The Devonian is a key period for the morphological evolution of the tracheophytes, particularly with the advent of the earliest arborescent taxa in the Cladoxylopsida, Lycopsida and lignophytes (Taylor et al. Reference Taylor, Taylor and Krings2009). Amongst the latter, the progymnosperm Archaeopteris/Callixylon has received much attention from palaeobotanists as the main component of most Late Devonian forests (Beck Reference Beck1964; DiMichele & Hook Reference DiMichele, Hook, Behrensmeyer, Damuth, DiMichele, Potts, Sues and Wing1992; Meyer-Berthaud et al. Reference Meyer-Berthaud, Soria, Decombeix, Vecoli, Clément and Meyer-Berthaud2010).
Zalessky (Reference Zalessky1911) established the genus Callixylon based on anatomically preserved stems from a Famennian locality of the Donetz Basin in the Ukraine and outlined the originality of its wood anatomy. It was to be almost 50 years before Beck (Reference Beck1960) discovered the connection between a conifer-like axis of Callixylon zalesskyi Arnold and a leafy branch of Archaeopteris (probably A. macilenta Lesquereux), a free-sporing genus erected by Dawson (Reference Dawson1871). This unique combination of characters led to the recognition of the progymnosperms, an extinct group of lignophytes containing the seed plant ancestor (Taylor et al. Reference Taylor, Taylor and Krings2009).
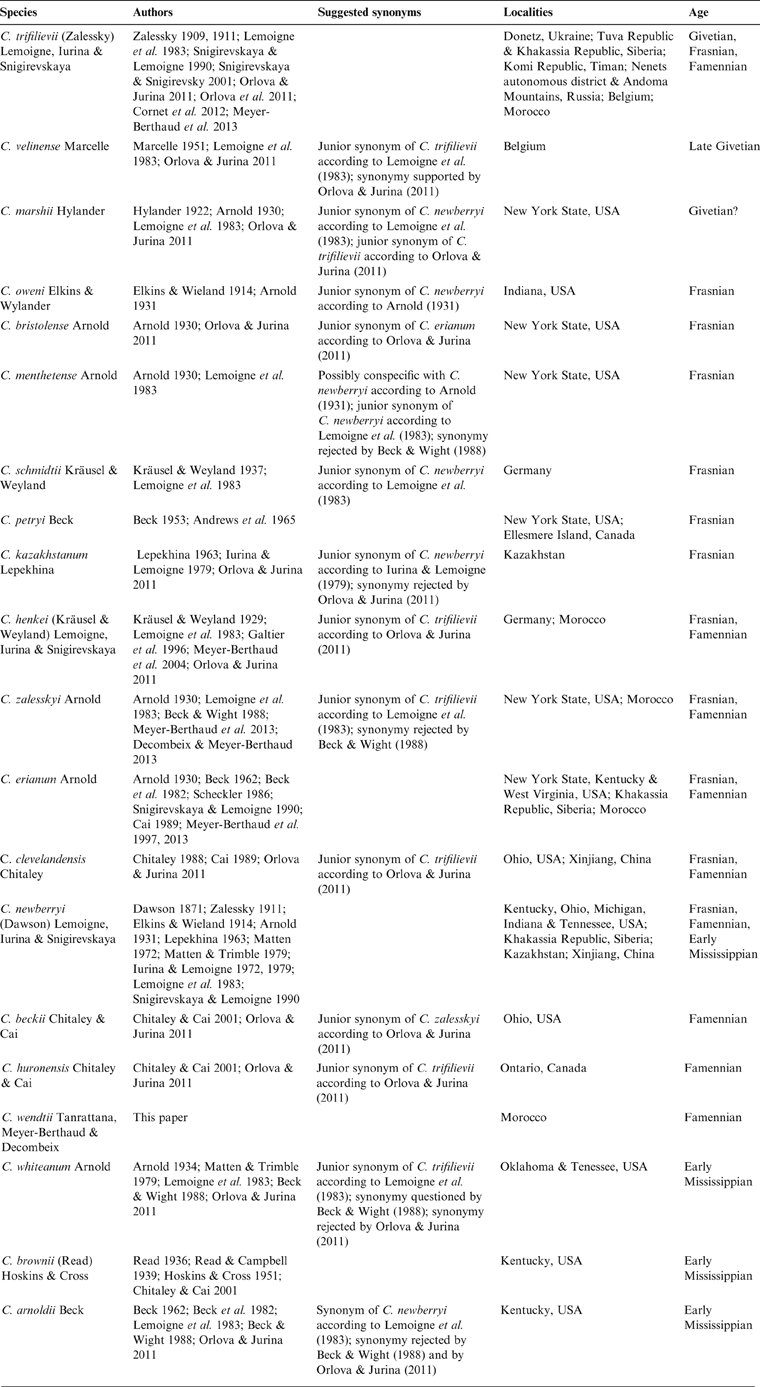
Many reports of Callixylon followed Zalessky's work, starting in 1914 when Elkins and Wieland transferred Dadoxylon newberryi Dawson from the Famennian black shales of Ohio to the new genus, and described a new species, Callixylon oweni, from a deposit of similar age in Indiana (Dawson Reference Dawson1871; Penhallow Reference Penhallow1900; Elkins & Wieland Reference Elkins and Wieland1914). During the next 100 years, no less than 16 additional species were described from late Middle Devonian to early Mississippian localities of North America, Europe, Xinjiang and several parts of Russia (Table 1), and three major reviews of the genus were conducted (Arnold Reference Arnold1930; Lemoigne et al. Reference Lemoigne, Iurina and Snigirevskaya1983; Orlova & Jurina Reference Orlova and Jurina2011). Arnold (Reference Arnold1930) increased the number of Callixylon species from four to eight, all but including C. trifilievii recorded from the eastern USA. Lemoigne et al. (Reference Lemoigne, Iurina and Snigirevskaya1983) provided the first diagnosis of the genus and of its type species C. trifilievii. They recognised seven legitimate species and synonymised seven others, a systematic treatment that was later disputed by both Beck & Wight (Reference Beck, Wight and Beck1988) and Orlova & Jurina (Reference Orlova and Jurina2011) (Table 1). Indeed, two factors, at least, hamper a satisfactory distinction of the species. One is that most Callixylon species have been mainly defined from characteristics of the wood, without