Introduction
A cholesteatoma is an epidermal inclusion cyst formed by keratin-producing squamous epithelium. It is characterised by hyperkeratosis and progressive accumulation of keratin debris. Cholesteatoma is histologically composed of an epithelial matrix and connective tissue (perimatrix) with associated inflammation. Clinically, it is a destructive lesion that may cause hearing loss, vestibular dysfunction, facial paralysis and terminal intracranial complications.Reference Semaan and Megerian1–Reference Bujia, Holly, Sudhoff, Antoli-Candela, Tapia and Kastenbauer3
Many classification systems have been proposed for cholesteatoma. At present, the most accepted classification system depends on aetiology, and comprises two main groups: primary (congenital) cholesteatoma and secondary (acquired) cholesteatoma.Reference Semaan and Megerian1, Reference Olszewska, Wagner, Bernal-Sprekelsen, Ebmeyer, Dazert and Hildmann4 Recent studies on the pathogenesis of acquired cholesteatoma have focussed on epithelial hyperproliferation, altered differentiation, apoptosis mechanisms and inflammation.Reference Semaan and Megerian1, Reference Bujia, Holly, Sudhoff, Antoli-Candela, Tapia and Kastenbauer3–Reference Choufani, Roper, Delbrouck, Hassid and Gabius6
In cholesteatoma, inflammation is always present as a response to tissue injury.Reference Olszewska, Wagner, Bernal-Sprekelsen, Ebmeyer, Dazert and Hildmann4 Langerhans cells have a role as antigen-presenting cells within cutaneous immune defence. They have been demonstrated to be an important prognostic factor for many cancers.Reference Gallo, Libonati, Gallina, Fini-Storchi, Giannini and Urso7 In cholesteatoma pathogenesis, Langerhans cells recognise antigens and present them to lymphocytes. These reactions activate the immune system, leading to a chronic inflammatory reaction to the cholesteatoma.Reference Gantz8
In normal tissue, there is a dynamic balance between cell proliferation and apoptosis (programmed cell death). However, this process is defective in cholesteatoma, and the resulting imbalance causes keratin accumulation.Reference Huisman, De Heer and Grote9
Monoclonal antibodies against Ki-67 protein can be used to assess the proliferative capacity of normal and pathological cells. This protein is a reliable, stable marker for proliferation within cholesteatoma.Reference Raynov, Moon, Choung, Hong and Park10 Hyperproliferation of keratinocytes has been found to be associated with more aggressive cholesteatoma.Reference Mallet, Nouwen, Lecomte-Houcke and Desaulty11
Studies of apoptosis in cholesteatoma pathogenesis have demonstrated that apoptosis plays a role in the differentiation and accumulation of keratin debris. The mechanism of and capacity for apoptosis, and the distribution of apoptotic cells within cholesteatoma layers, can predict recurrence of the disease.Reference Olszewska, Chodynicki and Chyczewski2, Reference Olszewska, Wagner, Bernal-Sprekelsen, Ebmeyer, Dazert and Hildmann4, Reference Choufani, Mahillon, Decaestecker, Lequeux, Danguy and Salmon12
This study sought to elucidate the role of Langerhans cells in cholesteatoma pathogenesis. Relationships between Langerhans cells, Ki-67 protein, apoptosis and the clinical features of cholesteatoma were investigated.
Materials and methods
This study was conducted by the departments of otolaryngology and pathology of Baskent University School of Medicine, with the support of the Baskent University School of Medicine Research Committee.
The study included 40 patients who had undergone surgery with a diagnosis of chronic otitis media with acquired cholesteatoma, at Baskent University Ankara and Adana Hospitals between November 2007 and June 2008.
The study was approved by Baskent University School of Medicine Local Ethics Committee. All patients included in the study were informed about it and gave their consent to participate in it.
The patients underwent tympanomastoidectomy surgery using an open or closed technique. The decision about whether to create an open or closed mastoid cavity was based upon the clinical and intra-operative impressions of the surgeon. In each case, disease exenteration was initially attempted via an intact canal mastoidectomy. An open mastoid cavity was created in the following instances: presence of a bony defect in the posterior canal wall; extensive disease (for any type (attic or tensa) or age (child or adult)); recurrent disease; erosion of the semicircular canal; lack of mastoid pneumatisation (e.g. sclerotic mastoids); risk to the patient's hearing status (e.g. cholesteatoma in an only-hearing ear); and poor patient compliance. Randomisation was not performed for ethical reasons. In each patient, a small piece of external auditory canal skin (approximately 2 × 4 mm) located away from the cholesteatoma was sampled as a control.
