al-Qumber and Tagg2006). Jiminez et al. (Reference Jimenez, Fernandez, Maldonado, Martin, Olivares, Xaus and Rodriguez2008) showed that lactobacilli reduce staphylococcal counts in human mastitis milk over a 14-d period, with no clinical signs of mastitis in the treatment group. However, Greene et al. (Reference Greene, Gano, Smith, Hogan and Todhunter1991) investigated the effects of treating bovine subclinical mastitis infections with intramammary infusions of lactobacillus and although an increase in somatic cell counts (SCC) occurred, no increase in intramammary cure rate was observed.
Lactococcus lactis DPC 3147 is a food grade organism that produces the bacteriocin lacticin 3147 (Ryan et al. Reference Ryan, Flynn, Hill, Ross and Meaney1999). This bacteriocin exhibits broad-spectrum antimicrobial inhibition against mastitis-causing pathogens in vitro (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998) and when combined with a bismuth-based teat seal, it provides protection against infection with Streptococcus dysgalactiae and Staph. aureus in dry cows (Ryan et al. Reference Ryan, Flynn, Hill, Ross and Meaney1999; Twomey et al. Reference Twomey, Wheelock, Flynn, Meaney, Hill and Ross2000). Klostermann et al. (Reference Klostermann, Crispie, Flynn, Ross, Hill and Meaney2008) recently demonstrated that a resuspended freeze-dried application of Lc. lactis is as effective as an antibiotic in curing clinical mastitis cases. Crispie et al. (Reference Crispie, Alonso-Gomez, O'Loughlin, Klostermann, Flynn, Arkins, Meaney, Ross and Hill2008) showed that administration of the lactococcal culture into the mammary glands of uninfected animals elicits an immunomodulatory effect, with substantial recruitment of polymorphonucleocytes (PMN) and lymphocytes to the infused quarters. The aim of this study was to investigate this immunomodulatory effect further, by describing the innate immune response, at the transcriptional level, to a deliberate infusion of Lc. lactis DPC 3147 into a healthy mammary gland.
Materials and Methods
Animal selection
For methodology set-up, a preliminary study was performed with a Holstein Friesian in her sixth lactation and a Norwegian Red in her second lactation. The animals were selected based on their low SCC and the healthy appearance of their udders and milk. The follow-up study consisted of four healthy Holstein Friesian cows (Cows H, J, K and L) in their first lactation and were selected using the same selection criteria as above. Quarter milk samples from all cows were collected aseptically for 7 d prior to experimental challenge. The milks were screened for the presence of pathogens by streaking 10 μl onto Aesculin Blood Agar (ABA) plates containing blood agar base No. 2 (Oxoid), supplemented with 7% citrated whole calf blood (v/v) and 0·1% aesculin (v/v) (Sigma, St. Louis MO, USA) and incubating overnight at 37°C. SCC was performed using a Somacount 300® (Bently Instruments Inc., Chaska MN, USA) somatic cell counter. Infusions and milk and blood sampling were performed under licence from the Irish Department of Agriculture and Food, and the cows' health was subsequently monitored by trained farm staff and veterinary personnel.
Preparation of Lc. lactis and intramammary challenge
Lc. lactis DPC 3147, isolated originally from a kefir grain (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998), was grown at 30°C in M17 broth (Difco Laboratories, Detroit MI, USA) supplemented with 0·5% lactose (LM17). Two millilitres of this culture was diluted with 3 ml of sterile Water for Injection B.P.® (Antigen Pharmaceuticals Ltd., Roscrea, Ireland) and this 5-ml suspension (containing 108 cfu Lc. lactis) was used for challenge. Immediately following the morning milking, one quarter from each animal was infused with this suspension into the teat sinus via the streak canal. The infusions were inoculated to a depth of 17 mm using a syringe with a blunted smoothed tip to prevent injury to the teat. Following infusion the culture was massaged upwards into the quarter. A second quarter from each animal, where possible the contralateral quarter, was selected as the control quarter. To minimize animal handling and conform to animal welfare best practices, no infusion was made in the control quarter.
Milk sampling
Following challenge, 10 ml of milk from each quarter was collected aseptically and 100 μl was plated on LM17 agar plates containing 0·5% lactose to determine Lc. lactis counts. One-hundred microlitres was also plated onto ABA plates for total microbiological analysis. Total quarter milk, (or up to a 2-l volume), was then collected from the infused quarter and the control quarter immediately prior to infusion and at 7 h, 24 h, 72 h, 7 d and 14 d post infusion (PI).
