Ichthyosaurs were a successful group of secondarily aquatic tetrapods that evolved a fish-shaped body plan (e.g., McGowan & Motani Reference McGowan and Motani2003; Motani Reference Motani2005). They appeared and rapidly diversified in the Early Triassic (e.g., Motani et al. Reference Motani, Jiang, Chen, Tintori, Rieppel, Ji and Huang2015, Reference Motani, Jiang, Tintori, Ji and Huang2017; Jiang et al. Reference Jiang, Motani, Huang, Tintori, Hu, Rieppel, Fraser, Ji, Kelley, Fu and Zhang2016; Moon & Stubbs Reference Moon and Stubbs2020) and remained important components of marine faunas until their extinction in the early Late Cretaceous (e.g., Fischer et al. Reference Fischer, Bardet, Benson, Arkhangelsky and Friedman2016). Among the key events in the evolutionary history of ichthyosaurians was the Late Triassic appearance of Parvipelvia, a clade diagnosed by a small pelvic girdle, a feature likely related to the evolution of an oscillatory mode of swimming that allowed parvipelvians to colonise the pelagic realm (Motani Reference Motani1999, Reference Motani2002a, Reference Motanib, Reference Motani2005, Reference Motani2008). Parvipelvia was the only ichthyosaurian clade that survived the Triassic/Jurassic extinction and flourished during the Early Jurassic (e.g., Motani Reference Motani2008; Benson & Butler Reference Benson and Butler2011; Thorne et al. Reference Thorne, Ruta and Benton2011; Kelley et al. Reference Kelley, Motani, Jiang, Rieppel and Schmitz2014). However, the divergence and early evolution of the group in the Late Triassic are still incompletely understood.
Early-diverging parvipelvians are represented by the early Norian Hudsonelpidia brevirostris and the middle Norian Macgowania janiceps, both from the Pardonet Formation, British Columbia, Canada (McGowan Reference McGowan1995, Reference McGowan1996, Reference McGowan, Callaway and Nicholls1997; Motani Reference Motani1999; Henderson Reference Henderson, Bininda-Emonds, Powell, Jamniczky, Bauer and Theodor2015). These taxa are known from only a few, incomplete specimens, and several important details of their morphology (e.g., morphology of the cranial sutures, the endocranium and the vertebral column) remain only partially known. In the majority of recent phylogenetic analyses (e.g., Ji et al. Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016; Jiang et al. Reference Jiang, Motani, Huang, Tintori, Hu, Rieppel, Fraser, Ji, Kelley, Fu and Zhang2016; Motani et al. Reference Motani, Jiang, Tintori, Ji and Huang2017; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019; but see Moon Reference Moon2019; Moon & Stubbs Reference Moon and Stubbs2020), Parvipelvia is consistently recovered as the sister-group to Toretocnemidae – a clade of small- to moderately-sized ichthyosaurians from the Middle–Late Triassic (Ladinian–Carnian) represented by two genera – Toretocnemus from Western North America and Qianichthyosaurus from South China (e.g., Merriam Reference Merriam1903, Reference Merriam1908; Motani Reference Motani1999; Li Reference Li1999; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013; Ji et al. Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016). Fragmentary ichthyosaurian remains with putative toretocnemid affinities were also described from the Middle Triassic of Svalbard by Maisch & Blomeier (Reference Maisch and Blomeier2009), but judging by the remarkably high and narrow neural spines, they represent mixosaurids rather than toretocnemids (e.g., compare [Brinkmann Reference Brinkmann1998, Reference Brinkmann2004; Schmitz et al. Reference Schmitz, Sander, Storrs and Rieppel2004] and [Nicholls et al. Reference Nicholls, Wei and Manabe2002; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013]). The North American fossil record of toretocnemids is scarce (Merriam Reference Merriam1903, Reference Merriam1908; Lucas Reference Lucas2002) in contrast to the abundant and relatively well-studied material of Qianichthyosaurus from China (Li Reference Li1999; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013). However, despite the availability of phylogenetically informative data on the dermatocranium and appendicular skeleton, the available data on the endocranium and the vertebral column of Qianichthyosaurus are limited, further hampering the comparison of toretocnemids and parvipelvians. Filling the gaps in our understanding of the morphological differences between these two clades is, therefore, crucial for reconstructing the assembly of the evolutionarily successful parvipelvian body plan.
Triassic ichthyosaurians from Russia are poorly known, despite the country's large territory and wide distribution of Triassic marine deposits in the Arctic and Siberia, including the Far East. This is mostly a consequence of the remote location and difficulties with access to outcrops of Triassic marine sediments. Compared to numerous localities with abundant and relatively complete marine reptile remains from the Late Jurassic and Early Cretaceous of European Russia, the known Russian localities of Triassic marine reptiles yielded only fragmentary and mostly undiagnostic specimens, collected during geological explorations, rather than dedicated palaeontological expeditions (e.g., Storrs et al. Reference Storrs, Arkhangel'skii, Efimov, Benton, Shishkin, Unwin and Kurochkin2000). These Triassic marine reptile records were summarised by Sennikov (Reference Sennikov2001) and later discoveries included a fragmentary, putative shastasaurid ichthyosaurian from the upper Ladinian of Cape Tsvetkov, Taymyr Peninsula (Efimov et al. Reference Efimov, Rogov, Khudolei, Verzhbitsky, Tuchkova and Zdobin2010), sauropterygian remains from the Norian of Franz Josef Land (Sennikov & Arkhangelsky Reference Sennikov and Arkhangelsky2010) and fragmentary ichthyopterygian jaws with durophagous teeth from the middle Anisian of Russky Island, Russian Far East (Arkhangelsky et al. Reference Arkhangelsky, Zverkov, Zakharov and Borisov2016). Here, we describe new ichthyosaurian specimens from the Upper Triassic (lower Carnian–middle Norian) of the New Siberian Islands. The material was collected during geological expeditions of the Geological Institute of the Russian Academy of Sciences (GIN RAS, Moscow) and the Trofimuk Institute of Petroleum Geology and Geophysics, Siberian Branch of the Russian Academy of Sciences (IPGG SB RAS, Novosibirsk) in 2006 and 2009, respectively. The specimens are mostly represented by isolated vertebrae and rib fragments. However, several specimens are of special interest, such as ZIN PH 5/250, a fragmentary skeleton of a small ichthyosaurian that includes cranial remains (parabasisphenoid, fragmentary quadrate, jugal, partial mandible and hyoids) and a series of eight anterior presacral vertebrae with associated neural arches and ribs, and several other bone associations with vertebrae highly similar to those of ZIN PH 5/250 (ZIN PH 3/250; 6/250; 20/250). These specimens have similarities to both toretocnemids and basal parvipelvians, and provide data on skeletal regions only partially known in Late Triassic representatives of these lineages.
Institutional abbreviations. AGM = Anhui Geological Museum, Hefei, Anhui Province, China; GIN RAS = Geological Institute of the Russian Academy of Sciences, Moscow, Russia; IPGG SB RAS = Trofimuk Institute of Petroleum Geology and Geophysics, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia; IVPP = Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; NGM = Nanjing Geological Museum, Nanjing, Jiangsu Province, China; NHMUK = Natural History Museum, London, UK; OUMNH = Oxford University Museum of Natural History, Oxford, UK; PIMUZ = Paleontological Institute and Museum, University of Zürich, Zürich, Switzerland; PMU = Palaeontological Collections, Museum of Evolution, Uppsala University, Uppsala, Sweden; ROM = Royal Ontario Museum, Toronto, Ontario, Canada; TMP = Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada; UCMP = University of California Museum of Paleontology, Berkeley, California, USA; ZIN PH = Zoological Institute of the Russian Academy of Sciences, St Petersburg, Russia.
1. Material and methods
A list of specimens from the New Siberian Islands examined as part of the present study is given in Table 1. The specimens are currently deposited in the Paleoherpetological Collection of the Zoological Institute of the Russian Academy of Sciences, St Petersburg, Russia (ZIN PH).
Table 1 Triassic marine reptile specimens from the New Siberian Islands

The material discussed here was prepared manually by DVG and NGZ, using pneumatic air scribe and dental scalers.
The phylogenetic framework used throughout this study follows Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019), a comprehensive and recently updated phylogenetic hypothesis for Ichthyosauriformes, previously published by Jiang et al. (Reference Jiang, Motani, Huang, Tintori, Hu, Rieppel, Fraser, Ji, Kelley, Fu and Zhang2016) and Ji et al. (Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016).
1.1. Micro-computed tomography (μCT) scanning methods
ZIN PH 5/250 was μCT scanned using Phoenix v|tome|x L 240/300 & L 450 (GE Measurement & Control Solutions) at the Skoltech Center for Hydrocarbon Recovery (Moscow, Russia) with the following parameters: voltage of 200 kV, current of 200 μA. A total of 2400 images (2024 × 2024 pixels in size) were acquired at a resolution of 33.592 μm of an isotropic voxel size. Images were resized using ImageJ (Abramoff et al. Reference Abramoff, Magelhaes and Ram2004). The image stack was processed by DVG. Digital segmentation was completed in Avizo 9.0.1 (FEI Visualization Sciences Group, Burlington, Massachusetts, USA) using a WACOM Bamboo CTL-470 tablet. Final visualisation was performed in Blender 2.83.3.
The three-dimensional models and μCT datasets were uploaded to Figshare.com (https://figshare.com/) and can be accessed at https://figshare.com/projects/Ichthyosaurs_from_the_Upper_Triassic_of_the_New_Siberian_Islands_Russian_Arctic/92831.
2. Geological setting
Triassic deposits crop out at the NW, S and central part of Kotelny Island (the biggest island of the New Siberian Islands archipelago). The largest outcropping area of these deposits is observed in the central part of the island, where they fill the gentle W–NW-trending synclines (Bragin et al. Reference Bragin, Konstantinov and Sobolev2012) (Fig. 1c). Triassic deposits unconformably overlap Upper Palaeozoic rocks and are probably connected by gradual transitions with the overlying Lower Jurassic deposits (Dagys et al. Reference Dagys, Arkhipov and Bychkov1979). They are characterised by a uniform clay and carbonate clay composition, absence of detrital admixtures and a complex of fossils dominated by nektonic, planktonic and pseudoplanktonic fauna. These lithological and palaeontological features, together with the absence of sessile benthos in the pre-Norian strata, indicate the formation of Triassic deposits in an open, relatively warm sea at a considerable distance from the coast (Egorov et al. Reference Egorov, Bogomolov, Konstantinov, Kurushin and Dagys1987).

Figure 1 Spatiotemporal distribution of marine reptiles in the Upper Triassic of the New Siberian Islands. (A) Stratigraphic column with levels of marine reptile occurrences. (B) Position of the study Area in the Arctic. (C) Geologic map of the study area on Kotelny Island, New Siberian Islands. Modified from Bragin et al. (Reference Bragin, Konstantinov and Sobolev2012); (A) updated according to Konstantinov (Reference Konstantinov2018b, Reference Konstantinov2019b). Levels with marine reptile remains: 1 – ZIN PH 16/250–18/250; 2 – ZIN PH 13/250; 3 – ZIN PH 1/250, 10/250–12/250; 4 – ZIN PH 24/250, ?ZIN PH 2/250; 5 – ZIN PH 5/250–9/250, 15/250, 19/250–23/250; 6 – ZIN PH 25/250.
In 2006, Nikita Yu. Bragin and Alexander B. Kuzmichev (GIN RAS, Moscow) studied the Triassic outcrops in the central part of Kotelny Island. Their fieldwork allowed for the refinement of the structure of the Upper Triassic sequence and added to its palaeontological characterisation by providing additional data on radiolarians (Bragin Reference Bragin2011; Bragin et al. Reference Bragin, Konstantinov and Sobolev2012). The remains of marine reptiles were also collected (Table 1; Bragin Reference Bragin2011). It was demonstrated that the fauna of radiolarians and cephalopods of the Carnian and Norian stages of this region included Tethyan and Boreal elements (with the predominance of the latter), which indicates that the region belonged at that time to the specific Novosibirsk subprovince of the Siberian Province of the Boreal Realm (Bragin et al. Reference Bragin, Konstantinov and Sobolev2012). In 2009, two of the authors (AGK and ESS) studied the Upper Triassic sections in the lower reaches of the Tikhaya River, as part of the exploration team of IPGG SB RAS, Novosibirsk. In addition to the collection of invertebrates (cephalopods, bivalves, brachiopods and gastropods), remains of marine reptiles were discovered in several stratigraphic levels (Fig. 1; Table 1).
In Figure 1a we provide the composite stratigraphic column of the Upper Triassic at the lower course of the Tikhaya River, central part of Kotelny Island (Fig. 1b), based on Egorov et al. (Reference Egorov, Bogomolov, Konstantinov, Kurushin and Dagys1987), Konstantinov et al. (Reference Konstantinov, Sobolev and Klets2003) and Bragin et al. (Reference Bragin, Konstantinov and Sobolev2012), with slightly updated ammonoid zonation according to Konstantinov (Reference Konstantinov2018a, Reference Konstantinovb, Reference Konstantinov2019a, Reference Konstantinovb). The stratigraphic distribution of levels with marine reptile remains is indicated by asterisks (Fig. 1a).