Before surgery, audiography and computed tomography (CT) of the temporal bone was undertaken for all patients.
Clinical variables
For all patients included in the study, we noted age, gender, duration of symptoms due to cholesteatoma (up to the date of surgery), and clinical status. We also recorded information on the following clinical parameters.
Degree of conductive hearing loss
Pure tone average and air–bone gap (in dB) were determined audiographically and recorded, prior to ear surgery.
Extent of cholesteatoma
The cholesteatoma status (i.e. within the tympanic cavity only, invasion towards the mastoid bone, spread to the petrous area, or outside the temporal bone) was determined for each patient from their CT and intra-operative findings. Patients were then divided into two groups based on their disease extent: group I, cholesteatoma contained within the temporal bone; and group II, disease extending beyond the temporal bone.
Severity of bone destruction
Computed tomography and intra-operative findings were used to determine: destruction of the middle-ear ossicles; destruction of the posterior wall of the external ear canal and mastoid cells; subsequent invasion of the mastoid bone border; otic capsule destruction; and invasion outside the temporal bone. During surgery, ossicular destruction was classified as follows: 1, ossicle system intact; 2, malleus destruction alone; 3, incus destruction alone; 4, malleus and incus destruction; 5, incus and stapes destruction; and 6, all ossicles destroyed. Following Mallet et al.,Reference Mallet, Nouwen, Lecomte-Houcke and Desaulty11 patients were recategorised based on the extent of ossicular destruction: either no or only single ossicle destruction, or destruction of more than one ossicle.
Aggressiveness of cholesteatoma
We recorded the presence of erosion of the facial nerve canal, loss of integrity at the tegmen mastoideum, patency of the lateral semicircular canal, erosion of bone tissue overlying the sigmoid sinus, and/or destruction of the posterior wall of the external ear canal. We then compared the pathological findings of patients with general complications of bone destruction (i.e. having any patency of the lateral semicircular canal, facial canal, tegmen or scutum) versus those without such complications.
Pathological variables
Epithelial thickness and inflammation intensity
Samples of cholesteatoma and external ear canal skin (as the control group) were obtained from patients during surgery, fixed in formalin and sent separately to the pathology department. All samples were stained with haematoxylin and eosin (H&E) and evaluated under a light microscope (Nikon, Tokyo, Japan). Cholesteatoma specimens were categorised according to their thickness (judged by viewing 10 high-power fields): either 2.5 mm or thicker, or 2.4 mm or thinner (Figure 1). Cholesteatoma samples were also categorised according to the number of inflammatory cells: diffuse inflammation was defined as the presence of inflammatory cells beneath the epithelium in every microscopic field (Figure 2a), while mild inflammation was defined as the presence of inflammatory cells in fewer than all microscopic fields (Figure 2b). In the control group samples, epithelial thickness and severity of inflammation were not taken into consideration.
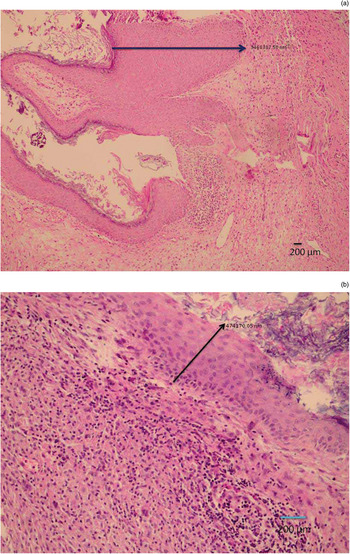
Fig. 1 Photomicrographs showing: (a) cholesteatoma epithelium thicker than 2.5 mm (arrow = 3 468 387.50 nm; and (b) cholesteatoma epithelium thinner than 2.5 mm (arrow = 474 170.05 nm). (H&E)