Harvesting milk somatic cells for RNA isolation
One millilitre of 0·5 m-EDTA (Sigma-Aldrich, Ireland Ltd., Dublin) was added per litre of milk (Boutinaud et al. Reference Boutinaud, Rulquin, Keisler, Djiane and Jammes2002) and the milk samples were then centrifuged at 1500 g at 4°C for 30 min. The fat layer was removed from each sample using a sterile spatula and the skim milk carefully decanted. The cell pellets were washed twice in phosphate-buffered saline (PBS, Sigma) pH 7·4 with EDTA at a final concentration of 0·5 mm. The washed cell pellets were then resuspended in 1 ml of TriPure isolation reagent (Roche Diagnostics, Bell Lane, East Sussex, UK) and pipetted up and down until fully homogenized.
Blood leucocyte isolation
Blood samples were taken at the same time points as the milk samples. Briefly, 10 ml blood was collected from the tail vein in a sampling tube containing potassium ethylenediaminetetraacetic acid (EDTA K3E 15%, 0·12 ml; BD Vacutainer™ BD Vacutainer Systems, Preanalytical solutions, Belliver industrial Estate, Plymouth, UK) and placed immediately on ice for subsequent RNA extraction. The samples were combined with 40 ml erythrocyte lysis buffer (ELB) from Qiagen (Qiagen House, Crawley, West Sussex, UK) and placed on ice for 15 min. Following centrifugation at 3000 g the supernatant was decanted and the leucocytes were washed in an additional 20 ml ELB. The cells were then resuspended in 1 ml of TriPure reagent (Roche Diagnostics).
RNA extraction and cDNA synthesis
Total RNA of milk cells and blood cells was extracted using TriPure (Roche Diagnostics) according to the manufacturer's instructions. RNA was quantified using optical density readings at 260 nm and the integrity was analysed following electrophoresis through glyoxyl gels (Ambion). One microgram of RNA was DNAse treated and reverse transcribed to cDNA using the QuantiTect® Reverse Transcription Kit (Qiagen, Crawley, West Sussex, UK) according to manufacturers instructions in a final volume of 20 μl.
Quantification by real-time PCR
Primers were designed for real-time PCR across intron/exon boundaries where possible, to minimize amplification of DNA. The primers were designed using data available in the Genbank database, and accession numbers are given with the primer sequences in Table 1. In addition to the immune genes under investigation a housekeeping gene, a ubiquitin conjugating enzyme (E2D2), was also included for analysis.
Table 1 Primers and conditions used for real-time PCR analyses (Ta is the annealing temperature)

Quantitative analysis of the genes of interest was performed in a LightCycler 480 instrument (Roche Diagnostics) using a dilution series of external plasmid DNA standards (Pfaffl, Reference Pfaffl2001). Plasmid standards were created for each gene by cloning a cDNA PCR product into pCR TOPO (Invitrogen, Life Technologies, Carlsbad CA, USA). Cloning was confirmed by sequencing. One microlitre of each dilution was used per 10 μl LightCycler reaction. The LightCycler 480 SYBR Green I Master kit (Roche Diagnostics) was used for quantification according to the manufacturer's instructions using 0·5 μm forward and reverse primer. Each programme began with initial denaturation at 95°C for 10 min, followed by 50 cycles of quantification consisting of 5-s denaturation at 95°C, 10-s annealing and 25-s elongation at 72°C. Annealing temperatures for each gene are given in Table 1. Melting curve analysis was performed on each product by heating from a temperature 5°C above the annealing temperature to 95°C in the continuous fluorescence acquisition mode to ensure specificity of Lightcycler products. For each gene, Lightcycler runs were performed in triplicate.