3. Descriptions and taxonomic affinities
Reptilia Laurenti, Reference Laurenti1768 Ichthyosauriformes Motani, Jiang, Chen, Tintori, Rieppel, Ji & Huang, Reference Motani, Jiang, Chen, Tintori, Rieppel, Ji and Huang2015 Ichthyopterygia Owen, Reference Owen1860
Ichthyosauria de Blainville, Reference de Blainville1835 Merriamosauria Motani, Reference Motani1999 Shastasauridae Merriam, Reference Merriam1902
Shastasauridae gen. et sp. indet. (Fig. 2)
Referred specimens. ZIN PH 1/250; ZIN PH 10/250; ZIN PH 13/250; ZIN PH 15/250; ZIN PH 19/250; ZIN PH 21/250; ZIN PH 24/250; see Figure 2 and Table 1 for details.

Figure 2 Remains of shastasaurid ichthyosaurs ZIN PH 1/250 (A–H, K–R), ZIN PH 10/250 (I, J), ZIN PH 13/250 (S), ZIN PH 21/250 (T–W), ZIN PH 19/250 (X–Z). (A–D) Posterior presacral centum; (E–J) anterior caudal centum; in articular (A, E), lateral (B, F, I), dorsal (C, H) and ventral (D, G) views and in cross-section (J). (K–P) Neural arches in right lateral (K–M), anterior (N), dorsal (O) and ventral (P) views. (Q–Z) Ribs in anterior/posterior (Q, S, T, W) and proximal (R, U, V, Y) views. Scale bar = 5 cm.
Description. Specimen ZIN PH 1/250 includes two large vertebral centra (Fig. 2a–h), several neural arches (Fig. 2k–p) and rib fragments (Fig. 2q, r). One vertebral centrum (Fig. 2a–d) has characteristic, dorsoventrally elongated rib facets (apophyses) being similar to those in the posterior presacral centra of shastasaurids (e.g., Merriam Reference Merriam1895, Reference Merriam1902, Reference Merriam1908; Camp Reference Camp1980; Nicholls & Manabe Reference Nicholls and Manabe2004). Ventrally, the apophysis is confluent with the anterior articular surface of the centrum, although this connection is dorsoventrally thin, unlike in cymbospondylids, in which it is proportionally broader (e.g., Merriam Reference Merriam1908). The intervertebral articular surfaces are oval in outline, narrowing dorsally, with their height exceeding their width. Dorsally, the centrum possesses parallel facets for the neural arch, which are widest at their mid-length, so that the floor of the neural canal located between them is hourglass-shaped (Fig. 2c).
Another nearly complete centrum of this specimen (Fig. 2e–h) bears much smaller rib facets, which are oval in outline, indicating its position in the caudal region of the vertebral column (Merriam Reference Merriam1908; Camp Reference Camp1980; Nicholls & Manabe Reference Nicholls and Manabe2004). Identical morphology is also observed in a fragment of another centrum, ZIN PH 10/250 (collected by ESS from the same horizon), that probably belonged to a different individual of comparable size (Fig. 2i–j). The vertebrae of ZIN PH 1/250 measure around 50 mm in anteroposterior length and 115–118 mm in dorsoventral height; therefore, they belonged to large ichthyosaurians, exceeding the largest specimens of Shastasaurus in size (e.g., Merriam Reference Merriam1902; note that ‘Shastasaurus careyi’ is considered as Shonisaurus sp. by Motani Reference Motani1999), but were not as large as Shonisaurus (e.g., Camp Reference Camp1980; Nicholls & Manabe Reference Nicholls and Manabe2004). These centra are also much larger than those of any other early-diverging Late Triassic euichthyosaurians (Callawayia neoscapularis, Californosaurus perrini), toretocnemids and basal parvipelvians, in all of which vertebral centra hardly exceed 50 mm in height and 20 mm in length in the largest-known specimens (e.g., Merriam Reference Merriam1902, Reference Merriam1903, Reference Merriam1908; McGowan Reference McGowan1994, Reference McGowan1995, Reference McGowan1996; Nicholls & Manabe Reference Nicholls and Manabe2001). Given the characteristic morphology and large size, the referral of ZIN PH 1/250 and 10/250 to shastasaurids seems most likely.
The preserved neural arches of ZIN PH 1/250 are massive, with thickened pedicles and neural spines (Fig. 2l, m). Considering the small and narrow neural canal and reduced zygapophyses, all of the neural arches likely originated from the caudal region (e.g., Merriam Reference Merriam1908; Camp Reference Camp1980). The spines are oval to lenticular in cross-section, dorsally convex in lateral view and bear a median groove in dorsal view. The anterior margins of the spines form sharpened crests similar to those of Shastasaurus (Merriam Reference Merriam1908). The zygapophyses are reduced (Fig. 2k–n).
Isolated fragments of large and massive ribs, representing different individuals, were also collected from several localities and different stratigraphic levels (ZIN PH 13/250; ZIN PH 15/250; ZIN PH 19/250; ZIN PH 21/250; ZIN PH 24/250; see Table 1 and Fig. 1). The dorsoventral width of the largest rib head, ZIN PH 21/250, is 70 mm (Fig. 2v, w). Based on their large size, and because their preserved proximal ends are all unicipital and, in some cases, demonstrate characteristic, dorsoventral expansion (Fig. 2v), we provisionally refer these ribs to Shastasauridae indet. (Merriam Reference Merriam1902, Reference Merriam1908; Camp Reference Camp1980; Nicholls & Manabe Reference Nicholls and Manabe2004).
Ichthyosauria gen. et sp. indet. (Fig. 3)
Referred specimens. ZIN PH 2/250; ZIN PH 7/250; ZIN PH 11/250; ZIN PH 12/250 (Fig. 3).

Figure 3 Remains of indeterminate ichthyosaurians (ZIN PH 11–12/250; ZIN PH 7/250, ZIN PH 2/250). (A–F) Anterior presacral centrum ZIN PH 11/250; (G–J) caudal centrum ZIN PH 12/250; (K–O) anterior presacral centrum ZIN PH 7/250. (P–V) ZIN PH 2/250, ribs with associated phalanges (P–R) and isolated phalanges (S–W). Vertebrae are depicted in dorsal (A, B, G, K, L), articular (C, H, M), lateral (D, E, I, N, O) and ventral (F, J) views. (B), (E), (L) and (O) are interpretive line drawings. Scale bar = 3 cm.
Description. Three vertebral centra represent medium- to large-bodied ichthyosaurians (but overall markedly smaller than the shastasaurid specimens described above) and display a primitive ichthyopterygian morphology found in cymbospondylids, shastasaurids and some basal euichthyosaurians (i.e., dorsoventrally high single apophyses confluent with the anterior surface of the centrum; e.g., Merriam Reference Merriam1908; Sander Reference Sander1989, Reference Sander1992; Engelschiøn et al. Reference Engelschiøn, Delsett, Roberts and Hurum2018).
Two centra – ZIN PH 11/250 and 12/250 (Table 1; Fig. 3a–j) – were collected in association and probably belonged to one individual. They are characterised by a specific texture of concentric wrinkles on their articular surfaces (Fig. 3c, h). ZIN PH 11/250, identified as an anterior presacral centrum, has an oval articular surface, the mediolateral width of which exceeds its dorsoventral height. The centrum is moderately long with a length/width ratio = 0.5 and a length/height ratio = 0.63, that are in the range reported for the presacral centra of Californosaurus perrini (Merriam Reference Merriam1902). The rib facet is situated in the dorsal half of the lateral surface of the centrum; it is obliquely oriented, being confluent anteriorly with the edge of the centrum and dorsally with the facet for the neural arch (Fig. 3e). A similar configuration of the rib facet in the anterior presacral centra is known in Cymbospondylus (e.g., Merriam Reference Merriam1908; Sander Reference Sander1989; Engelschiøn et al. Reference Engelschiøn, Delsett, Roberts and Hurum2018), Callawayia neoscapularis (Nicholls & Manabe Reference Nicholls and Manabe2001) and Californosaurus perrini (Merriam Reference Merriam1902, Reference Merriam1908; ASW, pers. obs. of UCMP 9082, November 2015). The floor of the neural canal is wide and nearly rectangular in outline with a slight mediolateral constriction at its midlength (Fig. 3b). The second centrum, ZIN PH 12/250, likely originated from the caudal region, which is indicated by the presence of a single, poorly pronounced rib facet and a relatively narrow floor of the neural canal. This centrum has articular surfaces of perfectly circular outline in anterior/posterior view (Fig. 3h), which is similar to the condition in the mid- to posterior caudal vertebrae of Callawayia neoscapularis (Nicholls & Manabe Reference Nicholls and Manabe2001:figs 4, 8). The centrum is proportionally short with a length/height ratio = 0.34. The floor of the neural canal is similar to that of ZIN PH 11/250 in its rectangular outline and the possession of a minor mediolateral constriction (Fig. 3g).
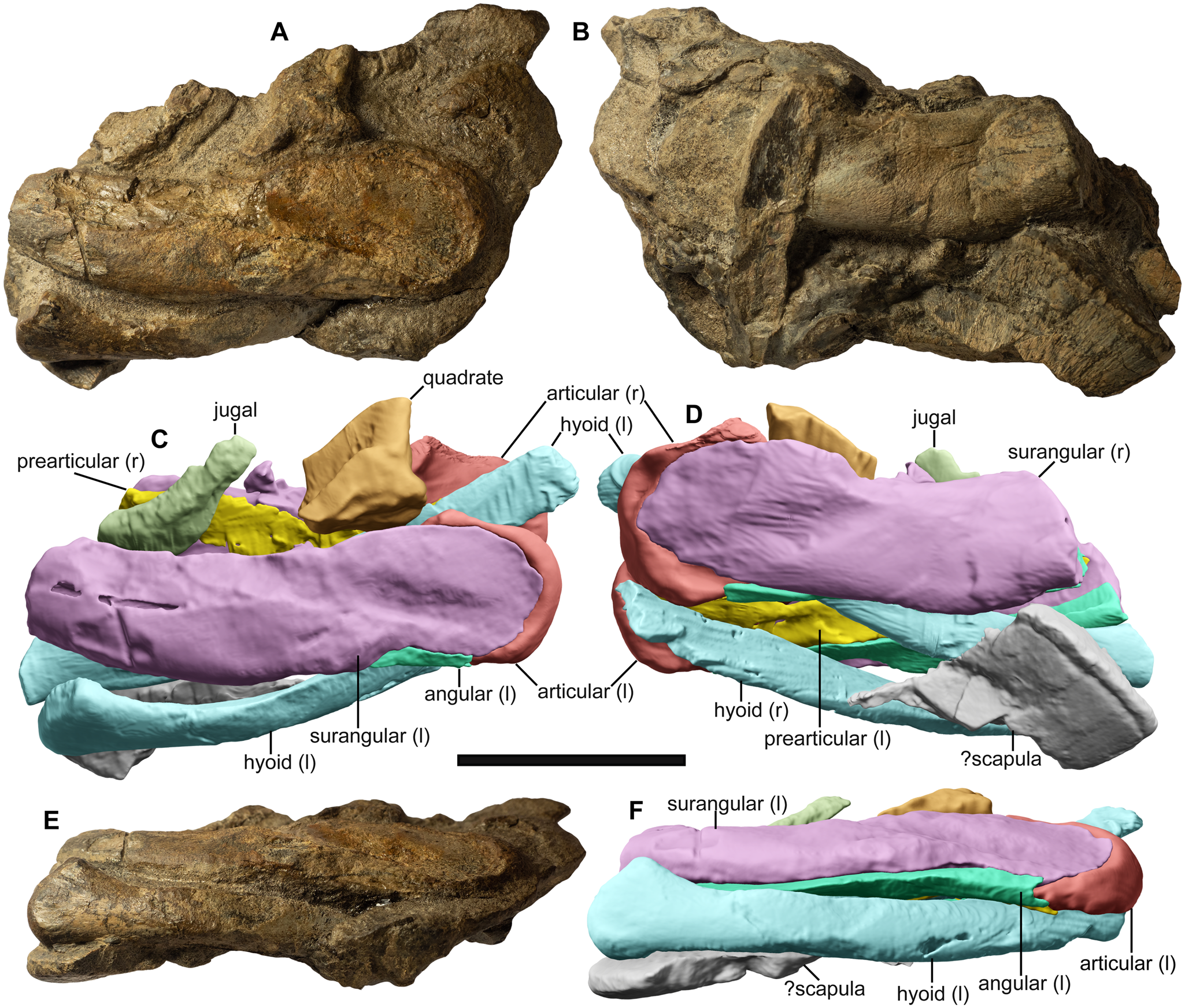
Figure 4 Associated cranial remains of the holotype (ZIN PH 5/250) of Auroroborealia incognita gen. et sp. nov., in left lateral (A, C), right lateral (B, D) and ventral (E, F) views. Scale bar = 3 cm.