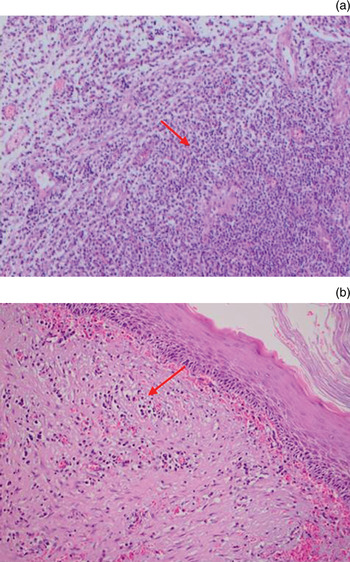
Fig. 2 Photomicrographs showing (a) dense and (b) mild inflammatory cell infiltration beneath cholesteatoma epithelium; arrows indicate inflammatory cells. (H&E; ×200)
Distribution of Langerhans cells
To investigate the distribution of Langerhans cells, all patient samples were fixed in 10 per cent formalin solution and embedded in paraffin blocks after routine preparation. Slices of 5 µm thickness were placed on slides coated with poly-l-lysine, and were then deparaffinised in an incubator at 37°C for 12 hours. The slices were placed in hydrogen peroxide for 15 minutes and thereafter rinsed with Tris-buffered saline (50 mM Tris–HCl, 150 mM NaCl, pH 7.6). They were then immersed in protein blocking solution and kept at room temperature for 10 minutes. Slides were incubated with cluster of differentiation 1a protein antibody (NeoMarkers, MS.1856, R7; Lab Vision, Fremond, California, USA) and Ki-67 protein antibody (SP6) (RM-9106-R7, NeoMarkers; Lab Vision) at room temperature for 2.5 hours to determine the presence of Langerhans cells. Slides were rinsed with Tris-buffered saline after incubation and were then incubated with biotin for 15 minutes and with streptavidin horseradish peroxidase for 15 minutes. Slides were rinsed again with Tris-buffered saline and then treated with 3-amino-9-ethylcarbazole for 15 minutes. After this process, they were background-stained with H&E then rinsed with water and covered with a coverslip (Figure 3). To create a positive control for cluster of differentiation 1a protein, a skin tissue sample was obtained and staining of intraepithelial dendritic cells evaluated. In order to evaluate Langerhans cell infiltration, the mean number of Langerhans cells stained with cluster of differentiation 1a protein antibody, out of 100 cells of all types, was determined for all stained fields, at suitable magnification (×40). Similarly, all stained fields were evaluated for Ki-67 (at ×40 magnification), and the mean number of stained epithelial cells per 100 epithelial cells was calculated. Dark nuclear staining was accepted as a positive result for Ki-67 protein (Figure 4), while cytoplasmic membrane staining was accepted as a positive result for cluster of differentiation 1a protein.
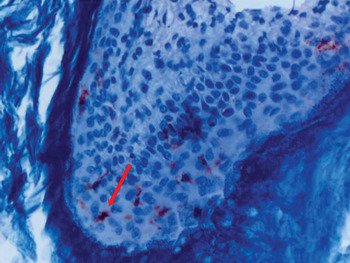
Fig. 3 Photomicrograph showing cholesteatoma epithelium stained for cluster of differentiation 1a protein (arrow). (H&E; ×400)
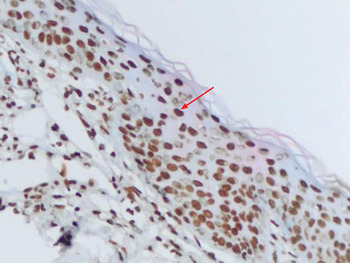
Fig. 4 Photomicrograph showing cholesteatoma epithelium stained for Ki-67 protein, demonstrating a nuclear staining pattern (arrow). (×400)
Apo2.7 protein
The presence of apoptosis was determined using antibodies against the Apo2.7 protein. This protein is located on the mitochondrial membrane and becomes detectable in the early stages of apoptosis. To detect apoptosis in our cases, paraffin blocks were treated with ApopTag antibody (ApopTag in situ assessment kit, S7101; Intergen, Purchase, New York, USA) using the terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick-end labelling (‘TUNEL’) immunohistochemical method. Under a light microscope at ×40 magnification, the mean number of Apo2.7-stained cells per 100 total cells, in all stained fields, was calculated (Figure 5). Cells with dark brown stained cytoplasm and a completely dye-stained nucleus were considered positive for Apo2.7; cells with pale and light staining were considered negative.