Statistical analysis
Results for the preliminary study were not included in the statistical analysis to exclude age and breed as a random effect. Gene expression data and SCC data were visually assessed for normality. Expression data and SCC data were then transformed by obtaining the natural log. SCS refers to the transformed variable of the SCC. A hierarchical mixed model (PROC MIXED; SAS Version 9.1, SAS Institute Inc., Cary NC, USA) was used to quantify the effect of treatment on SCS and gene expression. The dependent variable was transformed gene expression or SCS. Fixed effects included in the model were time, treatment, and time by treatment interaction. Where significant (P<0·05) a covariate, which was the gene expression or SCS for the control and infused quarters prior to the start of the experiment, was included as a fixed effect. This accounted for intra-cow variation. Time relative to the start of experiment was included as a repeated effect within udder quarter, and cow was included as a random effect. The most appropriate covariance structure among records was determined using Akaike information criterion. Least squares means were extracted from the analysis and differences between the control and infused quarters were considered significant at P<0·05. For graphical representation (Figs 2, 3 and 4) transformed gene expression data were back-transformed. Fold change was determined as the difference between peak gene expression and pre-infusion levels divided by pre-infusion expression for that gene.
Results
Recovery of viable bacteria from challenged quarters
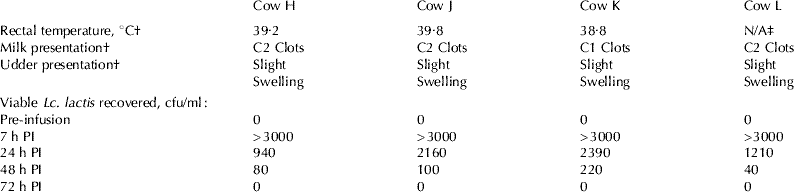
To establish whether Lc. lactis successfully survived following intramammary infusion, milk samples were taken aseptically 7 h, 24 h, 48 h, 72 h and 7 d PI. Viable Lc. lactis were recovered at 7 h and 24 h from all cows (H, J, K and L). The bacterium was recovered 48 h PI from Cows H, J, and L and at 72 h PI from Cow K (Table 2). No other bacteria were recovered from the infused quarters throughout the trial. Control quarters remained clear of bacteria for the duration of the trial.
Table 2 Rectal temperature, physical changes and viable Lactococcus lactis recovered in (a) infused quarters and (b) control quarters following intramammary Lc. lactis DPC 3147 infusion (PI=post infusion)
(a) Infused quarters
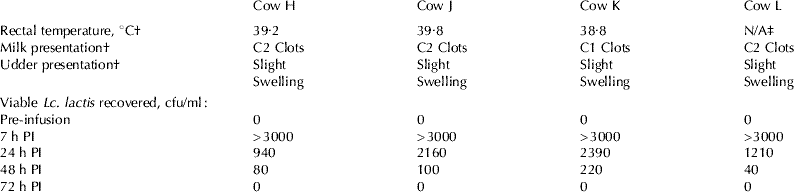
† 7 h PI; ‡ rectal temperature for this animal was not obtained
(b) Control quarters

† =7 h PI
Physical response and milk characteristics
All animals elicited signs of udder inflammation in the infused quarters 7 h PI. These included swollen infused quarters, an elevation in rectal temperature or an elevated SCS i.e. above 12·2 (198 789 cells/ml) SCS (see Table 2 and Fig. 1). At this time, clots were visible in the milk of all four cows. SCS of the animals was recorded as >16·12 (10 000 000 cells/ml) as an accurate estimation could not be made due to the presence of clots. All animals had a self-limiting infection which was completely cleared 7 d PI. Consequently, antibiotic intervention was not required. Statistical analysis of the four Holstein Friesian cows in their first lactation demonstrated that SCS remained at elevated levels until 72 h PI. SCS of the infused quarters were greater than control quarters at 7 (P<0·01), 24 (P<0·001), 48 (P<0·001) and 72 h (P<0·001) PI. At 7 d PI, the average SCS for infused quarters was <12·2, so the quarters were considered clear of infection at this time (Fig. 1) but was still different from the control quarters (P<0·01). Similar results were observed in the preliminary study (data not shown).

Fig. 1. Average somatic cell score (SCS) ±95% confidence intervals in quarters following infusion with Lactococcus lactis DPC 3147 compared with control quarters. *** P<0·001; ** P<0·01.