One severely weathered anterior presacral centrum (ZIN PH 7/250; Fig. 3k–o) is similar to ZIN PH 11/250 in having a dorsoventrally elongated rib facet, anteriorly connected to the articular surface of the centrum. However, in this specimen, unlike in ZIN PH 11/250, the articular surfaces are smooth. Furthermore, its rib facet tapers ventrally being similar in this respect to that of Californosaurus perrini (Merriam Reference Merriam1902, Reference Merriam1908). The length/height ratio of this centrum is 0.45, which is lower than that of presacral centra of Californosaurus perrini (0.55–0.75; Merriam Reference Merriam1902, Reference Merriam1908) but approaches the ratio values reported for Callawayia neoscapularis (0.48–0.53; Nicholls & Manabe Reference Nicholls and Manabe2001).
Specimen ZIN PH 2/250 (Fig. 3p–w) contains an isolated large autopodial element (?phalanx, ca. 45 mm in diameter) and multiple rib fragments of a moderately large ichthyosaurian with associated small discoidal elements that are interpreted as distal phalanges. The preservation is too poor for a detailed description of this specimen and its attribution to any of the less inclusive ichthyosaurian taxa. The large, isolated phalanx is rounded in outline with flat dorsal and ventral surfaces and an irregular, rugose peripheral margin indicating the presence of extensive cartilaginous coverage in vivo (Fig. 3v, w). The presumable small distal phalanges are all rounded in outline and have concave surfaces (Fig. 3p–u).
Merriamosauria Motani, Reference Motani1999
Euichthyosauria Motani, Reference Motani1999
Auroroborealia incognita gen. et sp. nov. (Figs 4–8)
ZooBank registration. LSID urn:lsid:zoobank.org:act:5EFAB647-9971-4361-925D-A64B665521E9.
Figure 5 Quadrate and parabasisphenoid of the holotype (ZIN PH 5/250) of Auroroborealia incognita gen. et sp. nov. (A–H) Partial left quadrate in lateral (A), posterior (B), anterolateral (C), posterolateral (D), anterior (E), dorsal (F, G) and ventral (H) views. (I–P) Parabasisphenoid in ventral (I, M), dorsal (J), anterior (K, O), right lateral (L, N) and posterior (P) views. Left basipterygoid process (shown semitransparent) on (M, O, P) is a reconstruction based on its mirrored left counterpart. Scale bar = 3 cm.

Figure 6 Mandible of the holotype (ZIN PH 5/250) of Auroroborealia incognita gen. et sp. nov. (A–D) Articulated left mandibular ramus in lateral (A), ventral (B), mediolateral (C) and medial (D) views. (E–I) Left surangular in oblique ventral view showing the passage of canals (E), in lateral (F), medial (G), ventral (H) and dorsal (I) views. (J–L) Right surangular in lateral (J), anterior (K) and medial (L) views. (M–R) Left articular in medial (M), dorsal (N), lateral (O), ventral (P), anterior (Q) and posterior (R) views. (S–W) Left angular in lateral (S), ventral (T), medial (U), anterior (V) and posterodorsal (W) views. (X–Z) Left prearticular in dorsal (X), lateral (Y) and ventral (Z) views. Abbreviations: ang.f = facet for the angular; art.f = facet for the articular; mame = preglenoid process for the attachment of the Musculus adductor mandibulae externus; pcp = paracoronoid process; sur.f = facet for the surangular. Scale bar = 3 cm.

Figure 7 Postcranial remains of the holotype (ZIN PH 5/250) of Auroroborealia incognita gen. et sp. nov. Articulated vertebrae and ribs in left lateral (A), posterior articular (B), right lateral (C, D) and dorsal (E) views. Scale bar = 3 cm.

Figure 8 Vertebrae of euichthyosaurians referred to Auroroborealia incognita gen. et sp. nov.: ZIN PH 3/250 (A–C; O–S), ZIN PH 20/250 (D–N; T–V), ZIN PH 6/250 (W–Y). Vertebrae are depicted in dorsal (A, F, I, M, R), articular (B, E, H, K, P, T, X), lateral (C, D, G, L, O, Q, U, W) and ventral (J, N, S, V, Y) views. Scale bar = 3 cm.
Etymology. The generic name refers to the aurora borealis polar lights that are typical for high-latitude regions, including the type locality of the taxon; the specific name emphasises the incomplete nature of the holotype that hinders its confident placement in a phylogenetic context.
Holotype. ZIN PH 5/250, fragmentary skeleton (see Table 1 and Figs 4–7).
Referred specimens. ZIN PH 3/250; ZIN PH 6/250; ZIN PH 8/250; ZIN PH 20/250; ZIN PH 23/250 (see Fig. 8 and Table 1 for details).
Diagnosis. Auroroborealia incognita is a small euichthyosaurian characterised by the following unique combination of characters: an extensive postglenoid portion of the surangular (reduced in Qianichthyosaurus; incomplete, but appears proportionally short, in Toretocnemus zitteli); angular largely obscured from lateral view (poorly visible in toretocnemids, well exposed in Macgowania); splenial not reaching the level of the paracoronoid process posteriorly (similar to Qianichthyosaurus zhoui, but unlike in T. zitteli); parasphenoid tapering posteriorly and terminating at the middle of the basisphenoid, anterior to the foramen for the internal carotid arteries (similar to many neoichthyosaurians, but different from other Triassic ichthyosaurians, in which the posterior extension of the parasphenoid reaches the posterior edge of the basisphenoid); a single foramen for the internal carotid arteries, similar to mixosaurids, but unlike the condition in many other Triassic ichthyosaurians and basal parvipelvians (Macgowania, Temnodontosaurus), which have paired foramina for the internal carotid arteries; an ossified region of the sella turcica (crista trabecularis and ossification from the pila metoptica) as in mixosaurids and probably all non-parvipelvian ichthyosaurians, but unlike in neoichthyosaurians; hyoids expanded anteriorly and narrow posteriorly (as in Hudsonelpidia but unlike in Qianichthyosaurus); at least four anteriormost presacral vertebral centra bearing the parapophysis only, whereas the diapophysis is located entirely on the neural arch, a condition also found in the mixosaurid Phalarodon callawayi, but not in any other mixosaurid in particular, and ichthyosaurian in general.
Occurrence. Lower Norian, Pinacoceras verchojanicum Ammonoid Biozone of the Kotelny Island, New Siberian Islands, Arctic Russia.
Remarks. The diagnosis is based solely on the holotype. Additional information from referred specimens is not considered.
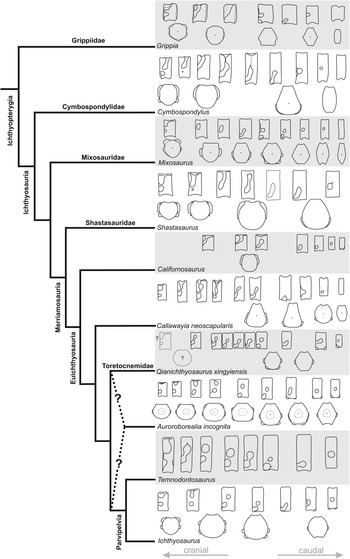
Description. The holotype of A. incognita (ZIN PH 5/250; Figs 4–7) is the most remarkable ichthyosaurian find from the New Siberian Islands. It is a partial skeleton consisting of a fragmentary jugal and quadrate, posterior portions of the mandible, hyoids, a parabasisphenoid and a series of eight anterior presacral centra associated with fragmentary neural arches and ribs, and a bone fragment interpreted as an element of the pectoral girdle.
Skull
Jugal (Fig. 4a, c). The jugal is preserved as a small fragment that shows few morphological details. Its position relative to the remaining skull elements implies the presence of a ventral notch between the cheek elements (jugal, postorbital and quadratojugal), resembling the poorly pronounced notch present in toretocnemids (Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; ASW, pers. obs. of the holotype of Toretocnemus zitteli UCMP 8099, November 2015), but differs from the proportionally larger notch in Macgowania janiceps (McGowan Reference McGowan1996; Henderson Reference Henderson, Bininda-Emonds, Powell, Jamniczky, Bauer and Theodor2015). In its slenderness and gentle curvature, the jugal of ZIN PH 5/250 resembles that of M. janiceps (McGowan Reference McGowan1996), but differs from the jugal in Qianichthyosaurus spp. and T. zitteli, in which the curvature of the jugal appears more pronounced, with the postorbital and suborbital rami forming an angle of up to about 90° (Merriam Reference Merriam1903; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013).
Quadrate (Figs 4a, c and 5a–h). The condylar portion of the left quadrate is preserved in articulation with the mandible. Despite a breakage of its lateral edge (part of the surangular boss), the condyle has a subcircular outline (Fig. 5h). The separation of the bosses for articulation with the articular and surangular is poorly pronounced. The condyle is anteromedially expanded and is separated from the rest of the quadrate by a periosteal ossification (Fig. 5c, d, e). Judging from the preserved portion, the posterior embayment of the quadrate was shallow. The natural cross-section of the quadrate is somewhat triangular in outline, forming a sharp crest anteromedially (Fig. 5f). The quadrate of ZIN PH 5/250 is difficult to compare with that of toretocnemids and Triassic parvipelvians, as in all described specimens this element is either missing or is largely covered by other elements and/or embedded in matrix (Merriam Reference Merriam1903; McGowan Reference McGowan1995, Reference McGowan1996; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013).
Parabasisphenoid (Fig. 5i–p). The parasphenoid and basisphenoid are fused producing no marked suture. It appears that the parasphenoid tapers posteriorly and terminates anterior to the foramen for the internal carotid arteries, thus differing from the plesiomorphic ichthyosauriform condition with an extensive, sheet-like posterior parasphenoid (e.g., Maisch & Matzke Reference Maisch and Matzke2000; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019; see Discussion, Section 4.2.1). The derived condition with a distinctly mediolaterally narrowed posterior parasphenoid is considered a feature of Neoichthyosauria, and possibly Parvipelvia (Moon Reference Moon2019).
The preserved portion of the cultriform process of the parasphenoid is dorsoventrally tall and bears a deep groove on its dorsal surface (Fig. 5j, k). The anterior part of the ventral surface of the parabasisphenoid is gently convex; it bears grooves that originate at the level of the foramen for the internal carotid arteries and diverge anterolaterally. These likely represent the grooves for the sphenopalatine arteries (Fig. 5i).
Only the right basipterygoid process is preserved. It is separated from the main body of the parabasisphenoid by a short, robust and slightly anteroposteriorly constricted shaft of smooth bone. Laterally, the basipterygoid process forms an extensive convex surface for articulation with the pterygoid, which is anteroposteriorly wider than dorsoventrally tall and oval in outline (Fig. 5l, n). Posteromedial to the basipterygoid process, the ventral surface of the parabasisphenoid bears a narrow groove that is commonly interpreted as the passage of the facial nerve (CN VII) (e.g., Kear Reference Kear2005; Moon & Kirton Reference Moon and Kirton2016). In the marked separation of the basipterygoid processes from the body of the parabasisphenoid and in the lateral orientation of their pterygoid facets, Auroroborealia incognita is similar, among Triassic ichthyopterygians, only to Callawayia neoscapularis and Phantomosaurus neubigi (McGowan Reference McGowan1994; Maisch & Matzke Reference Maisch and Matzke2006), whereas the majority of Triassic ichthyosaurians have anterolaterally facing basipterygoid processes that are not clearly demarcated from the body of the parabasisphenoid (e.g., Wiman Reference Wiman1912; Maisch & Matzke Reference Maisch and Matzke1997a, Reference Maisch and Matzkeb, Reference Maisch and Matzke2000; see also Section 4.2.3). The pronounced lateral protrusions of the basipterygoid processes and their separation from the body of the parabasisphenoid is more characteristic for neoichthyosaurians and especially for derived ophthalmosaurians (e.g., Maisch & Matzke Reference Maisch and Matzke2000; Moon Reference Moon2019; Zverkov & Efimov Reference Zverkov and Efimov2019).
The main body of the parabasisphenoid is anteroposteriorly shortened relative to the extensive basipterygoid processes. Anteriorly, it is bounded by a high vertical wall of the dorsum sellae (Fig. 5j, k); posteriorly, it forms a quadrangular facet with an irregular surface for articulation with the basioccipital. This posterior surface is slightly convex posterodorsally (Fig. 5l, n).