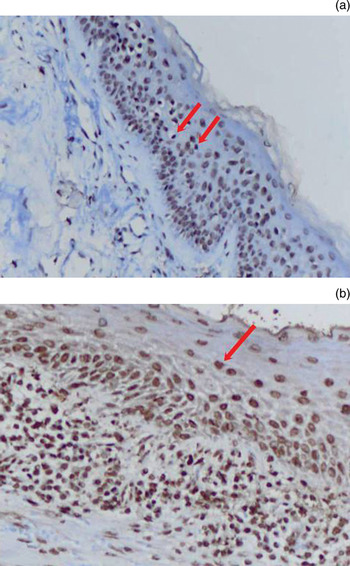
Fig. 5 Photomicrograph showing ApopTag antibody staining of Apo2.7 protein: (a) staining in cholesteatoma epithelium (arrows indicate stained cells) (×200); (b) staining of epithelial cell nuclei (arrow) (×400).
The pathologist assessing the staining distribution of Langerhans cells and Apo2.7 protein was blinded to each patient's clinical status.
For statistical assessment, the Levene test, Student's t-test (independent-samples t-test), Wilcoxon signed ranks test, Mann–Whitney U test and single directional analysis of variance test were used. A p value of less than 0.05 was regarded as statistically significant.
Results and analysis
Forty patients were included in the study, 17 (42.5 per cent) women and 23 (57.5 per cent) men. Patients' ages ranged from 7 to 62 years, with a mean ± standard deviation (SD) age of 35 ± 16 years.
The mean ± SD duration of patients' symptoms (up to the date of surgery) was 12.7 ± 10.7 years (range, 1–44 years). The mean ± SD pure tone average was 54 ± 19 dB (range, 8–90 dB) and the mean ± SD air–bone gap 31 ± 12 dB (range, 10–54 dB). On CT scan, 34 patients (85 per cent) had disease contained within the temporal bone, while 6 (15 per cent) had disease extending beyond the temporal bone.
Eleven patients (27.5 per cent) underwent surgery on the right ear and 29 (72.5 per cent) on the left ear. A closed surgical technique was used in 8 patients (20 per cent) and an open technique in 32 patients (80 per cent).
During surgery, the ossicular system was found to be intact in 2 patients (5 per cent), malleus destruction only was found in 1 patient (2.5 per cent), incus destruction only in 8 patients (20 per cent), malleus and incus destruction in 14 patients (35 per cent), incus and stapes destruction in 2 patients (5 per cent), and destruction of all ossicles in 13 patients (32.5 per cent).
When patients were recategorised based on the extent of ossicular destruction (i.e. none or only one ossicle destroyed, versus more than one ossicle destroyed),Reference Mallet, Nouwen, Lecomte-Houcke and Desaulty11 no statistically significant differences were found between the patients in the former group (n = 11, 27.5 per cent) and those in the latter group (n = 29, 72.5 per cent) as regards cluster of differentiation 1a protein scores (p = 0.567), Ki-67 index (p = 0.083) or apoptosis scores (p = 0.603).
An eroded facial canal was observed in 8 patients (20 per cent), an eroded lateral semicircular canal in 7 patients (17.5 per cent), a defect in the tegmen mastoideum in 12 patients (30 per cent) and an eroded scutum in 22 patients (55 per cent) (Table I). When patients were categorised according to the presence or absence of general complications of bone destruction (25 and 15 patients, respectively), we found no statistically significant differences between these two groups for any pathological finding (p > 0.05 for all) (Table II).
Table I Surgical findings
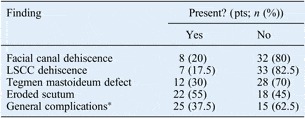
*Due to bone destruction. Pts = patients; LSCC = lateral semicircular canal
Table II Significance of difference in pathological findings, by clinical parameter: p values

All p values were calculated using Student's t-test, except *single directional analysis of variance. †p < 0.05, Student's t-test; **p < 0.05, Mann–Whitney U test (since homogeneity was not achieved in the Levene test). ‡Open versus closed technique. §Due to bone destruction. CD1a = cluster of differentiation 1a protein staining; CT = computed tomography; LSCC = lateral semicircular canal
Histopathological examination revealed that: Langerhans cells were significantly more numerous in cholesteatoma epithelium (mean ± SD, 25 ± 9 cells/field) than in normal external auditory canal skin (mean ± SD, 8 ± 5 cells/field) (p < 0.001); the Ki-67 index was significantly higher in cholesteatoma epithelium (mean ± SD, 36 ± 13 cells/field) than in external auditory canal skin (mean ± SD, 13 ± 8 cells/field) (p < 0.001); and apoptosis was significantly more prominent in cholesteatoma epithelium (mean ± SD, 39 ± 12 cells/field) than in external auditory canal skin (mean ± SD, 15 ± 10 cells/field) (p < 0.001) (Table III).
