Introduction
The association of congenital neurosensory auditory loss with an enlarged vestibular aqueduct is known as ‘large or wide vestibular aqueduct syndrome’. This syndrome was first described by Valvassori and Clemis in 1978.Reference Valvassori and Clemis1 This portion of the inner ear is the non-sensory part of the labyrinth. The non-sensory components of the labyrinthReference Mafee, Charletta, Kumar and Belmont2 are the endolymphatic duct and the endolymphatic sacReference Lo, Daniels, Chakeres, Linthicum, Ulmer, Mark and Swartz3 contained in the vestibular aqueduct.Reference Mafee, Charletta, Kumar and Belmont2 The functions of the vestibular aqueduct have not yet been demonstrated or completely clarified in humans, although certain functions have been widely demonstrated in animals.
The vestibular aqueduct is radiologically visualised by computed tomography (CT) scanning (Figures 1 and 2).Reference Mafee, Charletta, Kumar and Belmont2–Reference Levenson, Parisier, Jacobs and Edelstein4 However, CT scans do not provide visual definition of the ultrastructure of the membranous content of the osseous canal. In contrast, MRI protocol included axial T2-weighted fast-spin-echo sequence without and with gadolinium highlights not the osseous structure of the vestibular aqueduct but its content, i.e. the duct and the endolymphatic sac; in addition, it may magnify anomalies of the liquor spaces related to labyrinth structures and the normal portion of the endolymphatic duct and sac.Reference Mafee, Charletta, Kumar and Belmont2, Reference Oehler, Chakeres and Schmalbrock5, Reference Casselman, Majoor and Albers6

Fig. 1 Computed tomography scan of the left inner ear in case 6. The enlarged vestibular aqueduct is demonstrated (white arrow).

Fig. 2 Computed tomography scan of the right inner ear in case 2. The enlarged vestibular aqueduct is demonstrated (white arrow).
An increase in the volume of the vestibular aqueduct and endolymphatic sac not only causes structural anomalies of the inner ear but also anomalies of the auditory and vestibular physiology and mechanisms. In patients with this anatomical alteration, the signs and symptoms of auditory problems are well known, and are better represented in the literature than the signs and symptoms of vestibular complaints. To our knowledge, few descriptions of such vestibular signs and symptoms appear in the literature; existing reports range from mild instability to real, episodic vertigo.Reference Yetiser, Kertment and Ozkaptan7, Reference Ishida, Sugiura, Nakashima, Naganawa, Sato and Sugiura8
This prospective study of a case series in a tertiary care referral centre endeavoured to evaluate and to highlight a pattern of signs and symptoms which could be useful in the investigation of vestibular function in adult patients with anatomical alterations of the vestibular aqueduct and endolymphatic sac. The study tested the hypothesis that a combination of bedside and vestibular tests could detect such signs and symptoms. The aim of the study was to find a possible marker of such inner-ear pathology.
Materials and methods
The 15 subjects included in the study comprised nine women and six men, ranging in age from 21 to 68 years (mean age, 39.1 ± 6.25 years). All 15 patients had been referred to a tertiary otoneurological centre, with vestibular symptoms, between 1 January 2004 and 30 June 2006. Accurate case histories were recorded in all cases (Table I).
All symptoms were evaluated using a standardised set of tests, including the following performed at the bedside with a three-dimensional infrared video oculography. (50Hz sampling; Torsio VNG Ulmer; Synapsys, Marseille, France): observation and evaluation of spontaneous nystagmus and head-shaking nystagmus; tests to detect positional nystagmus (Dix-Hallpike and head-rolling test); and observation of nystagmus after the Valsalva manoeuvre, after hyperventilation, after exposure to 110 dB sound stimulation at a frequency of 3 KHz (to detect so-called Tullio's phenomenon) and after mastoid vibration at 100 Hz.