The ventral surface of the parabasisphenoid is perforated by a large circular foramen for the internal carotid arteries approximately in its center (Fig. 5i, m). The canal for the internal carotid arteries is circular in cross-section throughout its length within the body of the parabasisphenoid with no trace of separation. It opens anterodorsally, ventral to the dorsum sellae, at the posterior part of a circular depression, the sella turcica (also commonly termed as pituitary fossa; Fig. 5j). In having an unpaired ventral foramen for the internal carotid arteries, A. incognita is similar to mixosaurids (Wiman Reference Wiman1912; von Huene Reference von Huene1916; Maisch & Matzke Reference Maisch and Matzke1997a; Brinkmann Reference Brinkmann2004) and the majority of neoichthyosaurians (e.g., Maisch & Matzke Reference Maisch and Matzke2000; Moon Reference Moon2019), whereas the majority of non-parvipelvian ichthyosaurians with known parabasisphenoids, as well as the basal parvipelvians Macgowania janiceps and Temnodontosaurus spp., have paired ventral foramina for the internal carotid arteries (Maisch & Matzke Reference Maisch and Matzke1997b, Reference Maisch and Matzke2000, Reference Maisch and Matzke2006; see also Section 4.2.2).
The sella turcica is bounded laterally by paired ridges – the cristae trabeculares. These ridges have irregular edges, better pronounced on the right side where a marked lateral protuberance is present in the middle of the crista trabecularis (Fig. 5j, k, l, n). Anteriorly, the sella turcica is bounded by a raised process, an ossification from the pila metoptica (Fig. 5j). This process is triangular in lateral view and is recurved posterodorsally (Fig. 5l, n). Its posterior surface bordering the sella turcica is smooth and shallowly concave, whereas its anterior surface is deeply concave, separating the structure into two lobes. Anteriorly, this separation is continued as a groove on the dorsal surface of the cultriform process (Fig. 5j, k). The condition with an ossification from the trabeculae is absent in Neoichthyosauria, in which the trabecular cartilage is present in all ontogenetic stages so that only its posteriormost insertion areas are pronounced as two slightly protruding impressions ventral to the anterior foramen for the internal carotid arteries (e.g., Appleby Reference Appleby1961; McGowan Reference McGowan1973; Marek et al. Reference Marek, Moon, Williams and Benton2015; Moon & Kirton Reference Moon and Kirton2016). The ossification from the anterior trabeculae (and pila metoptica) was hitherto reported only for Mixosaurus (Appleby Reference Appleby1961; Maisch & Matzke Reference Maisch and Matzke1997a), thus suggesting that this condition is probably plesiomorphic for Ichthyosauria and was lost only in Neoichthyosauria, or possibly Parvipelvia.
Mandible
The posterior portions of both mandibular rami are preserved with all elements, which include the surangulars, angulars, articulars and prearticulars, in natural articulation. We have not identified the splenials, although their posterior portions were expected to be present in articulation with the other preserved mandibular elements. As a result, we suggest that the splenial terminated anterior to the preserved portion of the mandible. There is no evidence for the presence of the coronoid.
Surangular (Figs 4, 6a–l). The surangular forms most of the posterior mandible in lateral view (Fig. 6a). Throughout most of its length, the surangular is mediolaterally thick, with an oval cross-section and a shallow groove on its medial surface that forms the lateral wall of the Meckelian canal (Fig. 6c, k). The posteriormost portion of the surangular is mediolaterally compressed to a greater degree than the rest of the element and has a lenticular cross-section. It is dorsoventrally expanded and has a rounded posterior margin. The posterior portion of the surangular is raised above the remaining part of the element giving it a marked posteroventral curvature in lateral view (Fig. 6a, f, j).
The paracoronoid process is poorly pronounced and weakly raised above the mostly concave dorsal margin (Fig. 6a, f, g, j, l), similarly to toretocnemids (Merriam Reference Merriam1903; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008). A process that is commonly interpreted as a point for attachment of the Musculus adductor mandibulae externus (e.g., McGowan Reference McGowan1973; Moon & Kirton Reference Moon and Kirton2016) or termed ‘processus praeglenoidalis’ (Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008) is well developed, similarly to that of Qianichthyosaurus (Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008). In Early Jurassic parvipelvians, in which the anatomy of the glenoid region is preserved/exposed, this preglenoid process is less prominent, whereas the paracoronoid process is commonly much more pronounced than that of ZIN PH 5/250 (e.g., McGowan Reference McGowan1973; Maisch & Ansorge Reference Maisch and Ansorge2004; Marek et al. Reference Marek, Moon, Williams and Benton2015). The preglenoid processes are well-pronounced in both surangulars, are oriented approximately perpendicular to the element, point medially and are broken due to mediolateral diagenetic compression. In articulation, the preglenoid process would have bordered the articular condyle of the quadrate anteriorly.
Anteromedial to the preglenoid process, the surangular bears two foramina, which give rise to posterolaterally directed canals that open ventrally into a longitudinal canal passing through the middle of the element (Fig. 6e). This canal continues anteriorly within the bone, gradually approaching its lateral surface. It seems plausible that in the anterior part of the surangular (which is not preserved), this canal opened externally and continued as the fossa surangularis, like in other ichthyosaurians (e.g., McGowan Reference McGowan1973; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; Marek et al. Reference Marek, Moon, Williams and Benton2015). The fossa surangularis that emerges only in the anterior half of the surangular was also reported for Qianichthyosaurus (Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008). In neoichthyosaurians, this fossa emerges externally close to the paracoronoid process (e.g., McGowan Reference McGowan1973; Kear Reference Kear2005; Marek et al. Reference Marek, Moon, Williams and Benton2015), so the canal obliquely pierces the surangular but does not continue within its body as in toretocnemids and more basal ichthyosaurians (e.g., Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019).
Angular (Fig. 6a–d, s–w). In lateral view, the angular is almost entirely obscured by the surangular, being slightly visible only in its posteriormost part (Fig. 6a), similarly to toretocnemids (Merriam Reference Merriam1903; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008) but in contrast to the basal parvipelvian Macgowania, in which the angular is extensively exposed laterally (McGowan Reference McGowan1996). The angular of ZIN PH 5/250 forms most of the ventromedial part of the mandible. The preserved portion can be divided into two parts that are rotated one relative to another: a posterior, horizontally oriented trough-like portion, and an anterior vertically oriented sheet that articulates with the surangular medially. The posterior portion forms a trough that supports the anterior part of the articular and most of the prearticular (Fig. 6b, d). The anterior portion also bears a groove on its dorsal side, which forms the floor of the Meckelian canal. The lateral surface of the anterior portion is concave and articulates with the surangular (Fig. 6c, s).
Articular (Fig. 6a–d, m–r). The articular is a laterally compressed element that forms the posterior portion of the mandible. Its dorsoventral height nearly equals its anteroposterior length. It is very similar to the articular of Ichthyosaurus described by McGowan (Reference McGowan1973). It has a saddle-shaped surface medially, and a marked concavity laterally, for articulation with the surangular (Fig. 6o). Posteriorly, the articular is rounded and thickens peripherally (Fig. 6m–o, r). The anterior end, which articulated with the quadrate, possesses a semicircular, weakly convex surface (Fig. 6q). The ventral edge articulating with the angular is straight and sharpened (Fig. 6p), whereas the dorsal margin is slightly concave and proportionally thicker (Fig. 6n).
Preartiсular (Fig. 6c, d, x–z). The prearticular is a sheet-like element covering the articular medially and forming the posterior part of the medial wall of the Meckelian canal. The prearticular tapers anteriorly and terminates in a lanceolate process. This process is oval in cross-section and ends in a hollow funnel-like structure (Fig. 6c, d). Posteriorly, the dorsoventral height of the prearticular increases and the element forms a process that extensively covers the articular medially. This condition differs from the majority of ichthyosaurians, in which the prearticular decreases in dorsoventral height posteriorly (e.g., McGowan Reference McGowan1973; Camp Reference Camp1980; Moon & Kirton Reference Moon and Kirton2016).
Hyoid apparatus
Two hyoids (ceratobranchial I; Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013) are preserved in life position between the mandibular rami. The hyoids are gently curved ventrally and oval in cross-section, with their dorsoventral height exceeding their mediolateral width. In lateral view, the height of the hyoid bar is constant, increasing only in its anteriormost portion (Fig. 4c, f). The hyoids of Auroroborealia incognita differ from those of Qianichthyosaurus zhoui in having a marked expansion of the anterior end and lacking a posterior expansion, whereas in Q. zhoui the opposite condition is observed with the posterior ends more expanded than the anterior ends (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013: fig. 4a). It is possible that a condition similar to that in A. incognita is also present in the holotype of Hudsonelpidia brevirostris, in which two rod-like elements with expanded anterior and narrow posterior ends lie parallel and ventral to the posterior mandibular ramus (McGowan Reference McGowan1995, fig. 3; ASW, pers. obs. of the holotype ROM 41993, April 2015).
Postcranial skeleton
Vertebral column (Fig. 7). The preserved anterior presacral vertebral centra of ZIN PH 5/250 have a length/height ratio of ca. 0.5. This ratio is lower than that in presacral centra of Californosaurus perrini (0.55–0.75; Merriam Reference Merriam1902, Reference Merriam1908) and is within the range of the ratios reported for Callawayia neoscapularis (0.48–0.53; Nicholls & Manabe Reference Nicholls and Manabe2001). The articular surfaces of the centra in ZIN PH 5/250 are oval in outline with their mediolateral width slightly exceeding their height (Fig. 7b). The parapophyses and diapophyses are separated. In the four anteriormost preserved vertebrae, only the parapophysis is present on the lateral surface of the centrum, whereas the diapophysis is located entirely on the pedicle of the neural arch. A similar condition was reported for Phalarodon callawayi (Schmitz et al. Reference Schmitz, Sander, Storrs and Rieppel2004), but not for any other mixosaurid in particular, or ichthyosaurian in general (although some Early Triassic ichthyosauriforms – namely, Utatsusaurus, Chaohusaurus and Cartorhynchus – have a single rib facet in their cervical vertebrae; see e.g., character scores in Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019). However, the pattern of rib articulation in the anteriormost presacral vertebrae is currently unknown for the basal parvipelvian H. brevirostris and toretocnemids. Therefore, it is possible that the condition reported for ZIN PH 5/250 may turn out to have been more widespread among euichthyosaurians in the future. The minor contribution of the centrum to the formation of the diapophysis occurs on the fifth preserved centrum of ZIN PH 5/250; this contribution becomes greater in the subsequent centra. The parapophyses are connected to the anterior surface of the centra. The floor of the neural canal is narrowest at its anteroposterior mid-length and has an hourglass-shaped outline (Fig. 7d).
All the neural spines are broken and only the pedicels of the neural arches are preserved. The bases of the pedicels are anteroposteriorly and mediolaterally expanded with lateral protuberances representing the diapophyseal contributions of the neural arches. The prezygapophyses are fused along the dorsal midline to form a single rounded articular surface (Fig. 7e).
The preserved ribs are bicipital (Fig. 7a). They have a T-shaped cross-section in their proximal part and become 8-shaped in cross-section distally.
In addition to the holotype of Auroroborealia incognita (ZIN PH 5/250), several specimens comprising vertebral associations and isolated centra, that agree well in their size and morphology with the holotype, and were collected from the same stratigraphic horizon, most likely belong to the same taxon. These are ZIN PH 3/250, 6/250, 8/250, 20/250 and 23/250 (Fig. 8). Integrating anatomical information from the holotype and the five referred specimens of A. incognita allows for the reconstruction of a nearly complete vertebral column of this taxon.
The articular surfaces are oval to subhexagonal in outline (with their width exceeding the height) in all available anterior presacral centra referred to A. incognita (Fig. 8b, e, h). Along the length of the vertebral column, the diapophysis becomes gradually separated from the neural arch facet and the parapophysis becomes separated from the anterior articular surface (Fig. 8c, d, g). In the posterior presacral centra, the outline of the articular surface tapers dorsally and its height equals its width (Fig. 8k). The diapophysis and parapophysis are located ventral to the centrum mid-height (Fig. 8l, o). The parapophysis is confluent with the articular surface of the centrum; it is located at the transition of the ventral and lateral surfaces of the centrum. In ventral view, the ventral surface of the centrum bears a flattened surface that is trapezoidal in outline, being narrowest anteriorly, between the parapophyses, and widest posteriorly (Fig. 8n). The lateral ridges bordering this surface contribute posteriorly to the formation of the anterior edge of the parapophysis of the subsequent centrum (Fig. 8n). The anterior caudal centra of ZIN PH 3/250 have single apophyses that are oval in outline, markedly dorsoventrally higher than anteroposteriorly long (Fig. 8q); their articular surfaces are subhexagonal in outline with their height exceeding their width and being narrowest dorsally (Fig. 8p). The caudal centa of ZIN PH 20/250 and ZIN PH 6/250 are subhexagonal in articular outline (Fig. 8t, x). The single rib facet is circular in outline and located approximately at centrum mid-height (Fig. 8u, w). Ventrally, in all available caudal centra referred to A. incognita, there are well-pronounced anterior and posterior chevron facets, all pointing obliquely medially (Fig. 8s, v, y). Caudal centra are proportionally short with their length/height ratio in the anterior to middle caudal centra being less than 0.5 (0.44–0.46; supplementary Table S1 available at https://doi.org/10.1017/S1755691021000372), which is lower than in toretocnemids (0.63 in Toretocnemus zitteli and ca. 0.7 in Toretocnemus californicus; Merriam Reference Merriam1903) and Californosaurus perrini (0.65; Merriam Reference Merriam1902), but is similar to the proportions reported for Callawayia neoscapularis (0.47; Nicholls & Manabe Reference Nicholls and Manabe2001) and those typically found in neoichthyosaurians (e.g., McGowan & Motani Reference McGowan and Motani2003).