Table III Langerhans cells, Ki-67 and Apo2.7 results in EAC skin and cholesteatoma epithelium
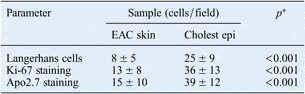
Results represent means ± standard deviations unless otherwise indicated. *p < 0.05, Wilcoxon signed ranks test (as homogeneity was not achieved with Levene test). EAC = external auditory canal; Cholest epi = cholesteatoma epithelium
We compared patients with diffuse versus mild inflammation intensity, and with thicker versus thinner epithelial thickness, as regards Langerhans cells, Ki-67 protein and Apo2.7 protein results. We found that patients with diffuse inflammation had statistically significantly higher Ki-67 results, compared with patients with mild inflammation (p = 0.001).
Table II shows the significance of cluster of differentiation 1a protein, Ki-67 protein and Apo2.7 protein results, for each of the clinical parameters assessed. We observed significant differences for both Ki-67 protein (p = 0.046) and ApopTag results (p = 0.037), comparing patients undergoing open versus closed technique surgery.
Discussion
The immune system is known to play an important role in the pathology of cholesteatoma. The role of the perimatrix layer in the immune response has already been shown, and the role of the cholesteatoma epithelium in this process has been investigated in studies of Langerhans cells located in the epidermis.Reference Ottaviani, Neglia and Berti13, Reference Sudhoff, Bujia, Holly, Kim and Fisseler-Eckhoff14
Gantz studied the presence of Langerhans cells in the normal tympanic membrane, external ear canal skin and cholesteatoma matrix.Reference Gantz8 Gantz reported that cholesteatoma matrix displayed more Langerhans cells than either external ear canal skin or tympanic membrane. The Langerhans cell population was observed to be more intensely developed in cholesteatoma epithelium than in normal tissue, and this was concluded to be a consequence of a chronic inflammatory process. Takahashi and Nakano also reported more Langerhans cell involvement in samples of chronic otitis with cholesteatoma and otorrhoea, compared with samples from patients without otorrhoea.Reference Takahashi and Nakano15 Based on this result, they argued that Langerhans cells accounted for the chronic inflammatory process seen in cholesteatoma. Kamide et al. studied the effect of Langerhans cells on keratinocytes in vitro, and concluded that Langerhans cells prompted proliferation and differentiation of keratinocytes.Reference Kamide, Sasaki, Abramson and Huang16
Inflammation is a normal immune response to tissue injury. Cytokines and growth factors are secreted from immune cells located within the inflammatory tissue accompanying cholesteatoma, and account for the increased proliferation of cholesteatoma epithelium.Reference Ottaviani, Neglia and Berti13 Previously reported studies have shown that Langerhans cells play an active role in the establishment of the cell-mediated immune response seen in cholesteatoma, and also in the proliferation and differentiation of keratinocytes.Reference Kamide, Sasaki, Abramson and Huang16 Therefore, it can be assumed that increased numbers of Langerhans cells might correlate with increased epithelial proliferation and hence with more aggressive cholesteatoma types. However, in our study we did not observe any difference between Langerhans cell numbers in cholesteatoma samples with and without intense inflammation. Moreover, increased numbers of Langerhans cells were not found in cholesteatoma samples showing clinically more aggressive properties. However, cholesteatoma epithelium samples showed increased Langerhans cell numbers compared with controls (normal external ear canal skin). In conclusion, it is thought that Langerhans cell numbers increase in cholesteatoma, but that this has no clinical significance.