- - - - - - - - denotes threshold value of 12·2 SCS (198 789 somatic cells/ml), where quarters with values below this were considered healthy and free of infection
Cytokine changes in infused quarters
The panel of immune genes investigated consisted of Toll-like receptor (TLR) 2, TLR4, cluster of differentiation (CD) 14, interleukin (IL)-1β, IL-8, IL-10, IL-12, tumour necrosis factor (TNF)-α, nuclear factor-kappa B (NF-κB) and chemokine receptor CXCR1. Statistical analysis of the four cows in their first lactation demonstrated that all ten immune genes investigated were significantly upregulated 7 h post challenge. The greatest increase was noticed in IL-1β, IL-8 and CXCR1 expression, which underwent a 7000-fold, 4400-fold and 2700-fold average increase within 7 h PI respectively (P<0·001, Fig. 2a, b, c). Expression of all three genes in the infused quarters differed from the control quarters up to 72 h PI (P<0·05); however, there was no significant difference 7 d PI.

Fig. 2. Gene expression profiles in infused quarters compared with control quarters. (a) IL-1β; (b) IL-8; (c) CXCR1. Values are given as the exponential of transformed data ±95% confidence intervals. *** P<0·001; ** P<0·01; * P<0·05.
For TLR2, the highest levels were detected 7 h PI (average 600-fold increase; P<0·001; see Fig. 3a) with a second, albeit lesser peak at 72 h PI (P<0·01). Levels of TLR2 in the infused quarters were still greater (P<0·05) than in the control quarters 7 d PI; however, there was no significant difference between the control and infused quarters 14 d (2 weeks) PI. TLR4 showed a greater fold increase within 7 h of challenge. Expression levels were on average 1000-fold greater than pre-infusion levels (P<0·001; see Fig. 3b). Expression in the infused quarters was not significantly different from the control quarters 7 d PI.

Fig. 3. Gene expression profiles in infused quarters compared with control quarters. (a) TLR2; (b) TLR4; (c) TNF-α. Values are given as the exponential of transformed data ±95% confidence intervals. *** P<0·001; ** P<0·01; * P<0·05.
TNF-α expression was greatest at 7 h PI with, on average, almost a 450-fold increase (P<0·001) within that time (Fig. 3c). Gene expression levels remained elevated until 72 h PI (P<0·01) when compared with control quarters. The highest levels of NF-κB were also observed in all animals 7 h PI (P<0·001). The fold change in all animals did not vary as much as other genes, with on average a 45-fold increase in RNA levels. Transcript abundance in the infused quarters remained greater (P<0·05) than in the control quarters until 7 d PI (Fig. 4a). CD14 was also greater 7 h PI, with an average 500-fold increase (P<0·001) within that time. Elevated levels (P<0·05) were still observed 7 d PI; however, there was no significant difference between the control and infused quarters 14 d PI (Fig. 4b). IL-12 gene expression in the infused quarters was greater than the control quarters at 7, 24 and 48 h PI (P<0·001). Peak in expression levels occurred at 7 h PI (>300-fold increase). The increase of expression of this gene was short-lived, however, and there was no difference between the control and infused quarters by 72 h PI (Fig. 4c).

Fig. 4. Gene expression profiles in infused quarters compared to control quarters. (a) NF-κB; (b) CD14; (c) IL-12; (d) IL-10. Values are given as the exponential of transformed data ±95% confidence intervals. *** P<0·001; ** P<0·01; * P<0·05.
IL-10 expression also peaked at 7 h PI (average 400-fold up-regulation) and infused quarters were greater than control quarters (P<0·001). Elevated levels remained different (P<0·05) from control quarters expression until 7 d PI, with no difference 14 d PI (Fig. 4d).
There was no significant difference in expression levels of the housekeeping gene, E2D2, throughout the challenge (data not shown). Results from the preliminary study showed similar gene expression profiles, with notable increases in IL-1β and IL-8 observed (data not shown).
Cytokine expression in control quarters and blood
Control quarters acted as internal controls for the infused quarters in each animal. Throughout the trial, there were some slight, non-significant increases in gene expression in control quarters at 7 h and 24 h PI, relative to immediately prior to the start of the experiment; however, these increases were much less pronounced than in the infused quarters. An increase in pro-inflammatory cytokine gene expression was also observed 14 d PI; however, this was not significantly different from pre-infusion levels (Figs 2, 3, 4). No significant changes in gene expression were observed in the blood of any of the animals in this study (data not shown).