The neural canal floor in centra from all regions of the vertebral column is hourglass-shaped with a central mediolateral constriction (Fig. 8a, f, i, m, r).
Two fragmentary neural arches and several rib fragments are preserved in association with the posterior presacral vertebrae of ZIN PH 3/250 (Fig. 8o). The neural arches have well-developed but mediolaterally narrow prezygapophyses. The preserved portions of the neural spines are anteroposteriorly wide and lenticular in cross-section with sharp anterior and posterior flanges.
Unidentified elements. A fragment of a flattened bone presumably from the pectoral girdle (scapula or coracoid) is preserved in association with the cranial remains of ZIN PH 5/250 (Fig. 4b, d), but it is too fragmentary for description. Two additional, fragmentary elements are associated with specimen ZIN PH 3/250 – these elements possibly represent rib heads from a much larger ichthyosaur specimen, but they are more likely propodials referable to the same small-bodied taxon. However, because of their fragmentary nature and uncertain anatomical identity, these elements are not included within the hypodigm of A. incognita (see Supplementary Information for details).
Euichthyosauria gen. et sp. indet., taxon B
Some vertebral centra belong to individuals larger than those referred to Auroroborealia incognita (i.e., ZIN PH 14/250; ZIN PH 22/250; see supplementary Table S1) or differ in some morphological aspects from the vertebrae of A. incognita. Therefore, some of these centra might represent older ontogenetic stages of A. incognita, or more likely, belong to other euichthyosaurian taxa.
Referred specimens. ZIN PH 14/250 and probably ZIN PH 22/250 (see Fig. 9 and Table 1 for details).

Figure 9 Vertebrae of Euichthyosauria gen. et sp. indet. ZIN PH 16/250 (A–C), ZIN PH 17/250 (D–F), from the lower Carnian; ZIN PH 14/250 (G–I), ZIN PH 9/250 (J–L), ZIN PH 22/250 (M–S), from the Norian. Vertebrae are depicted in articular (A, D, G, K, N, Q), lateral (B, E, H, L, O, R), dorsal (C, F, I, J, M, P) and ventral (S) views. Scale bar = 3 cm.
Description. Centrum ZIN PH 14/250 (Fig. 9g–i) differs too markedly from the centra of A. incognita to allow referral to this taxon. This centrum was collected ex situ, so its precise stratigraphic position is uncertain. It was collected in an area with middle Norian exposures, but it could have originated from the lower Norian horizon exposed upstream as well. The articular surfaces of the centrum are circular with their height equal to their mediolateral width (Fig. 9g), being in this regard dissimilar to the anterior presacral vertebrae of A. incognita (cf. Figs 7b, 8b, e, h and 9g). The centrum is anteroposteriorly short, with a length/height ratio of 0.44, although this shortening could be in part due to diagenetic compression. The diapophyses are confluent with the facets for the neural arch and are somewhat triangular in outline, tapering ventrally (Fig. 9h). The parapophyses are oval with their height greater than their length; they are confluent with the anterior edge of the centrum (Fig. 9h). The parapophyses are located at the centrum mid-height, unlike the more ventrally positioned parapophyses in A. incognita (cf. Figs 7, 8c, d and 9h). The floor of the neural canal is hourglass-shaped in dorsal view, as the facets for the neural arch are widest in their mid-length and become less wide anteriorly and posteriorly (Fig. 9i).
Two caudal centra, ZIN PH 22/250 (Fig. 9m–s), are slightly larger than those of A. incognita, but smaller than the centrum ZIN PH 14/250 described above; they also differ from caudal centra referred to A. incognita in that their rib facets are confluent with the anterior articular surface of the centrum (Fig. 9o, r), although this can simply imply their more anterior position in the vertebral column.
Euichthyosauria gen. et sp. indet., taxon C
Referred specimens. ZIN PH 9/250; ZIN PH 16/250; ZIN PH 17/250 and ZIN PH 18/250 (see Fig. 9 and Table 1 for details).
Description. Three presacral centra (ZIN PH 16/250, 17/250 and 9/250; Fig. 9a–f, j–l) are generally similar to Auroroborealia incognita. However, ZIN PH 16/250 and 17/250 are stratigraphically older, originating from the lower Carnian deposits. A middle presacral centrum, ZIN PH 17/250, differs from A. incognita in that its rib facets are widely spaced so that the diapophysis is located close to the dorsal surface of the centrum, whereas the parapophysis is located at the transitional level between the lateral and ventral surfaces (Fig. 9e). This is similar to the Carnian centrum from Mexico described by Lucas (Reference Lucas2002) and referred to Toretocnemus sp. It is, therefore, possible that the small vertebrae from the Carnian of the New Siberian Islands belong to toretocnemids.
4. Discussion
4.1. Taxonomic assignment of ZIN PH 5/250
The holotype of Auroroborealia incognita, ZIN PH 5/250, represents a small individual. With the anterior presacral vertebrae 21.5–28 mm in height and the posterior mandible 21 mm in maximum height, it is comparable in size with toretocnemids and the basal parvipelvian Hudsonelpidia, and was likely also approximately 1–1.5 m in length (McGowan Reference McGowan1995; Li Reference Li1999). Given its incompleteness, the ontogenetic state of ZIN PH 5/250 can hardly be assessed with confidence. However, the abundance of specimens of comparable small size in the same stratigraphic horizon suggests that these are more likely to represent a small-bodied taxon, common in the Norian of the New Siberian Islands, rather than abundant juveniles.
ZIN PH 5/250 and other specimens that probably belong to A. incognita are early Norian (Pinacoceras verchojanicum Ammonoid Biozone) in age. Therefore, they are younger than any known toretocnemid (Ladinian–Carnian; McGowan & Motani Reference McGowan and Motani2003; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013; Lu et al. Reference Lu, Jiang, Motani, Ni, Sun, Tintori, Xiao, Zhou, Ji and Fu2018) and approximately contemporaneous with the early Norian parvipelvian Hudsonelpidia (McGowan Reference McGowan1995). A comparison with Hudsonelpidia is difficult due to poor preservation of the holotype and specimens referred to this taxon (McGowan Reference McGowan1995, Reference McGowan, Callaway and Nicholls1997). It is possible that A. incognita is closely related or even referable to Hudsonelpidia; however, better preserved specimens with sufficient anatomical overlap between both taxa are required to support or reject such a referral.
The mandible of ZIN PH 5/250 is highly similar to that of toretocnemids in its slenderness, posteroventral curvature of its posterior portion, restricted lateral exposure of the angular, a well-developed preglenoid process and an elongated canal within the surangular (Merriam Reference Merriam1903; Maisch et al. Reference Maisch, Jiang, Hao, Sun, Sun and Stöhr2008). In the restricted lateral exposure of the angular, ZIN PH 5/250 differs from the parvipelvian Macgowania and many other Triassic merriamosaurians (McGowan Reference McGowan1996; Maisch & Matzke Reference Maisch and Matzke2000; Nicholls & Manabe Reference Nicholls and Manabe2001; Ji et al. Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016). However, it cannot be ruled out that the ‘toretocnemid’ morphology of the mandible may also turn out to be present in basal parvipelvians such as Hudsonelpidia, given the incomplete data on mandibular morphology for this taxon. Furthermore, since different ichthyosaurian clades kept re-evolving similar feeding ecologies (e.g., Moon Reference Moon2019; Huang et al. Reference Huang, Motani, Jiang, Ren, Tintori, Rieppel, Zhou, Hu and Zhang2020), mandible proportions could be convergent, making it less taxonomically informative.
In summary, the holotype of A. incognita, ZIN PH 5/250 (and other small ichthyosaurians from the New Siberian Islands similar to it), may represent the stratigraphically youngest-known toretocnemid, or one of the stratigraphically oldest and earliest-diverging parvipelvians, or even another yet unrecognised euichthyosaurian lineage closely related to both toretocnemids and parvipelvians. With this in regard, we tentatively place this taxon within Euichthyosauria.
4.2. Evolution of the parabasisphenoid in ichthyosauriforms
The first detailed overview of the evolution of the ichthyosaurian parabasisphenoid was provided by Maisch & Matzke (Reference Maisch and Matzke1997b, Reference Maisch and Matzke2000). They proposed four phylogenetic characters describing the major evolutionary transitions in this skeletal element: the contribution of the basisphenoid and parasphenoid to the formation of the basicranium (Maisch & Matzke Reference Maisch and Matzke1997b, character 15; Maisch & Matzke Reference Maisch and Matzke2000, character 41); the pattern of the internal carotid circulation (Maisch & Matzke Reference Maisch and Matzke1997b, character 14; Maisch & Matzke Reference Maisch and Matzke2000, character 42); the constriction at the base of the cultriform process (Maisch & Matzke Reference Maisch and Matzke2000, character 43); and the ventral exposure of the basicranium between the pterygoids (Maisch & Matzke Reference Maisch and Matzke2000, character 44). Several additional characters for the basisphenoid were later proposed by Maxwell (Reference Maxwell2010), Fischer et al. (Reference Fischer, Masure, Arkhangelsky and Godefroit2011), Maxwell et al. (Reference Maxwell, Dick, Padilla and Parra2016); Zverkov & Efimov (Reference Zverkov and Efimov2019) and Zverkov & Jacobs (Reference Zverkov and Jacobs2021); these, however, are largely applicable for post-Triassic taxa, especially ophthalmosaurians, and are of limited relevance for ichthyosaur evolution in the Triassic and Early Jurassic, so they will not be discussed further.
The unique data on the parabasisphenoid of Auroroborealia incognita allow for discussion of its implications for the existing knowledge of the evolution of this cranial element in ichthyosaurians.
4.2.1. Posterior flange of the parabasisphenoid
The relative contribution of the parasphenoid and basisphenoid to the formation of the basicranium was first considered by Maisch & Matzke (Reference Maisch and Matzke2000), who revealed a transition from the parasphenoid-dominated basicranium in most Triassic ichthyosaurians to the basisphenoid dominated basicranium in neoichthyosaurians. The euichthyosaurian Callawayia neoscapularis, in their opinion, demonstrated a somewhat transitional condition (Maisch & Matzke Reference Maisch and Matzke2000). In the majority of Triassic ichthyosauriforms (Chaohusaurus, mixosaurids, Besanosaurus, Phantomosaurus, Shastasaurus and some other Triassic merriamosaurians) (Maisch & Matzke Reference Maisch and Matzke1997a, Reference Maisch and Matzkeb, Reference Maisch and Matzke2000, Reference Maisch and Matzke2006; Bindellini et al. Reference Bindellini, Wolniewicz, Miedema, Scheyer and Dal Sasso2021) the posterior parasphenoid is an extensive mediolaterally broad sheet of bone, forming the basicranium ventrally and representing the retention of the ancestral amniote state (Fig. 10; Maisch & Matzke Reference Maisch and Matzke2000). Based on our observations of the holotype of C. neoscapularis (ROM 41993, ASW, pers. obs. July 2017), the parasphenoid of this taxon widens posterior to the carotid foramina. However, the extent of its posterior portion cannot be determined because of preservation. It is unclear if there is a long posterior sheet of the parasphenoid subjacent to the basioccipital, as in other Triassic non-parvipelvian taxa, or whether the posterior protrusion of the parasphenoid is short and terminates at the level of the posterior basisphenoid as interpreted by Maisch & Matzke (Reference Maisch and Matzke2000), based on their observations on the cast of the holotype specimen.

Figure 10 Evolution of the parabasisphenoid in ichthyosauriforms; phylogeny compiled from Ji et al. (Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016) and Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019). Basicranial elements redrawn from Peabody (Reference Peabody1952) for Petrolacosaurus, Maisch (Reference Maisch2001) and Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019) for Chaohusaurus, Bindellini et al. (Reference Bindellini, Wolniewicz, Miedema, Scheyer and Dal Sasso2021) for Besanosaurus, Fraas (Reference Fraas1913) and Maisch (Reference Maisch2002) for Temnodontosaurus. Mixosaurus and Ichthyosaurus are based on personal observations of NGZ of the specimen figured by Wiman (Reference Wiman1912, taf. 11, fig. 1; PMU uncatalogued) and OUMNH J2242; Cymbospondylus buchseri, Guizhouichthyosaurus tangae and Callawayia neoscapularis are based on ASW's personal observations of PIMUZ T 4838, IVPP V11853 and ROM 41993, respectively. Macgowania janiceps is based on DVG's personal observations of TMP 2009.121.1. Scale bars = 1 cm.