any detailed information on the primary vascular architecture, or on other tissues like the pith and bark. The other is that the intra- and inter-individual variability of these characteristics has been rarely analysed in past studies. Orlova & Jurina (Reference Orlova and Jurina2011) acknowledged the artificial character of the specific divisions in Callixylon. They validated nine species and proposed organising them within four morphotaxa, the Trifilievii, Erianum, Newberryi and Arnoldii groups being separated from each other by wood characteristics such as ray size (narrow/wide), ray composition (with/without ray tracheids), radial pit structure (with/without circular apertures) and radial pit arrangement (uni/multiseriate).
Table 1 Occurrence, age and suggested synonymies of all Callixylon species described to date, including C. wendtii.
Whilst Gondwana was the largest landmass in the Devonian, its floras are still poorly known. Archaeopteridaleans from Gondwana are mostly represented by remains of Archaeopteris from South Africa, South America and Australia (Dun Reference Dun1897; White Reference White, Ulbrich and Rocha Campos1988; Anderson et al. Reference Anderson, Hiller and Gess1995; Berry et al. Reference Berry, Morel, Mojica and Villarroel2000; Hammond Reference Hammond2004; Moreno-Sanchez Reference Moreno-Sanchez2004). Apart from one specimen under investigation from the Famennian locality of Barraba (New South Wales, Australia), all the Gondwanan specimens of Callixylon published to date come from Late Devonian localities of Anti-Atlas, Morocco. The first report of the genus in this area documented a trunk occurring just above the Frasnian–Famennian boundary at El Atrous (Galtier et al. Reference Galtier, Paris and El Aouad-Debbaj1996). This trunk, referred to as Callixylon sp., showed affinities with the species C. henkei from Germany and C. velinense from Belgium. These similarities were taken as evidence for the proximity of Laurussia and Gondwana during the Late Devonian. Subsequent investigations led to the discovery of early Frasnian specimens in the Dra Valley. They represent the oldest occurrence of the genus in Gondwana (Meyer-Berthaud et al. Reference Meyer-Berthaud, Rücklin, Soria, Belka and Lardeux2004). The richest assemblage of Callixylon from Anti-Atlas was recorded in the Late Devonian locality of Mader el Mrakib, which yielded remains of trunks, branches and roots assignable to three of Orlova & Jurina's (Reference Orlova and Jurina2011) morphotaxa, the Trifilievii, Erianum and Newberryi groups. These specimens provided new information on secondary phloem and branching patterns in stems and roots (Meyer-Berthaud et al. Reference Meyer-Berthaud, Wendt and Galtier1997, Reference Meyer-Berthaud, Scheckler and Bousquet2000, Reference Meyer-Berthaud, Decombeix and Ermacora2013; Decombeix & Meyer-Berthaud Reference Decombeix and Meyer-Berthaud2013).