Discussion
This study was initiated to determine the effect of a deliberate intramammary infusion with a food-grade bacterium, Lc. lactis DPC 3147, in healthy lactating dairy cows. Experimental trials have previously shown that treatment with Lc. lactis live culture is effective for cases of clinical and subclinical mastitis (Klostermann et al. Reference Klostermann, Crispie, Flynn, Ross, Hill and Meaney2008). We describe a massive immune response, with an increase in all pro-inflammatory genes investigated. The most significant difference was observed in expression of IL-1β, IL-8 and CXCR1, where a 7000-fold, 4400-fold and 2700-fold increase, respectively, was observed within 7 h of infusion. The magnitude of the response is particularly noteworthy as Lc. lactis does not colonize within the udder and bacterial counts recovered from milk decrease to zero 72 h PI. All animals experienced an increase in SCC and swollen udder quarters. However, the immune response was short-lived and SCC, as well as expression of most pro-inflammatory genes had returned to pre-infusion levels within 1 week.
As a therapeutic, the immune profile elicited by this Gram-positive bacterium is distinctly different from a pathogen assault. The Gram-positive pathogen Staph. aureus fails to upregulate expression of IL-8 and TNF-α at both gene and protein level (Bannerman et al. Reference Bannerman, Paape, Lee, Zhao, Hope and Rainard2004b; Yang et al. Reference Yang, Zerbe, Petzl, Brunner, Gunther, Draing, von Aulock, Schuberth and Seyfert2008). Str. uberis induces a late TNF-α response and a sustained elevated expression of IL-1β protein (Bannerman et al. Reference Bannerman, Paape, Goff, Kimura, Lippolis and Hope2004a). Str. dysgalactiae infusion caused a subdued immune response with IL-8 gene up-regulation typically peaking at a 10–100-fold increase per 30 000 cfu/ml bacteria recovered (data not shown). Escherichia coli, a Gram-negative pathogen induces a much more acute response with an increase >50-fold and >100-fold increase of IL-8 and TNF-α gene expression, respectively,within 12 h of challenge, and an increase in abundance of these proteins within 16 h (Bannerman et al. Reference Bannerman, Paape, Lee, Zhao, Hope and Rainard2004b; Yang et al. Reference Yang, Zerbe, Petzl, Brunner, Gunther, Draing, von Aulock, Schuberth and Seyfert2008). However, the magnitude and speed of the response is still less than that to Lc. lactis. Up-regulation of cytokines and chemokines is necessary to mount a successful defence against mammary pathogens. Lc. lactis is capable of providing a substantial immune stimulation.
As Lc. lactis is a Gram-positive bacterium and TLR2 binds to lipotechoic acid, the observed increase in TLR2 expression was to be expected. However, the increase in TLR4 and CD14, whose gene products are involved in LPS recognition, was of the same magnitude and, in the case of TLR4 in some animals, greater than the up-regulation of TLR2. Ozinsky et al. (Reference Ozinsky, Underhill, Fontenot, Hajjar, Smith, Wilson, Schroeder and Aderem2000) proposed that TLRs are recruited to all phagosomes of macrophages to sample the contents, identify the bacteria and initiate the most effective response. Indeed, Goldammer et al. (Reference Goldammer, Zerbe, Molenaar, Schuberth, Brunner, Kata and Seyfert2004) also observed an increase in both TLR2 and TLR4 RNA in mastitic tissue of cows infected by the Gram-positive Staph. aureus.
Once a bacterium is recognized through TLR signalling, cells usually secrete TNF-α and IL-1β to induce an acute phase response, activate NF-κB and increase IL-8 protein abundance. Our data set described a massive burst of IL-1β and IL-8 gene expression and a significant up-regulation of TNF-α at the first PI sampling time. The up-regulation of IL-8 gene expression was supported by a concomitant increase in IL-8 protein concentration in a representative milk sample (P Rainard, personal communication), as measured by ELISA (Rainard et al. Reference Rainard, Riollet, Berthon, Cunha, Fromageau, Rossignol and Gilbert2008). In addition the CXCR1 gene, which codes for an IL-8 receptor on neutrophils, is significantly up-regulated. Further circumstantial evidence that IL-8 expression is considerably increased is the observation of a large influx of neutrophils to the site of infusion in a comparable study by Crispie et al. (Reference Crispie, Alonso-Gomez, O'Loughlin, Klostermann, Flynn, Arkins, Meaney, Ross and Hill2008). The massive stimulation of IL-1β by Lc. lactis may be one of the immunomodulatory mechanisms in which the bacterium confers its therapeutic effect. Oviedo-Boyso et al. (Reference Oviedo-Boyso, Cardoso-Correa, Cajero-Juarez, Bravo-Patino, Valdez-Alarcon and Baizabal-Aguirre2008) has shown administration of the pro-inflammatory cytokines, TNF-α and IL-1β, increases the endocytic activity of the bovine endothelial cells (BEC) for Staph. aureus and enhances the ability of BEC to eliminate intracellular Staph. aureus and Staph. epidermidis in vitro. Wedlock et al. (Reference Wedlock, Denis, Lacy-Hulbert and Buddle2008) state that administration of recombinant bovine IL-1β to mammary glands at drying off results in sterile mastitis (i.e. increased SCC) but lowers the incidence of new intramammary infection by Streptococcus uberis.