The transition from a parasphenoid-dominated to a basisphenoid-dominated basicranium is marked by the mediolateral narrowing of the posterior parasphenoid into a central strip underlying the basisphenoid (Maisch & Matzke Reference Maisch and Matzke2000, character 43). This central strip of the parasphenoid reaching the posterior edge of the basisphenoid and separating the paired carotid foramina is well discernible in a referred specimen of Macgowania janiceps (TMP 2009.121.1) and in Temnodontosaurus spp. (Fig. 10; Fraas Reference Fraas1891; Maisch Reference Maisch2002; Henderson Reference Henderson, Bininda-Emonds, Powell, Jamniczky, Bauer and Theodor2015). In other parvipelvians, the posterior parasphenoid terminates anterior to the central carotid foramen and does not reach the posterior edge of the basisphenoid (e.g., Maisch & Matzke Reference Maisch and Matzke2000). This derived, otherwise typically parvipelvian, condition is also present in A. incognita.
4.2.2. Patterns of carotid circulation in ichthyosauriforms
Auroroborealia incognita is the only Late Triassic ichthyosaurian with a documented unpaired posterior (ventral) foramen for the internal carotid arteries. The presence of a single and circular carotid foramen is typical for derived neoichthyosaurians (e.g., Maisch & Matzke Reference Maisch and Matzke2000); however, it shows a complex pattern of distribution in ichthyosauriforms, implying a homoplastic nature of this character.
The stratigraphically oldest ichthyosauriform with a documented parabasisphenoid is Chaohusaurus (Maisch & Matzke Reference Maisch and Matzke2000; Maisch Reference Maisch2001; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019). Maisch (Reference Maisch2001; see also Maisch & Matzke Reference Maisch and Matzke2000) reported a single central foramen (wider than long and somewhat kidney-shaped in outline) for Chaohusaurus (Chaohusaurus chaoxianensis, specimen NGM P45-H85-21, according to Zhou et al. Reference Zhou, Jiang, Motani, Tintori, Ji, Sun, Ni and Lu2017). This, however, was depicted only as a line drawing with no photographs of the specimen provided. The recent description of the parasphenoid and basisphenoid of Chaohusaurus brevifemoralis by Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019) differs in some aspects from the interpretations of Maisch (Reference Maisch2001); therefore, in Figure 10 we provide both the drawing of Maisch (Reference Maisch2001) and the photograph from Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019). Huang et al. (Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019) have not described the foramen(ina) for the internal carotid arteries. The seemingly circular ?foramen located posterior to the base of the cultriform process of the parasphenoid in AGM AGB7403 (Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019, fig. 6) was not described and discussed by the authors and could be a preservational artefact. In summary, it is not clear if Chaohusaurus preserved an ancestral amniote condition with paired foramina for the internal carotid arteries at the base of the basipterygoid process, commonly obscured in ventral view (e.g., Müller et al. Reference Müller, Sterli and Anquetin2011; Ford & Benson Reference Ford and Benson2019), or developed a derived ichthyosaurian condition with medially shifted and closely spaced foramina for the internal carotids exposed ventrally, rather than laterally. Considering the interpretation of Maisch (Reference Maisch2001), the latter seems more likely.
In mixosaurids, a single circular foramen located at the base of the cultriform process, on the ventral surface of the parabasisphenoid, is the typical condition (Wiman Reference Wiman1912; von Huene Reference von Huene1916; Maisch & Matzke Reference Maisch and Matzke1997a; Brinkmann Reference Brinkmann2004). In contrast, cymbospondylids and Triassic representatives of Merriamosauria, which are generally larger than mixosaurids, have paired and markedly spaced foramina for the internal carotids (Fig. 10; Maisch & Matzke Reference Maisch and Matzke1997b, Reference Maisch and Matzke2006).
Paired ventral carotid foramina are known for the Late Triassic and Early Jurassic parvipelvians Macgowania (Fig. 10) and Temnodontosaurus (Fraas Reference Fraas1913; Godefroit Reference Godefroit1993; Maisch Reference Maisch2002; Martin et al. Reference Martin, Fischer, Vincent and Suan2012). However, in the majority of parvipelvians, a condition with a single foramen prevails (e.g., McGowan & Motani Reference McGowan and Motani2003). Some Early Jurassic parvipelvians (e.g., Stenopterygius and some species of Ichthyosaurus) have a single foramen leading into a canal, which is partially separated dorsally by a longitudinal ridge, demonstrating the transitional condition of confluence of carotid canals within the basisphenoid (Fig. 10; Fraas Reference Fraas1891; McGowan Reference McGowan1973). This is especially evident from the ontogeny of Stenopterygius (Miedema & Maxwell Reference Miedema and Maxwell2019), in which the embryonic and juvenile stages demonstrate a well pronounced ridge demarcating the carotid canals.
In summary, it appears that a single ventral (posterior) foramen for the internal carotid arteries evolved three to four times (depending on the correctness of interpretation for Chaohusaurus, the phylogenetic hypothesis used and the inferred phylogenetic position of A. incognita) in the evolutionary history of ichthyosauriforms, indicating high homoplasy of this trait. Considering the relatively smaller sizes of Triassic ichthyosaurian taxa with an unpaired posterior carotid foramen compared to coeval taxa with paired foramina, we hypothesise that this condition could be somehow related to the miniaturisation that is commonly paralleled by the reduction and simplification of various structures (e.g., Hanken & Wake Reference Hanken and Wake1993). Even though patterns of cranial arterial circulation in amniotes are considered to contain phylogenetically useful information at least for some groups (e.g., Jamniczky Reference Jamniczky2008; Müller et al. Reference Müller, Sterli and Anquetin2011; Zverkov et al. Reference Zverkov, Averianov and Popov2018; Rollot et al. Reference Rollot, Evers and Joyce2021), their potential for ichthyosaurian phylogenetics is still to be fully realised as more data become available.
4.2.3. Basipterygoid processes
Many Triassic ichthyosauriforms demonstrate weak development of basipterygoid processes. In mixosaurids, the basipterygoid processes are not demarcated from the parabasisphenoid and are anterolaterally directed in Mixosaurus cornalianus (Fig. 10; Wiman Reference Wiman1912), but seem slightly more pronounced in Phalarodon atavus (von Huene Reference von Huene1916). In the merriamosaurian Besanosaurus, the condition is highly similar to that of M. cornalianus (Fig. 10; Maisch & Matzke Reference Maisch and Matzke1997b; Bindellini et al. Reference Bindellini, Wolniewicz, Miedema, Scheyer and Dal Sasso2021). The data for cymbospondylids and shastasaurids are too scarce to provide an informative comparison at present. Among other Triassic taxa, well-developed laterally protruding basipterygoid processes, clearly demarcated from the parabasisphenoid, are known for Phantomosaurus (Maisch & Matzke Reference Maisch and Matzke2006) and Callawayia neoscapularis (Fig. 10; McGowan Reference McGowan1994). In parvipelvians, the basipterygoid processes are overall more strongly pronounced, compared to other ichthyosaurians, although are not as markedly demarcated from the main body of the posterior basisphenoid in basal Triassic and Early Jurassic parvipelvian taxa (Fig. 10).
In this regard, the well-developed basipterygoid processes of Auroroborealia incognita, distinctly demarcated from the main body of the basisphenoid by a constriction formed of smooth bone, are most similar to those of C. neoscapularis and, to a lesser degree, to those of parvipelvians, but not to any other Triassic ichthyosauriform. This further supports a likely derived phylogenetic position of A. incognita within Euichthyosauria.
4.2.4. Loss of ossification of the sella turcica
The most interesting and hitherto rarely discussed condition is the ossification of the trabeculae bounding the sella turcica in the parabasisphenoid of ichthyosaurians. Although the basiscranium is well known for many Jurassic and Cretaceous ichthyosaurians (e.g., Romer Reference Romer1968; McGowan Reference McGowan1973; Maisch & Matzke Reference Maisch and Matzke2000; McGowan & Motani Reference McGowan and Motani2003; Kear Reference Kear2005; Marek et al. Reference Marek, Moon, Williams and Benton2015; Moon & Kirton Reference Moon and Kirton2016), among Triassic forms, the only taxon for which the morphology of the dorsal surface of the parabasisphenoid was described is Mixosaurus cornalianus (Maisch & Matzke Reference Maisch and Matzke1997a). In the only specimen with exposed dorsal parabasisphenoid, however, the preservation is not exceptional and some structures (i.e., the impressions of the posterior ends of the trabeculae), as interpreted by Maisch & Matzke (Reference Maisch and Matzke1997a), are uncertain. In A. incognita, the dorsal surface of the parabasisphenoid is well preserved and, among ichthyosaurians, is most similar to that of M. cornalianus. In both taxa, the region anterior to the dorsum sellae, the sella turcica, is well ossified, unlike in any post-Triassic ichthyosaur. The typical condition for all post-Triassic ichthyosaurians is the loss of ossification of the trabeculae, so that only small impressions located ventral to the anterior carotid foramen and dorsal to the strap-like cultriform process are present (Fig. 11; e.g., McGowan Reference McGowan1973; McGowan & Motani Reference McGowan and Motani2003). This indicates that the sella turcica remained cartilaginous in neoichthyosaurians.
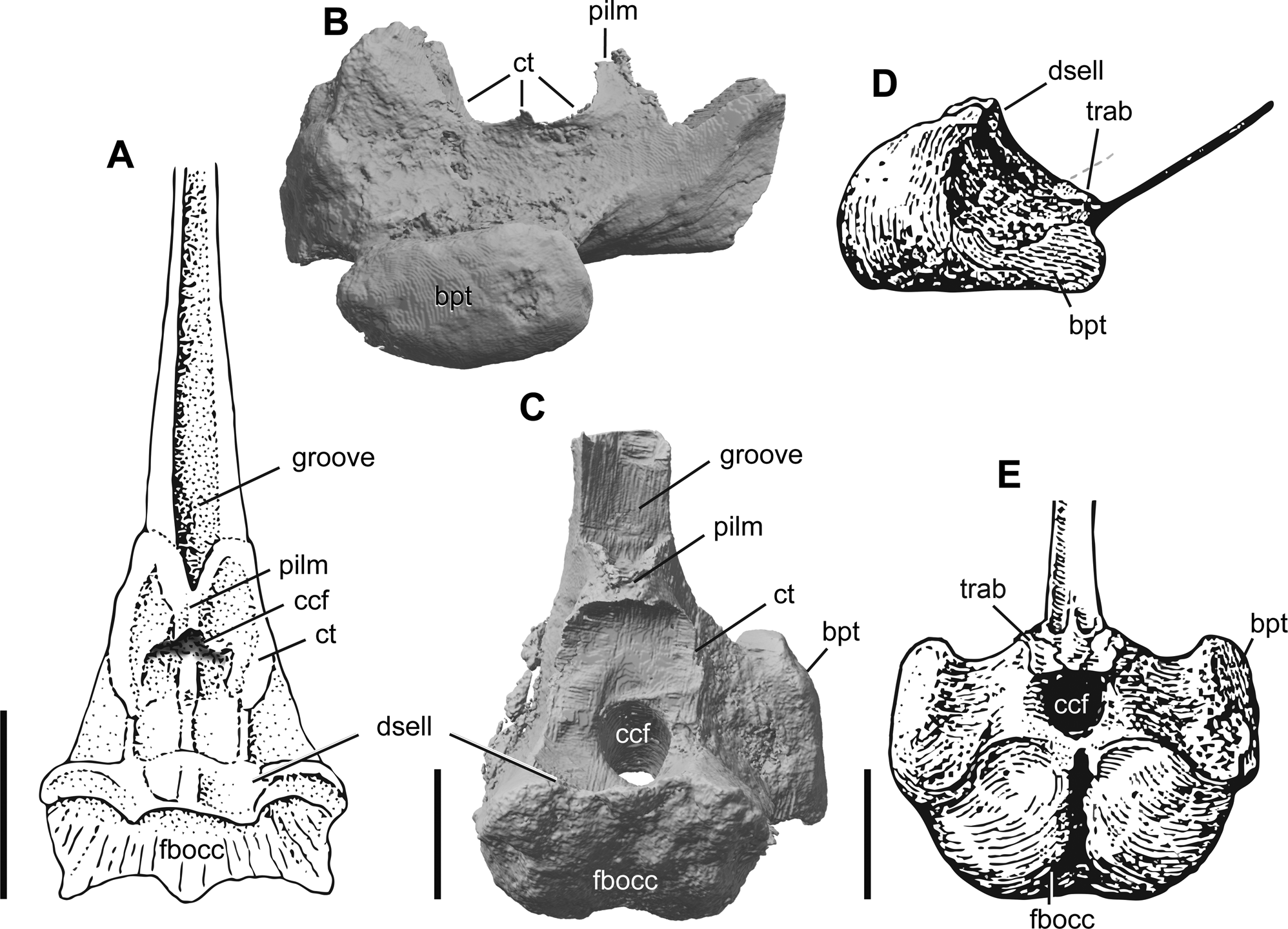
Figure 11 Comparison of ichthyosaurian parabasisphenoids: (A) dorsal view of the parabasisphenoid of Mixosaurus cornalianus modified from Maisch & Matzke (Reference Maisch and Matzke1997a); parabasisphenoid of Auroroborealia incognita gen. et sp. nov. in right lateral (B) and dorsal (C) views; the parabasisphenoid of Ichthyosaurus sp. modified from Romer (Reference Romer1968), in right lateral (D) and dorsal (E) views. Abbreviations: bpt = basipterygoid process; ccf = cerebral carotid foramen; ct = crista trabecularis; dsell = dorsum sellae; fbocc = facet for basioccipital; pilm = ossification from the pila metoptica; trab = impressions of the posterior ends of the trabeculae. Scale bars = 1 cm.