All patients then underwent a specific set of audiovestibular tests: caloric tests (Fitzgerald–Hallpike stimulation); rotational chair tests (according to a previously described protocol);Reference Arriaga, Chen and Cenci9 audiometry; stapedial reflex testing; and auditory brainstem response (ABR) and vestibular evoked myogenic potential testing to evaluate the amplitude, latency and threshold of the primary biphasic complex.
After this thorough otoneurological assessment, patients underwent CT scanning of the temporal bone (with 0.3-mm contiguous sections also in the plane of the superior semicircular canal) and MRI scanning (T2 height-weighted fast spin echo) of the labyrinth and cerebello-pontine angle, in order to allow accurate evaluation of the middle ear, labyrinthine capsule and internal auditory canals.
The criteria used to classify a vestibular aqueduct as large have been defined elsewhere, and have led to a number of controversies. For the present study, the result of a review of six existing studies, which established a normal range of 1.5 to 2.00 mm for the opening of a non-sensory labyrinthine structure, was taken as an appropriate cut-off value for abnormality.Reference Arriaga, Chen and Cenci9
All patients' records, audiometric test results and vestibular evoked myogenic potential responses were reviewed in order to classify and identify anomalies associated with the disorder in question. None of the patients considered eligible for inclusion showed signs or symptoms of intracranial hypertension or symptoms of papilloedema.
Results
The enlarged vestibular aqueduct was unilateral in six patients (40 per cent) and bilateral in nine (60 per cent). The symptoms reported by some patients during anamnesis are reported in Table I. The two most striking and frequent symptoms were migraine-related vertigo and motion sickness.
Table I Summary of Patients' Symptoms

No = number; yr = year; MRV = migraine-related vertigo; MS = motion sickness; F = female; M = male; L = left; Bi = bilateral; R = right
The term ‘migraine’ refers both to a syndrome and a disorder. Migraine symptoms include a neurological aura associated with headache or a mixture of symptoms, including unilateral, disabling, throbbing pain associated with sensitivity to noise and light or with nausea.Reference Su, Huang, Young and Cheng10, 11 In addition to these defining features, the study patients often described a variety of associated symptoms. One of the most common symptoms accompanying migraine was dizziness or instability. Dizziness has been reported to occur in 28–30 per cent, and vertigo to occur in 25–26 per cent, of patients with a primary complaint of migraine.Reference Zalzal, Tomaski, Vezina, Bjornsti and Grundfast12, Reference Gupta13
The bedside clinical signs elicited are reported in Table II. None of the patients had a history of otological disorders or pathology. In five patients (33.3 per cent), the symptoms had appeared after concussion. Routine biochemical analysis revealed autoimmune disease (i.e. elevated antinuclear antibodies) in one patient (case 12). Nine patients (60 per cent) had a normal response to the caloric test, while four (26.6 per cent) showed unilateral vestibular paresis and two (13.3 per cent) showed bilateral hyporeflexivity. Rotatory test results showed an elevated visually enhanced vestibulo-ocular reflex (VVOR) gain in 11 cases (73.3 per cent).
Table II Clinical data for Patients with Volumetric Abnormalities of Vestibular Aqueduct
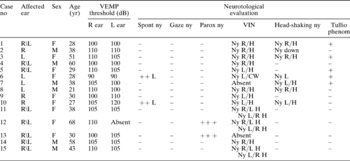
No = number; yr = year; VEMP = vestibular evoked myogenic potential; R = right; L = left; spont = spontaneous; ny = nystagmus; gaze = gaze-evoked nystagmus; paross = paroxysmal; VIN = vibratory induced nystagmus (100 Hz); phenom = phenomenon; F = female; M = male; R/H = nystagmus with fast component to right + horizontal; L/H = nystagmus with fast component to left + horizontal; L/CW = nystagmus with fast component to left + clockwise; R/L H = nystagmus evoked from right mastoid, with fast component to left + horizontal; L/R H = nystagmus evoked from left mastoid, with fast component to right + horizontal
All four patients who complained of Tullio's phenomenon during audiometric testing showed a transmission type deficit of low tones, whereas six patients (40 per cent) had neurosensory auditory loss, especially in the high frequencies.