In earlier studies using antibodies against Ki-67 protein, it has been shown that cholesteatoma epithelium has higher proliferation rates than external ear canal skin.Reference Bujia, Holly, Sudhoff, Antoli-Candela, Tapia and Kastenbauer3, Reference Sudhoff, Bujia, Fisseler-Eckhoff, Schulz-Flake, Holly and Hildmann17 In a study conducted to examine hyperproliferative features of cholesteatoma epithelium, Olszewska et al. found greater release of Ki-67 in cholesteatoma epithelium than in normal skin.Reference Olszewska, Chodynicki, Chyczewski and Rogowski18 They demonstrated a high level of proliferative activity in cholesteatoma epithelium, particularly in the basal and suprabasal layers; however, this was unrelated to epithelial thickness. In our study, we did not find any relationship between epithelial thickness and cell proliferation, supporting Olszewska and colleagues' findings.Reference Olszewska, Chodynicki, Chyczewski and Rogowski18
Mallet et al. studied the correlation between cholesteatoma aggressiveness and features of epithelial hyperproliferation.Reference Mallet, Nouwen, Lecomte-Houcke and Desaulty11 In that study, patients were divided into two groups on the basis of ossicular destruction. The first group included patients with single bone destruction, while the second group included patients with destruction of two or more bones, facial nerve canal erosion due to cholesteatoma, loss of integrity in the tegmen mastoideum, lateral semicircular canal dehiscence, or erosion of bone tissue overlying the sigmoid sinus. Active proliferation was higher in the second group compared with the first group. It was also reported that, in samples with high levels of active proliferation, inflammation was also more intense.Reference Mallet, Nouwen, Lecomte-Houcke and Desaulty11
Like Mallet et al., we also classified our patients into two groups: those with general complications of bone destruction (including dehiscence of the lateral semicircular canal, facial canal, tegmen or scutum) and those without such complications. When we compared the pathological findings of these groups (i.e. Langerhans cell numbers, Ki-67 and Apo2.7 protein staining, inflammation intensity, and epithelial thickness), we found no statistically significant differences. We then recategorised patients according to the number of ossicles destroyed: either none or one, or more than one. When we compared the staining of cluster of differentiation 1a, Ki-67 and Apo2.7 proteins in these two new groups, we also found no statistically significant differences.
When the pathological parameters of inflammation intensity and epithelial thickness were compared with Langerhans cell numbers and Ki-67 and Apo2.7 protein staining, it was found that Ki-67 staining was statistically significantly greater in cholesteatoma samples with more intense inflammation. This result is consistent with similar studies, and supports the thesis that increased inflammation is responsible for cell proliferation. More research in needed to elucidate the relationship between inflammation and cell proliferation, as regards inflammatory regulators of cell proliferation.
In cholesteatoma, the dead cells generated during keratinocyte differentiation accumulate as keratin debris. Compared with the external ear canal skin, increased cell death among keratinocytes is the reason for the accumulation of such keratin debris. This process results from terminal differentiation of keratinocytes and is explained by apoptosis.Reference Olszewska, Wagner, Bernal-Sprekelsen, Ebmeyer, Dazert and Hildmann4 Studies of apoptosis in cholesteatoma support the theory that apoptosis increases in cholesteatoma, and that the accumulation of keratin debris is due to increased terminal differentiation.Reference Olszewska, Chodynicki and Chyczewski2, Reference Huisman, De Heer and Grote9
• In cholesteatoma, Langerhans cells recognise antigens and present them to lymphocytes
• This study found denser Langerhans cell infiltration in cholesteatoma epithelium versus external ear canal skin
• Indicators of cholesteatoma proliferation and apoptosis were higher in patients receiving open versus closed surgery
• Open surgery is thus appropriate where clinical severity correlates with histological evidence of cholesteatoma
In previous studies of cholesteatoma, parameters such as Ki-67 protein have been used to investigate the proliferative process, and the rate of epithelial apoptosis has been accepted to reflect this hyperproliferative process.Reference Olszewska, Chodynicki and Chyczewski2 In contrast, our study assessed parameters relating both to proliferation and to apoptosis, in an attempt to determine the association between these processes and the link with clinical parameters. Although we found increased cell proliferation and apoptosis rates in cholesteatoma epithelium, we observed no relationship between increased epithelial thickness, inflammation and apoptosis. In our study, the only statistically significant relationship between histological and clinical parameters was that patients who underwent open-technique surgery had greater Ki-67 protein staining (p = 0.046) and Apo2.7 staining (p = 0.037) than those undergoing closed-technique surgery. Since the choice of surgical technique was related to the level of clinical aggression of the patient's cholesteatoma, this finding indicates that our choices of surgical technique were appropriate.
Conclusion
This study found that middle-ear cholesteatomas were associated with intense Langerhans cell infiltration, and this response was confirmed by results for Ki-67 and Apo2.7 protein staining, both of which are accepted, indirect indicators of bone destruction. The open technique of surgery was the treatment of choice for patients in whom clinical severity correlated with histological evidence of cholesteatoma.