The ossified region of the sella turcica in A. incognita, similar in morphology to that in M. cornalianus, represents a plesiomorphic condition that was likely also present in many Triassic ichthyosaurian taxa. In dorsal view, the parabasisphenoids of A. incognita and M. cornalianus are similar in that the sella turcica is laterally bounded by ridges (cristae trabeculares) extending posteriorly around the dorsum sellae and merging anteriorly to form a central ascending process (ossification from the pila metoptica). The latter central process of M. cornalianus was originally interpreted as ‘the impressions of the posterior ends of the trabeculae’ (Maisch & Matzke Reference Maisch and Matzke1997a, p. 728).
A dorsoventrally high, trough-like structure of the cultriform process anterior to the sella turcica is also a feature shared by A. incognita and M. cornalianus, whereas in neoichthyosaurians the cultriform process is commonly subtle and strap-like at its base (Fig. 11). Together with the ossified sella turcica, this indicates a more robust structure of the basicranium in these Triassic compared to post-Triassic taxa.
Despite the plesiomorphic condition of an ossified sella turcica, the presence of ossification from the pila metoptica and a dorsoventrally high trough-like base of the cultriform process, the parabasisphenoid of A. incognita differs from that of Mixosaurus and other Triassic ichthyosaurians in the restricted posterior parasphenoid terminating anterior to the unpaired foramen for the internal carotids and well-developed basipterygoid processes (discussed in detail above), thus representing an interesting combination of plesiomorphic and derived character states.
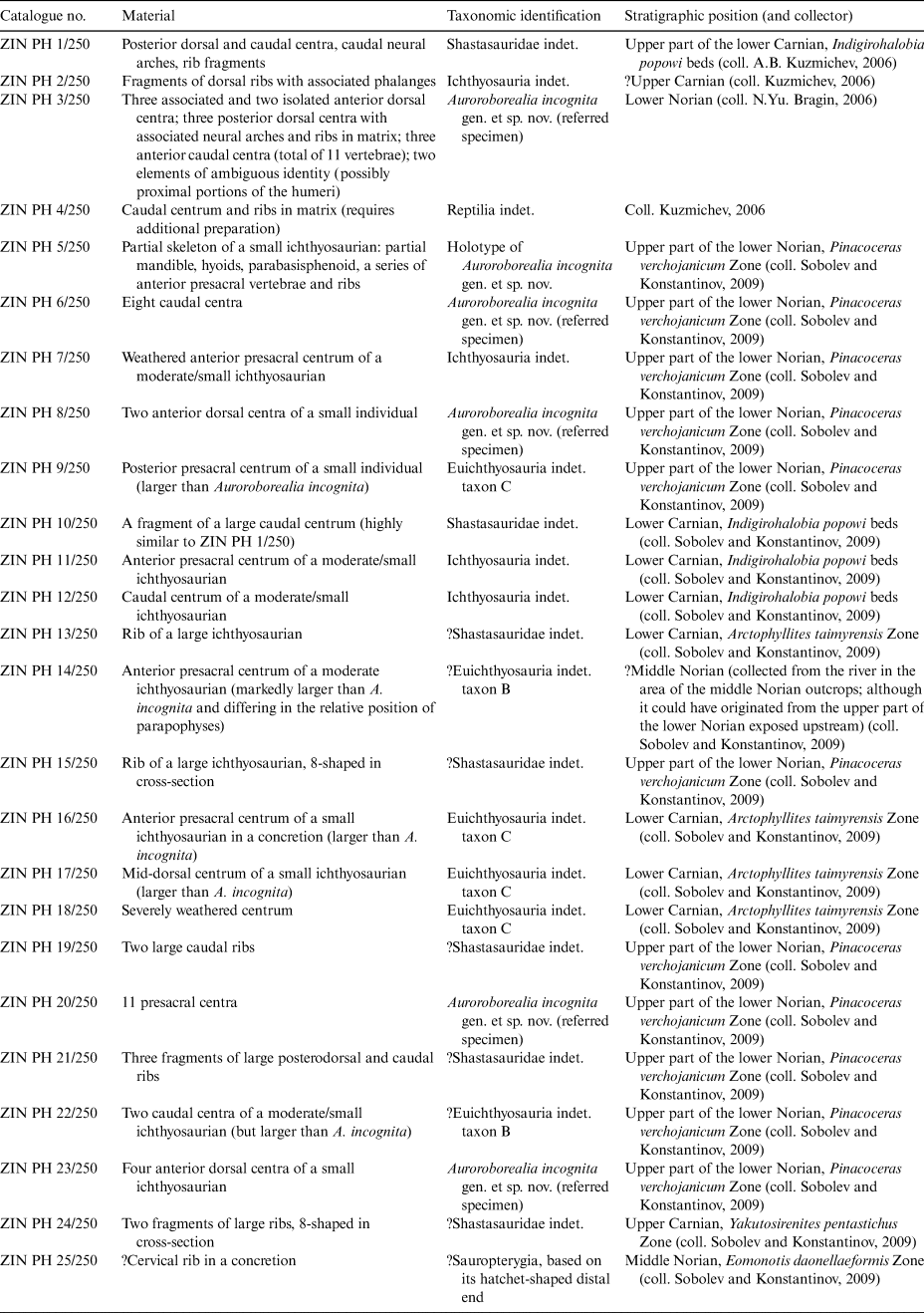
4.3. Evolution of the vertebral column in Ichthyopterygia
The morphology and evolution of the vertebral column of ichthyopterygians receives little attention in the literature, compared to cranial and appendicular elements. As a result, not much data (illustrations, in particular) on the morphology of vertebrae are available for a number of ichthyosaurian taxa and their phylogenetic and taxonomic implications appear somewhat underappreciated at present. In Figure 12, we summarise the patterns of vertebral centra variation throughout the column in different ichthyopterygian lineages. It can be noted that in the majority of ichthyopterygians, the anteriormost presacral vertebrae (commonly termed as ‘cervical’, despite the fact that the extent and even presence of a neck, especially in derived ichthyosaurians, is somewhat problematic to define) have two rib facets, although the parapophysis is commonly weakly developed in basal taxa and rapidly disappears in more posterior vertebrae. In the post-‘cervical’ vertebrae of basal ichthyosaurians, rib facets are single and dorsoventrally elongated up to the caudal region, where they become smaller and rounded in outline. In grippiids, mixosaurids and toretocnemids, the posteriormost presacral (and probably anteriormost caudal) vertebrae attain double rib facets (e.g., Maisch & Matzke Reference Maisch and Matzke2000). However, according to the most recent phylogenetic contexts (e.g., Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019; Moon Reference Moon2019), this condition appears convergent in these groups. Double rib facets present throughout the entire presacral region are unambiguously known only for post-Triassic parvipelvians (Neoichthyosauria). This condition is, most likely, also present in Auroroborealia incognita (Fig. 12). However, given the incomplete data on the vertebral column of toretocnemids and basal parvipelvians (Merriam Reference Merriam1903, Reference Merriam1908; McGowan Reference McGowan1995; Li Reference Li1999; Lucas Reference Lucas2002; Nicholls et al. Reference Nicholls, Wei and Manabe2002; Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013), it is difficult to infer the affinity of A. incognita based on this vertebral character.

Figure 12 Evolution of the vertebral column in Ichthyopterygia; phylogeny follows Ji et al. (Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016); vertebrae redrawn from Wiman (Reference Wiman1933), Ekeheien et al. (Reference Ekeheien, Delsett, Roberts and Hurum2018) for Grippia; Repossi (Reference Repossi1902), von Huene (Reference von Huene1916) and Brinkmann (Reference Brinkmann1998) for Mixosaurus; Merriam (Reference Merriam1895, Reference Merriam1902, Reference Merriam1908) for Cymbospondylus, Shastasaurus and Californosaurus perrini; Nicholls & Manabe (Reference Nicholls and Manabe2001) for Callawayia neoscapularis; Yang et al. (Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013) for Qianichthyosaurus xingyiensis; Owen (Reference Owen1881) and McGowan & Motani (Reference McGowan and Motani2003) for Ichthyosaurus spp. and Temnodontosaurus sp.; and based on NGZ's personal observations on PMU specimens of Grippia (PMU 24690 and 24704) and mixosaurids (numerous specimens including articulated and isolated vertebrae, e.g., PMU 24576, partly uncatalogued as of May 2016) and NHMUK specimens of Temnodontosaurus (NHMUK PV OR 481) and Ichthyosaurus (e.g., NHMUK PV R 44, 216, 1162, 2013; December 2018); and ASW's personal observations of C. perrini (UCMP 9119 and 9082, November 2015).
Below is an overview of the patterns of rib articulation in the vertebrae of toretocnemids and basal parvipelvians, based on the available data.
The stratigraphically oldest known toretocnemid is Qianichthyosaurus xingyiensis from the upper Ladinian of China (Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013; Lu et al. Reference Lu, Jiang, Motani, Ni, Sun, Tintori, Xiao, Zhou, Ji and Fu2018). It was described based on two specimens, both providing some information on the morphology of the vertebral column. From the photographs (Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013, fig. 1), it appears that the presacral vertebrae in this taxon largely bear single rib articulations with 8-shaped rib facets with incompletely separated diapophyses and parapophyses; this inference is also supported by the numerous single-headed dorsal ribs preserved (Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013, fig. 1). Only in the posteriormost presacral region, several vertebrae with two markedly separated rib facets occur (Yang et al. Reference Yang, Ji, Jiang, Motani, Tintori, Sun and Sun2013, fig. 1). The anteriormost presacral (cervical) region is insufficiently known for this taxon. For the Carnian Qianichthyosaurus zhoui, Li originally reported a double rib articulation, but without mentioning any variation and without specifying in which regions of the vertebral column this condition was observed (Li Reference Li1999, p. 1332). Judging from the published photographs (Li Reference Li1999, fig. 1), this observation is relevant for the posterior presacral region of the paratype (IVPP V11838), whereas in the anterior presacral region, the morphology of rib facets cannot be determined. In the holotype (IVPP V11839), the morphology of rib facets appears largely obscured (Li Reference Li1999, fig. 1b). More information on the vertebral column anatomy of this species was later reported by Nicholls et al. (Reference Nicholls, Wei and Manabe2002), who identified a double rib articulation in ‘cervical’ and middle to posterior-dorsal regions and noted that, in the anterior dorsal region, rib articulations were not visible, but the ribs in this region were single headed. It appears that at least some mid-dorsal vertebrae of Q. zhoui possessed single rib facets, although it seems the general proportion of vertebrae with two rib articulations increased in both anterior and posterior presacral regions of this taxon.
In the type species of Toretocnemus, Toretocnemus californicus from the Carnian of California, Merriam (Reference Merriam1903) reported ‘middle dorsal ribs with widely forking heads articulating on small and widely separated diapophyses and parapophyses’ (Merriam Reference Merriam1903, p. 260); and also ‘near the middle of the dorsal region the diapophyses are small and round and lie above the middle of the side of the centrum, while prominent parapophyses with backwardly directed facets are present low down and nearer the anterior margin’ (Merriam Reference Merriam1903, p. 260). The presacral vertebrae are unknown for Toretocnemus zitteli. However, Merriam reported single-headed dorsal ribs for this taxon (Merriam Reference Merriam1903, Reference Merriam1908). If this interpretation is correct, it implies that at least some dorsal vertebrae of T. zitteli may have had confluent rib facets. Lucas & González-León (Reference Lucas and González-León1995) and later Lucas (Reference Lucas2002) reported several small posterior presacral vertebrae of toretocnemids from the Carnian of Sonora, Mexico. In having widely separated diapophyses and parapophyses, these vertebrae agree well with the description and figures of the posterior presacral vertebrae of T. californicus (Merriam Reference Merriam1903). Notwithstanding, more complete materials are still needed for clarification if the diapophysis and parapophysis were separate in Toretocnemus throughout the entire presacral region, or if this condition occurred only in the anterior and posterior parts of the presacral series.