The aim of this paper is to describe a new species of Callixylon from two specimens collected at Mader el Mrakib and provide information on the variability of its anatomical characters. These specimens, which correspond to aerial axes (probably two orders of branches), are characterised by the presence of sclerotic nests in the pith, a character that has never been described previously in the genus.
1. Geological setting and stratigraphy
The two specimens described here were found at Mader el Mrakib, on the northwest slope of Jebel el Mrakib in the eastern part of Anti-Atlas, southern Morocco (Fig. 1A, B). This plant locality, which was discovered by Professor J. Wendt in the 1990s, yielded the 5 m-long trunk of Callixylon erianum reported by Meyer-Berthaud et al. (Reference Meyer-Berthaud, Wendt and Galtier1997, Reference Meyer-Berthaud, Scheckler and Bousquet2000). In addition to numerous stems and roots assignable to Callixylon, this locality also yielded a new species of the cladoxylopsid genus Pietzschia, P. levis (Soria et al. Reference Soria, Meyer-Berthaud and Scheckler2001; Soria & Meyer-Berthaud Reference Soria and Meyer-Berthaud2004) and a few remains of lycopsid axes. The plant locality occurs on the border of the Mader Basin (Fig. 1B), in the upper part of a marine facies of dark shales interbedded with black limestones and concretions. The associated fauna indicates an early Famennian age (Upper crepida conodont Zone) (Fig. 1C). For further information on the geological context and stratigraphy, see Meyer-Berthaud et al. (Reference Meyer-Berthaud, Wendt and Galtier1997).

Figure 1 (A) Map of Morocco showing location of eastern Anti-Atlas (in box). (B) Palaeogeography of eastern Anti-Atlas. (C) Givetian to lower Famennian section at Mader el Mrakib. Dark grey=Mesozoic to Quaternary deposits; star=Callixylon locality. Modified from Meyer-Berthaud et al. Reference Meyer-Berthaud, Wendt and Galtier1997.
Three transverse, three tangential and four radial thin-sections (at different levels) were prepared from specimen MD106 and two transverse, five tangential and two radial sections from specimen 600/2/3. Observations and photography were conducted with Sony XCD-U100CR digital cameras attached to an Olympus SZX12 stereomicroscope and to an Olympus BX51 compound microscope. Images were captured using Archimed software (Microvision Instruments) and plates were composed with Adobe Photoshop CS5 version 12.0 (Adobe Systems Inc.). Cell and tissues measurements were made with ImageJ version 1.45 (Schneider et al. 2012).
Specimen MD106 is deposited in the AMAP Research Unit, Palaeobotany Collections, University of Montpellier. Specimen 600/2/3, temporarily deposited at AMAP, is part of the Institut und Museum für Geologie und Paläontologie Collections, University of Tübingen.
2. Systematic palaeobotany
Class Progymnospermopsida Beck, Reference Beck1960
Order Archaeopteridales Zimmermann, Reference Zimmermann1930
Family Archaeopteridaceae Schmalhausen, Reference Schmalhausen1894
Genus Callixylon Zalessky, Reference Zalessky1911
Species Callixylon wendtii Tanrattana, Meyer-Berthaud & Decombeix sp. nov.