Table II also shows patients' vestibular evoked myogenic potential results, in terms of the threshold of the early biphasic complex. Significantly, amplitude was normal in all patients. Thyroid function was altered in only two patients (cases 12 and 15), both of whom had autoimmune thyroid disease. Magnetic resonance imaging, with and without intravenous gadolinium, was performed in all patients and was normal, thus excluding intracranial pathology and specific diseases of the central nervous system. Dilation of the cerebral ventricles was not found in any case. Computed tomography, with programmed reconstructions in the axial, coronal and parasagittal planes, was performed in all patients in order to visualise the labyrinthine capsule and the non-sensory portion of the labyrinth, and demonstrated increased volume in all cases. Superior semicircular canal dehiscence and perilymphatic fistula were not found in any patient.
Discussion
Recently, increasing attention has been focused on investigating aspects of the vestibular system, partly due to the contributions of imaging (CT and MRI) and new methods, specifically bedside study of static signs induced by physical manoeuvres or by low-cost instruments (such as vibratory stimulation to evoke nystagmus).Reference Lo, Daniels, Chakeres, Linthicum, Ulmer, Mark and Swartz3, Reference Oehler, Chakeres and Schmalbrock5, Reference Casselman, Majoor and Albers6 Many new observations are also establishing connections between vestibular signs and symptoms and dysmorphisms or alterations of the labyrinthine capsule. A major contribution to knowledge of vestibular phenomena has been the description of dehiscence of the superior semicircular canal by Minor et al. Reference Gupta13 It has also been interesting to observe the efforts made to define much-debated clinical conditions, such as migraine-related vertigo, over the last 20 years.
The purpose of this study was to search for a possible marker of inner-ear pathology, in patients treated in a tertiary otoneurology referral centre. This clinical series was interesting because it sheds light on clinical aspects, such as signs useful for diagnosis, while also introducing new points for discussion.
The results of the critical examination of these patients raise some interesting possibilities. Taken together, the records of patients' symptoms suggest that certain symptoms may be considered expressions of anatomical alteration (i.e. volumetric increase, lengthening and dehiscence from nearby structures (Figure 3) of the vestibular aqueduct and endolymphatic sac). The most significant finding was that, according to the International Headache Society diagnostic criteria (Table III),11 most patients (12 of 15, 80 per cent) in the present study had definite symptoms of migraine-related vertigo. This is even more significant when related to the fact that most patients (12 of 15, 80 per cent) had a history of motion sickness. A possible explanation of motion sickness and consequently of migraine-related vertigohasrecentlybeen formulated.Reference Gupta13

Fig. 3 Computed tomography scan of the left inner ear in case 11. The enlarged vestibular aqueduct dehiscent with the giugular gulf is demonstrated (white arrow).
Table III International Headache Society diagnostic definition and criteria for migraine

The other interesting result was the finding of oscillopsia in nine out of 15 (60 per cent) patients and dizziness (postural instability) in 14 out of 15 (93.3 per cent). Oscillopsia often affects patients with alterations of the labyrinthine capsuleReference Minor, Solomon, Zimreich and Zee14, Reference Karlberg, Aw, Black, Todd, MacDougall and Halmagyi15 as well as anatomical alterations of the vestibular aqueduct and endolymphatic sac.
Finally, it is worth mentioning that atypical paroxysmal positional vertigo was found in two patients (cases 12 and 13).
In one patient (case 12) (Figure 4), the absence of a vestibular evoked myogenic potential on the affected side can be explained by the evocation method used (air vestibular evoked myogenic potential), which obviously failed to evoke a primary biphasic complex in this case, possibly due to the patient's age.Reference Su, Huang, Young and Cheng16

Fig. 4 Computed tomography scan of the left inner ear in case 12. The enlarged vestibular aqueduct is demonstrated (white arrow).