In the Triassic parvipelvians from the Pardonet Formation (British Columbia, Canada), the morphology of the vertebrae is extremely poorly known due to their preservation (McGowan Reference McGowan1995, Reference McGowan1996). For Hudsonelpidia, McGowan (Reference McGowan1995, p. 302) reported that ‘rib articulations cannot be seen, and the only indication of their form comes from what appears to be a bicipital rib head at the level of vertebra 31’. However, this is insignificant for any further conclusions, other than that bicipital rib articulations were present in the posterior presacral vertebrae of this taxon. In the holotype of Macgowania janiceps (ROM 41992), several anteriormost presacral vertebral centra are present and in at least four of these the well pronounced and separated diapophyses and parapophyses can be observed (ASW, pers. obs., April 2015). In the referred specimen of Macgowania (TMP 2009.121.1; Henderson Reference Henderson, Bininda-Emonds, Powell, Jamniczky, Bauer and Theodor2015), several exposed postaxial presacral vertebrae demonstrate well the same condition with the extensively developed diapophyses confluent with the anterior edge of the centrum and the neural arch facet and parapophyses confluent with the anterior edge of the centrum and separated from the diapophyses (DVG, pers. obs. on TMP 2009.121.1, April 2019). This is consistent with the majority of ichthyosaurians, especially neoichthyosaurians, but is dissimilar to the peculiar condition in Auroroborealia incognita (ZIN PH 5/250), in which the anteriormost presacral vertebral centra bear only parapophyses, whereas diapophyses are located on the neural arches. This was also reported for the mixosaurid Phalarodon callawayi (Schmitz et al. Reference Schmitz, Sander, Storrs and Rieppel2004); however, in all other mixosaurids in particular, and ichthyosauriforms in general, the diapophyseal facet is present in the anteriormost presacral vertebral centra (e.g., McGowan & Motani Reference McGowan and Motani2003; Ekeheien et al. Reference Ekeheien, Delsett, Roberts and Hurum2018; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019).
In the Jurassic parvipelvians, the presacral vertebral centra typically bear two distinct rib facets (e.g., McGowan & Motani Reference McGowan and Motani2003); however, this is not without exceptions. McGowan & Motani (Reference McGowan and Motani2003) reported a peculiar condition in a series of 65 vertebrae of a large ichthyosaurian from the Lower Lias Group of Lyme Regis (NHMUK PV OR 481; referred to Temnodontosaurus). In this specimen, the diapophyses and parapophyses are confluent for vertebrae 3–6; by vertebra 7, the facets are confluent on the left side, but separate on the right. By vertebra 10, the facets are separate on both sides; however, they are confluent in several posterior presacral centra (McGowan & Motani Reference McGowan and Motani2003, p. 12; NGZ, pers. obs. of NHMUK PV OR 481, December 2018). Furthermore, the confluent diapophyses and parapophyses are present in some anterior presacral centra of Leptonectes tenuirostris (NGZ, pers. obs. of OUMNH J10309, December 2018) and confluent rib facets in the presacral vertebrae were recently described and figured for Suevoleviathan integer (Maisch Reference Maisch2020). Maisch & Matzke (Reference Maisch and Matzke2000, p. 37) also noted that for Temnodontosaurus, Leptonectes, Eurhinosaurus and Suevoleviathan, ‘although most presacral vertebral centra show clearly subdivided articulatory facets, the rib heads are not bicephalic but the two articulatory facets are connected so that the ribs appear rather holocephalic’. Therefore, the conditions of double rib articulations and double-headed/single-headed ribs in the presacral region can be quite variable in basal parvipelvians.
In summary, the pattern of rib articulation in the presacral region appears variable in euichthyosaurians, with parallel trends towards separation of parapophyses and diapophyses throughout the presacral series in both toretocnemids and parvipelvians. Although derived parvipelvians have separated diapophyses and parapophyses throughout the entire presacral series, it is still unclear if toretocnemids evolved bicipital rib articulations in the mid-dorsal region. Furthermore, given the presence of basal Early Jurassic parvipelvians with confluent diapophyses and parapophyses in some presacral vertebrae, it is difficult to suggest the phylogenetic placement of A. incognita based on the presence of the derived condition of this character. Further attention to this and other characters of the vertebral column of ichthyosaurians is needed in order to clarify its potential phylogenetic and taxonomic implications.
4.4. Late Triassic transitional ichthyosaurian faunas
The New Siberian Islands are the only locality in the world for which a sequence of early Carnian to Norian ichthyosaurian faunas is known. Furthermore, although the palaeolatitude of the New Siberian Islands in the Triassic is imprecisely known, from the existing palaeogeographic reconstructions, they appear to have been situated at very high palaeolatitudes in the Late Triassic (Fig. 13), which makes their ichthyosaurian faunas even more intriguing. In both the Carnian and Norian strata of the New Siberian Islands, the remains of ichthyosaurians representing three size classes and different taxonomic groups are found: the largest specimens are referable to shastasaurids, with vertebral centra 50 mm long and 115 mm high; the medium-sized taxa, with centra of around 50 mm in height, are referable to indeterminate ichthyosaurians (although several Norian specimens are most similar to euichthyosaurians); and small taxa, with centra 20–35 mm high and 10–20 mm long, most likely represent euichthyosaurians. Even though the taxonomic composition of ichthyosaurian assemblages from the New Siberian Islands is far from being completely understood, these assemblages are broadly similar to what McGowan (Reference McGowan, Callaway and Nicholls1997) characterised as a ‘transitional ichthyosaur fauna’ of the Pardonet Formation of British Colombia, Canada, that includes small- and medium-bodied parvipelvians, medium-bodied euichthyosaurians and gigantic shastasaurids. Globally, similar faunas are in fact present in other localities with sufficiently abundant and well-known Carnian and Norian ichthyosaurian fossils (Fig. 13; supplementary Table S2). The best-known Carnian ichthyosaurian faunas are those of the eastern coast of Panthalassa (California and Nevada in the USA and Sonora in Mexico) and eastern Tethys (Guanling, China), all situated at low tropical palaeolatitudes (Fig. 13). The Shasta fauna from the Hosselkus Limestone includes the large-bodied shastasaurid Shonisaurus sp., the moderately large Shastasaurus pacificus, the medium-sized euichthyosaurian Californosaurus perrini and the small toretocnemids Toretocnemus californicus and Toretocnemus zitteli (e.g., Merriam Reference Merriam1908; Motani Reference Motani1999). A similar fauna, but represented by far less abundant fossils, is reported from El Antimonio district, Sonora, Mexico, and includes the large Shonisaurus sp. and the small Toretocnemus sp. (Callaway & Massare Reference Callaway and Massare1989; Lucas & González-León Reference Lucas and González-León1995; Motani Reference Motani1999), whereas the Luning Formation of Nevada yielded gigantic Shonisaurus popularis exclusively (Camp Reference Camp1980; McGowan & Motani Reference McGowan and Motani1999). The Guanling fauna from the Xiaowa Formation of Guizhou Province, China, includes the large-bodied and small-headed shastasaurids Guanlingsaurus liangae and Typicusichthyosaurus tsaihuae (species inquirenda), the large-bodied and large-headed Ghuizhouichthyosaurus tangae (with evidence for megapredation; Jiang et al. Reference Jiang, Motani, Tintori, Rieppel, Ji, Zhou, Wang, Lu and Li2020) and the medium-sized ‘Callawayia’ wolonggangense, as well as the small toretocnemid Qianichthyosaurus zhoui (e.g., Li Reference Li1999; Yin et al. Reference Yin, Zhou, Cao, Yu and Luo2000; Ji et al. Reference Ji, Jiang, Motani, Rieppel, Hao and Sun2016). Other Carnian occurrences of ichthyosaurians are rare and mostly represented by findings of isolated vertebrae (supplementary Table S2; Fig. 13). With the presence of large shastasaurids (ZIN PH 1/250; 10/250), medium-sized indeterminate ichthyosaurians (ZIN PH 11 and 12/250) and small euichthyosaurians (ZIN PH 16–18/250) in the Carnian strata, the New Siberian Islands, therefore, represent one of the very few localities globally with several co-occurring ichthyosaurian taxa of different size classes, demonstrating that this was likely a typical Carnian ichthyosaur faunal composition regardless of its supposed position at high latitudes.

Figure 13 Palaeobiogeographic distribution of Carnian and Norian ichthyosaurs. For numbers of localities, list of taxa and references, see supplementary Table S2. Palaeogeographic reconstruction modified from Martindale et al. (Reference Martindale, Corsetti, James and Bottjer2015).
Norian occurrences of ichthyosaurians are even rarer and scarcer than Carnian ones, with hitherto the only but sound exception being the diverse ichthyosaurian fauna from the Pardonet Formation of north-eastern British Columbia, Canada, that includes the gigantic shastasaurid Shonisaurus sikanniensis, the medium-sized euichthyosaurian Callawayia neoscapularis and parvipelvian Macgowania janiceps, as well as the small parvipelvian Hudsonelpidia brevirostris (McGowan Reference McGowan1994, Reference McGowan1995, Reference McGowan1996, Reference McGowan, Callaway and Nicholls1997; Nicholls & Manabe Reference Nicholls and Manabe2001, Reference Nicholls and Manabe2004; Henderson Reference Henderson, Bininda-Emonds, Powell, Jamniczky, Bauer and Theodor2015). The contemporary Hound Island Volcanics assemblage is also promising but still requires further study (Adams Reference Adams2009). The New Siberian Islands Norian ichthyosaurian fauna also includes large shastasaurids (although much smaller than S. sikanniensis; see Section 3.1), medium-sized ichthyosaurians similar to C. neoscapularis (ZIN PH 7/250) and, the most common, small euichthyosaurians that are here referred to a new taxon, Auroroborealia incognita, that show some anatomical resemblance to toretocnemids and Hudsonelpidia. Furthermore, a vertebra of a medium-sized taxon from the ?middle Norian (ZIN PH 14/250) has a parvipelvian-like appearance and might have belong to a taxon similar to the middle Norian M. janiceps, thus making the Norian faunas of the New Siberian Islands and British Columbia even more similar. It appears that during the Carnian–Norian interval ichthyosaurian faunas were transitioning globally, with small- to medium-sized parvipelvians gradually replacing small toretocnemids (either as a result of competition or anagenetic evolution), and with shastasaurids evolving increasingly larger body sizes. However, elucidating the details of this transition in terms of changes in ecological niche occupation and taxonomic turnover requires further sampling of the global Late Triassic marine fossil record.
5. Supplementary material
Supplementary material is available online at https://doi.org/10.1017/S1755691021000372.
6. Acknowledgements
We are immensely grateful to Nikita Yu. Bragin, Alexander B. Kuzmichev, Maria K. Danukalova and Pavel A. Nikolsky for providing the material of Triassic marine reptiles from the New Siberian Islands for our study. We thank Evgeny E. Baraboshkin (Skoltech Center for Hydrocarbon Recovery, Moscow, Russia) for CT scanning of ZIN PH 5/250 and Dmitry I. Pashchenko (Paleontological Institute, Moscow, Russia) for help with the formation of the Latin name of the new taxon. We thank the following curators for providing us access to specimens under their care (arranged in alphabetical order): Sandra Chapman (NHMUK), Li Chun and Qinghua Shang (IVPP), Donald Henderson, Brandon Strilisky and James Gardner (TMP), Pat Holroyd (UCMP), Ben Kear (PMU), Hilary Ketchum (OUMNH), Christian Klug and Torsten Scheyer (PIMUZ), Kevin Seymour and Brian Iwama (ROM). We also thank Michael Maisch, Neil Kelley and two anonymous reviewers for their comments that helped improve previous versions of this manuscript. The work of NGZ was supported by the ‘grant for young researchers of the Geological Institute of the Russian Academy of Sciences’ and by the state program 0114-2021-0003. The work of DVG was supported by the Russian Science Foundation (19-14-00020). The work of ASW was partially supported by a Natural Environment Research Council (NERC) PhD Studentship (cohort grant NE/L501530/1) carried out at the Department of Earth Sciences, University of Oxford (2013–2017) and the Doris O. and Samuel P. Welles Research Fund from UCMP. The work of AGK and ESS is a contribution to the fundamental research project of the Ministry of Science and Higher Education of Russia No. 0331-2019-0004.
7. Data availability statement
The μCT dataset and 3D models used in this study can be freely accessed at https://figshare.com/projects/Ichthyosaurs_from_the_Upper_Triassic_of_the_New_Siberian_Islands_Russian_Arctic/92831 (3D models of cranial elements of ZIN PH 5/250 are available at doi:10.6084/m9.figshare.13251572 and μCT dataset for cranial remains of ZIN PH 5/250 – at doi:10.6084/m9.figshare.13249718).