(Figs 2–5)

Figure 2 Callixylon wendtii, early Famennian, Morocco: (A) general view and (B) stele of specimen MD106 in transverse section; composite drawing from slides MD106 2BI.1 (left side) and MD106 1AS.1 (right side); (C) general view of specimen 600/2/3 in transverse section; slide 600/2/3 AS.1; (D) stele of specimen 600/2/3 in transverse section; slide 600/2/3 BS.1; (E) tangential section showing the arrangement of three vascular traces in the wood of specimen 600/2/3; slide 600/2/3 AT.5.

Figure 3 Callixylon wendtii, early Famennian, Morocco. (A, B) General view of specimen MD106 in transverse section: (A) MD106 2BI.1; (B) MD106 1AS.1. (C) General view of specimen 600/2/3, showing a vascular trace (VT) crossing the wood in transverse section; slide 600/2/3 AS.1. (D) Pith of specimen 600/2/3, showing sclerotic nests in radial section; slide 600/2/3 AL.1.

Figure 4 Callixylon wendtii, early Famennian, Morocco, specimen MD106: (A) wood with a ring boundary in transverse section; slide MD106 1AS.1; (B) pith with three sclerotic nests in radial section; slide MD106 1BR.1; (C) vascular trace and its corresponding cauline bundle (arrow) in transverse section; slide MD106 1AS.1; (D) inner portion of wood and part of stele showing three primary xylem strands (arrows) and a sclerotic nest (SN) in transverse section; slide MD106 1AS.1; (E) detail of previous view showing two mesarch primary xylem strands; slide MD106 1AS.1; (F) detail of vascular trace in transverse section; slide MD106 1AS.1; (G–I) Wood in tangential section, showing narrow rays, many with variously arranged ray tracheids (arrows); slide MD106 1BT.1; (J–L) wood in radial section, showing ray tracheids (arrows) in rays of variable height; slide MD106 1BRG1.

Figure 5 Callixylon wendtii, early Famennian, Morocco, specimen 600/2/3: (A) wood with a ring boundary in transverse section; slide 600/2/3 BS.1; (B) vascular trace and its corresponding cauline bundle (arrow) in transverse section; slide 600/2/3 BS.1; (C) inner portion of wood and part of stele, showing two primary xylem strands (arrows) and a sclerotic nest (SN) in transverse section; slide 600/2/3 BS.1; (D) elongate primary xylem strand, showing two poles of protoxylem (arrows); slide 600/2/3 BS.1; (E) sclerotic nests surrounded by radiating parenchymatous cells in transverse section; slide 600/2/3 BS.1; (F) circular pattern of wood tracheids and rays linked to the emission of a vascular trace in tangential section; slide 600/2/3 AT.2; (G) wood in tangential section, showing uniseriate rays, some with ray tracheids (white arrows) and one biseriate ray (black arrow); slide 600/2/3 AT.1; (H) wood in tangential section, showing tangential pitting on some tracheids; slide 600/2/3 AT.1; (I) wood in tangential section, showing axial parenchyma at arrow; slide 600/2/3 AT.1; (J) wood in tangential section, showing a septate tracheid at arrow; slide 600/2/3 AT.1; (K) wood in radial section, showing numerous cross-field pits; slide 600/2/3 AR.3; (L) wood in radial section showing a four-cell-high ray containing a ray tracheid; slide 600/2/3 AR.3.
Diagnosis. Axes with a large eustele characterised by a heterocellular pith consisting of parenchyma with interspersed sclerotic nests of variable sizes. Cells of sclerotic nests smaller and with thicker walls than parenchyma cells. Mesarch strands of primary xylem numerous, either in contact with the secondary xylem or separated from it by no more than a few rows of parenchyma cells. Secondary xylem showing faint growth rings. Most rays uniseriate; few rays partly or entirely biseriate; scarce rays partly triseriate. Rays up to 60 cells high, the majority ten cells or less in height. Rays comprised of ray tracheids arranged unevenly between parenchyma cells, shorter than the latter in tangential section. Ray tracheids either scattered or in uninterrupted rows in radial section. Pits on the radial walls of tracheids showing oval oblique apertures. Occasional ungrouped pits on the tangential walls of tracheids. Occasional occurrence of septate tracheids and vertical parenchyma. Course of leaf traces short, within first two growth rings.