The pathophysiological mechanism responsible for vestibular dysfunction in these patients therefore seems to be threefold.Reference Couloigner, Teixeira, Sterkers, Rask-Andersen and Ferrary17 Firstly, there is the role of the endolymphatic sac in maintaining correct homoeostasis of the cochleovestibular endolymph. Indeed, it seems that the non-sensory part of the labyrinth responds to plasma hyperosmolarity in order to maintain osmolar endolymphatic equilibrium. Secondly, there is the role played by the endolymphatic duct and sac as immunodefenders of the inner ear.Reference Couloigner, Teixeira, Sterkers, Rask-Andersen and Ferrary17, Reference Tomiyama and Harris18 Thirdly, there is the role of the endolymphatic duct and sac in eliminating material contaminating the endolymph. This material may include degradation products of erythrocytes, plasmaReference Jansson and Rask-Andersen19 and otoconia.Reference Yamane, Imoto, Nakai, Igasashi and Rask-Andersen20
Dysfunction of the endolymphatic duct and sac seems to contribute to vestibular damage in patients with volumetric abnormalities of the vestibular aqueduct. This occurs partly via an increase in the susceptibility of the anatomical formations involved in transmitting variations in cerebrospinal fluid pressure waves (i.e. the ‘third mobile window’) towards the labyrinth, a mechanism also involved in other pathologies of the labyrinthine capsule.Reference Minor, Solomon, Zimreich and Zee14 Another theory suggests that hyperosmolar proteins in the endolymphatic sac flow back to the labyrinth through a defective endolymphatic duct.
A series of aetiological factors may affect these dysfunctions. As highlighted in cases one, six, seven and nine, post-traumatic damage may be involved.Reference Jackler and De La Cruz21, Reference Belenky, Madgy, Leider, Becker and Hotaling22 However, the possibility of secondary damage due to viral infection was also considered, as shown in cases two, four and five.Reference Gianoli, Goebel, Mowry and Poomipannit23, Reference Gacek24 In these patients, the actual damage to the labyrinth could be secondary to a sudden prejudicial event affecting the neuro-labyrinthine structures.
The finding of a lowered vestibular evoked myogenic potential threshold in the present study group is also significant, constituting a sign in patients with balance problems and structural defects of the labyrinthine capsule. Indeed, it has been hypothesised and demonstrated that this inner-ear clinical finding indicates an alteration of the labyrinthine capsule and is definitely responsible for a mechanism described as third mobile window.Reference Gupta13
In other words, non-physiological stimulation, such as sound or pressure, applied to the vestibular portion of the inner ear, may activate the vestibular system in an inappropriate way. Tullio's phenomenon is historically associated with different diseases of the inner ear. However, since 1998, and with the discovery of a third mobile window through the membranous labyrinth, it has become evident that transmission of sound or vibratory waves themselves may modify endolymphatic dynamics, producing a sensation of vertigo.
During otoneurological evaluation, diagnosis is facilitated by the data obtained, especially the results of vestibular evoked myogenic potential measurement and 100 Hz mastoid vibration testing (a method of evoking nystagmus recently included among routine bedside tests). Results for the former test were considerably modified in 13 out of 15 cases (86.6 per cent). A lowered threshold was identified in the affected ear in 21 out of 24 cases (87.5 per cent of ears). This finding is in line with reports in the literature.Reference Sheykholeslami, Schmerber, Habiby Kermany and Kaga25
Such clinical tests, included in the bedside examination, have increased the otoneurologist's diagnostic sensitivity. When interpreted correctly, certain clinical signs in this group of patients suggest peripheral alteration of the vestibular system. The vibratory test is a particularly sensitive nystagmus detection technique. It has recently been introduced into the bedside otoneurological examination. In the present patient group, it succeeded in evoking nystagmus in 10 out of 15 cases (66.6 per cent). This proportion suggests facilitation of vibratory stimulation to the sensory structures of the inner ear (especially the utricle and sacculus).Reference Karlberg, Aw, Black, Todd, MacDougall and Halmagyi15
Patients with right-sided and left-sided enlarged vestibular aqueduct (cases one, four, five and 14) had right-sided horizontal nystagmus in response to vibration. This is explained by a unilateral left vestibular deficit, defined by caloric testing.