NF-κB expression was also up-regulated following Lc. lactis challenge, but the fold change was not as noticeable as for other genes. This may be explained by the relative abundance of cytoplasmic NF-κB protein awaiting activation. IL-10 was included in the gene panel to describe an anti-inflammatory response in the mammary gland due to the presence of Lc. lactis. Peak levels of IL-10 were observed 7 h PI with an average 400-fold change from pre-infusion levels. As distinct from the majority of the other genes investigated, IL-10 remained elevated beyond one week PI.
Control quarters exhibited a negligible increase in SCS and expression of a number of pro-inflammatory genes. These increases were most likely due to cross-talk between quarters (Berry & Meaney, Reference Berry and Meaney2006). No response was observed at the systemic level. No infusion was administered to the control quarter. While we cannot rule out the possibility that the process of infusion in this study is the cause of the inflammatory reaction, we believe that it is highly unlikely. Previous and repeated trials by our research team has shown that infusion of sterile water into the control quarter does not cause irritation or inflammation as measured by gene expression, SCC and physical appearance. The immune response to Lc. lactis is also dose-dependent with a lower dose of 103 cfu eliciting no response (Crispie et al. Reference Crispie, Alonso-Gomez, O'Loughlin, Klostermann, Flynn, Arkins, Meaney, Ross and Hill2008) and no change in immune gene expression (K Klostermann, unpublished observations).
Treatment with Lc. lactis compares very favourably with other therapies recently investigated to treat mastitis. Cytokine therapy has been investigated, but only as a prophylactic treatment and use of some cytokines was found to have serious side-effects, especially at certain times of year (Alluwaimi, Reference Alluwaimi2004; Wedlock et al. Reference Wedlock, Denis, Lacy-Hulbert and Buddle2008; Zecconi et al. Reference Zecconi, Piccinini, Fiorina, Cabrini, Daprà and Amadori2008). Vaccination strategies have produced varying results and many require repeated dosing or boosters over a series of months (Middleton et al. Reference Middleton, Luby and Adams2009). LPS treatment was found to give only a transient decrease in bacterial numbers, but not to improve cure rates. Also, repeat dosing might be required, eventually reducing efficacy (Kauf et al. Reference Kauf, Vinyard and Bannerman2007). Lactoferrin has proved effective, but only in combination with antibiotics (Lacasse et al. Reference Lacasse, Lauzon, Diarra and Petitclerc2008). Bacteriophage therapy has been hampered by the discovery that phage activity against Staph. aureus was inhibited in bovine milk (O'Flaherty et al. Reference O'Flaherty, Coffey, Meaney, Fitzgerald and Ross2005; Gill et al. Reference Gill, Sabour, Leslie and Griffiths2006b). However, Lc. lactis DPC 3147 may prove to be a successful non-antibiotic treatment for mastitis because of is ability to (a) produce a bacteriocin with broad spectrum antibacterial activity against Gram-positive pathogens (Ryan et al. Reference Ryan, Meaney, Ross and Hill1998) and (b) elicit a rapid and substantial innate immune response.
The authors gratefully acknowledge Dr Stuart Childs, Dr Frank Buckley and Ballydague farm staff. We also wish to sincerely thank Dr Pascal Rainard, INRA for ELISA data. This work was funded by the Irish Dairy Research Trust, the Teagasc Retooling Programme under the National Development Plan and the Teagasc Walsh Fellowship.