Holotype. Specimen MD106 (Figs 3A–B, 4A–L).
Paratype. Specimen 600/2/3 (Figs 3C–D, 5A–L).
Repository. Specimen and slides currently stored in the AMAP Research Unit. Specimen MD106 and corresponding slides belong to the Palaeobotany Collections, University of Montpellier, France. Specimen 600/2/3 and corresponding slides belong to the Institut und Museum für Geologie und Paläontologie Collections, University of Tübingen, Germany.
Type locality. Mader el Mrakib, eastern Anti-Atlas, Morocco.
Stratigraphic horizon. upper part of Kellwasser member, early Famennian (Upper crepida conodont Zone).
Derivation of specific name. after Professor Jobst Wendt (University of Tübingen) who discovered the fossil plant locality.
3. Description
3.1. General features
Specimen MD106 was flattened during fossilisation and shows numerous cracks (Figs 2A, 3A–B). It is 110 mm long and 85×65 mm wide. It consists of a 5.5×24 mm wide stele (Fig. 2B) surrounded by a 30 mm-thick ring of secondary xylem. Specimen 600/2/3 represents the 66 mm-long portion of a smaller axis measuring 35×24 mm in cross-section (Figs 2C, 3C) and bearing a branch base about 20 mm wide. It shows an incomplete stele measuring 9.3×12 mm in width (Fig. 2D) and bordered along a third of its circumference by an 18 mm-thick sector of secondary xylem. In both specimens, the wood shows indistinct growth rings (Fig. 3A–C). The outer part of the wood and all external tissues are missing. A summary of the measurements made on the two specimens and detailed below is presented in Table 2.
Table 2 Measurements of anatomical features in specimens MD106 and 600/2/3. X1=primary xylem; X2=secondary xylem; n=number of measurements; sd=standard deviation.

3.2. Stele
In transverse section, the stele of specimen MD106 comprises 26 preserved primary xylem strands arranged at the periphery of a wide pith (Figs 2B, 4D). Based on the spacing of the well-preserved strands, their total number may have slightly exceeded 30. The preserved portion of the stele of specimen 600/2/3 shows at least 15 primary xylem strands arranged at the periphery of the pith, which seems to have undergone little deformation (Figs 2D, 5C). The original number of xylem strands in this specimen may have ranged between 20 and 30. Primary xylem strands are mesarch in the two specimens (Figs 4E, 5D). In cross-section, the smallest strands tend to be round, the largest oval. They range from 138 x 147 µm to 139 x 477 µm in specimen MD106. They are larger, measuring 157 x 298 µm to 324 x 805 µm, in specimen 600/2/3. Tangentially elongated primary xylem strands showing more than one protoxylem pole may correspond to the fusion of smaller individual strands (Fig. 5D). The primary xylem strands are either in contact with the secondary xylem (Fig. 4E), or separated from it by no more than 1–2 rows of thin-walled cells (Fig. 5D). Primary xylem tracheids have comparable dimensions in the two specimens, with a mean diameter of 33 µm for specimen MD106 and 35 µm for specimen 600/2/3 (Table 2).
The pith consists of thin-walled parenchymatous cells and interspersed sclerotic nests (Figs 2B, D, 3D, 4B, D, 5C, E). The parenchymatous cells of the two specimens are polygonal and range from 45 µm to 150 µm in both transverse and longitudinal sections. They tend to be smaller at the periphery of the pith. They are radially elongated near the primary xylem strands and around the largest sclerotic nests (Figs 4B, 5E). In longitudinal section, the external part of the pith of specimen 600/2/3 shows evenly spaced lacunae, bounded above and below by horizontally stretched cells (Fig. 3D).
Sclerotic nests are generally localised at a distance from the secondary xylem of 200–4000 µm for specimen MD106 and 530–1675 µm for specimen 600/2/3. The smallest nests are ovoid to spherical in shape, the largest ones being more irregular (Fig. 5E). Sclerotic nests in specimen MD106 range from 83×124 µm to 364×625 µm wide in transverse section and from 105×127 µm to 349×832 µm in longitudinal section (Fig. 4B). Those in specimen 600/2/3 may be larger, ranging from 121×135 µm to 778×902 µm in transverse section (Fig. 5E) and from 128×221 µm to 712×1355 µm in longitudinal section. In the best-preserved nests, the individual cells of both specimens are 20–80 µm wide in transverse and longitudinal sections. They show thickened walls. The parenchymatous cells surrounding the largest nests form a characteristic stellate pattern (Fig. 5E).