Significantly, in the head-shaking test, seven out of 15 patients (66.6 per cent) showed an increase in the volume of the vestibular aqueduct and endolymphatic sac. In cases six and 10, spontaneous horizontal nystagmus to the left was found at the first evaluation. It is conceivable that this was related to sudden, uncompensated right vestibular loss.
• The association of congenital neurosensory auditory loss with an enlarged vestibular aqueduct is known as ‘large or wide vestibular aqueduct syndrome’
• An increase in the volume of the vestibular aqueduct and endolymphatic sac not only causes structural anomalies in the inner ear but also anomalies of the auditory and vestibular physiology and mechanisms
• The following symptoms can suggest dysfunction of the vestibular aqueduct: instability or recurring oscillopsia, a history of migraine-related vertigo or motion sickness, and nystagmus induced by the mastoid vibration and head-shaking tests
The audiometric profiles of the patients give rise to some speculation. Not all patients showed significant auditory loss (eight of 15; 53.3 per cent). This suggests that, even with normoacusis, alteration of the vestibular aqueduct is possible. In these cases, labyrinthine damage had presumably not yet affected the cochlea. The clinical course may clarify the moment when this damage manifests.
Caloric testing was performed in all patients. Seven patients (46.6 per cent) showed normal results, six (40 per cent) showed unilateral vestibular paresis and two (13.3 per cent) showed bilateral hyporeflexivity. Unilateral vestibular paresis is defined as more than 25 per cent asymmetry between right- and left-sided responses.Reference Honrubia and Herdman26 Pursuit, saccades and the optokinetic nystagmus (OKN) test were normal in all subjects.
Rotational chair tests showed a significant modified VVOR gain, according to a previous study.Reference Arriaga, Chen, Hillmann, Kunschner and Arriaga27
Conclusions
These study findings show that dysfunction of the vestibular aqueduct can be suggested by symptoms and signs characterised by: instability or recurring oscillopsia; lowered vestibular evoked myogenic potential threshold; hypoacusis; a history of migraine-related vertigo or motion sickness; nystagmus induced by mastoid vibration and head-shaking tests; and VVOR gain elevation on rotational chair testing. The present clinical series indicates that CT and MRI scanning can confirm the clinical evidence of volumetric increase of the non-sensory portion of the labyrinth, vestibular aqueduct and endolymphatic sac.
The main functions attributed to the aqueduct and sac are endolymph homoeostasis, immune defence of the inner ear and elimination of endolymph debris.Reference Couloigner, Teixeira, Sterkers, Rask-Andersen and Ferrary17–Reference Yamane, Imoto, Nakai, Igasashi and Rask-Andersen20 The mechanisms and regulation of these tasks have now been amply demonstrated in laboratory animals, although they remain controversial in humans. However, there appear to be two basic mechanisms which reflect dysfunction of the vestibular aqueduct and endolymphatic sac: excess secretion or defective elimination of osmotically active components of the endolymph;Reference Arriaga, Chen, Hillmann, Kunschner and Arriaga27–Reference Thalmann and Thalmann29 and decreased resorption of endolymph due to impaired epithelial ultrastructure of the aqueduct and endolymphatic sac (as seen in the patient with autoimmune pathology (case 12)). These two mechanisms seem to underlie most of the vestibular alterations observed in the current patients.
Concussion was another event provoking dysfunction of the aqueduct and endolymphatic sac (as seen in cases one, six, seven and nine). Thyroid dysfunction, which is often involved in alterations of the labyrinthine capsule and which correlates with a volumetric increase in the vestibular aqueduct, was only highlighted in cases 12 and 15. This haemochemical alteration is therefore unlikely to be the only element responsible for the structural anomaly.
Further anatomical and laboratory studies are necessary in order to further clarify the mechanism of dysfunction of the vestibular apparatus in patients with dysmorphism of the non-sensory portion of the labyrinth.