3.3. Secondary xylem
In transverse section, the transition between the stele and the secondary xylem is not as sharp in specimen 600/2/3 as in specimen MD106. This is due to the presence, in the former, of larger amounts of parenchyma cells intercalated between the innermost rows of secondary xylem tracheids (Fig. 5C). The secondary xylem is comprised of tracheids and rays. Vertical parenchyma (Fig. 5I) and septate tracheids (Fig. 5J) are only recorded in specimen 600/2/3. Evidence for vertical parenchyma is inconclusive and probably artefactual in specimen MD106. All secondary xylem elements are best observed in transverse and tangential sections; therefore, most measurements have been made in these planes rather than in radial section.
3.3.1. Transverse section
Specimen MD106 shows nine growth rings ranging from 1.6 mm to 5.6 mm in thickness (Fig. 2A); specimen 600/2/3 shows ten growth rings, possibly more, the innermost ones being hardly distinguishable (Fig. 2C); growth rings in the latter specimen are up to 3.3 mm wide (Table 2). Growth rings in the two specimens are characterised by subtle boundaries comprised of 1–4 tangential rows of tracheids with a reduced radial diameter for MD106, and up to ten tangential rows of smaller tracheids for specimen 600/2/3 (Figs 4A, 5A).
Tracheids appear mostly polygonal. Those of specimen MD106 are larger in tangential dimension than those of specimen 600/2/3 (Table 2). Tracheids are homogeneous in size within any radial file, but often heterogeneous when considering several contiguous files (Fig. 5A).
Rays are separated by 1–13, more often 2–6, radial files of tracheids (Figs 4A, 5A). They are mostly uniseriate and relatively long, crossing several growth rings. They are comprised of parenchymatous cells and horizontal ray tracheids. The parenchymatous cells range from 29 µm to 195 µm in length in specimen MD106 (mean: 115 µm; standard error: 37 µm; n=50), and from 49 µm to 156 µm in length in specimen 600/2/3 (mean: 108 µm; standard error: 27 µm; n=50). The ray tracheids show conspicuous bordered pits (Figs 4A, 5A).
3.3.2. Tangential section
Rays are relatively abundant, 23–35 per mm² in specimen MD106, 39–49 per mm² in specimen 600/2/3 (Figs 4G, 5G). The rays of specimen MD106 are 47–1510 µm high (n=100) and 1–59 cells in height (Table 2). 54 % of rays are less than ten cells in height, 41 % 10–29 cells and 5 % 30–59 cells. These rays are uniseriate, very rarely biseriate (Fig. 4G–I). Rays of specimen 600/2/3 are shorter. They are 45–620 µm high (n=100) and 1–21 cells in height (Table 2). 95 % of these rays are 1–10 cells high and more than 90 % are uniseriate (Fig. 5G–I). The remaining ones are biseriate (Fig. 5G), rarely triseriate, either partially or over their whole height.
The ray parenchymatous cells are square to rectangular, except those at the top and bottom of the rays, which are triangular. The ray tracheids differ from the parenchymatous cells in their thickened walls and smaller vertical dimensions (Figs 4H–I, 5H–I). Ray cell measurements are detailed in Table 2. Tracheids generally occur in rays exceeding four cells in height. In specimen MD106, their number increases with ray height, although exceptions can be observed (e.g., a three-cell-high ray showing one tracheid or a nine-cell-high ray lacking tracheids). The distribution of ray tracheids in this specimen is variable. Some rays are composed of tracheids regularly alternating with 1–3 rows of parenchyma cells. In other rays, tracheids are randomly distributed along the rays (Fig. 4G). Two ray tracheids may lie directly above each other, or be arranged side by side (Fig. 4H). In specimen 600/2/3, ray tracheids are fewer, their number rarely exceeding three in a ray, even in the highest ones (Fig. 5G). They are randomly distributed, sometimes arranged in pairs of tracheids lying side by side. The tangential walls of tracheids occasionally show bordered pits in localised parts of the wood (Fig. 5H). These pits are arranged in 2–3 vertical rows and cover high portions of the tangential walls, without any separation by unpitted zones. Their diameter ranges from 7 µm to 13 µm.
In specimen 600/2/3, two additional types of elements are occasionally observed in tangential section. One consists of long cells showing transverse walls and tangential pits. The septa are slightly thinner than the tracheid walls and perpendicular to the latter. These cells are interpreted as septate tracheids (Fig. 5J). The second type is represented by vertical rows of unpitted cells showing transverse walls (Fig. 5I). They range from 26 µm to 36 µm in width and from 100 µm to 230 µm in height and represent axial parenchyma.
The tangential sections made in specimen 600/2/3 show a circular pattern of tracheids linked to the course of a vascular trace (Fig. 5F). Rays in the portions of wood above and below the trace are shorter and their parenchyma cells are larger than in the other parts of the wood. Such cells measure 23–44 µm in width (mean: 33 µm, standard error: 4 µm; n=100) and 22–47 µm in height (mean: 32 µm, standard error: 6 µm; n=100).
3.3.3. Radial section
The radial walls of tracheids are characterised by bordered pits in groups of 6–18, separated from each other by unpitted zones ranging from 3 µm to 17 µm (Figs 4J, 5K–L). The radial pits are arranged alternately in one to three vertical rows and measure 8–17 µm in diameter. Their aperture is oblique. Horizontal tracheids in rays occur either isolated or in uninterrupted rows (Figs 4K–L, 5L). They may show pointed extremities. Cross-field pits seem to be arranged in groups of 2–8 (Figs 4J–K, 5K).
3.4. Vascular traces
Several vascular traces are observed in the wood of the two specimens in transverse section (Figs 2B, D, 3C). They are radially aligned with the cauline strands of the primary xylem from which they derive (Figs 4C, 5B). None have been observed beyond the second growth ring. In the sections of specimen MD106 illustrated in Figure 2B, a trace occurs at about 770 µm from the inner border of the secondary xylem, the other at 1.7 mm. Both are contained within the innermost growth ring. The outermost trace shows the largest transverse section (Fig. 4C). It consists of a 310×480 µm-wide strand of primary xylem surrounded by secondary xylem, the entire trace measuring 710×1200 µm in transverse section. A crack runs through the middle but, given the arrangement of the primary xylem tracheids, it seems reasonable to assume that, at this level, this trace contained a single pole of protoxylem (Fig. 4F).
Longitudinal sections of specimen 600/2/3 show small vascular traces crossing the wood at 10–15 mm intervals (Fig. 2E). In transverse section, the best preserved vascular trace occurs at 2.8 mm from the inner border of the secondary xylem (Figs 2D, 5B). It consists of a 520×650 µm wide strand of primary xylem surrounded by secondary xylem, the whole trace measuring 1670×2350 µm in transverse section. Specimen 600/2/3 also shows a large branch trace on its external surface. This axis thus produced lateral organs of different sizes. The small ones which are interpreted as leaf traces were closely arranged and in spiral (Fig. 2E). The short length of their course in the wood indicates that they did not have a long lifespan.
4. Discussion
4.1. General features and intra-specific variability
Specimens MD106 and 600/2/3 are both defined by a eustele with mesarch primary xylem strands, leaf traces departing radially from cauline bundles, and secondary xylem tracheids with radial pits arranged in groups separated by un-pitted zones. These features are diagnostic of the genus Callixylon Zalessky. The wood of the two specimens shows faint growth rings. It is characterised by uniseriate, rarely wider rays. A high proportion of them are ten cells or less high (54 % in specimen MD106, 95 % in specimen 600/2/3). Rays are comprised of ray tracheids that generally alternate randomly with the parenchyma cells and are shorter in tangential sections than the latter. The eustele of the two specimens is characterised by a peripheral ring of primary xylem strands lying against the secondary xylem, or separated from it by no more than 1–2 rows of parenchyma cells. The pith of both specimens is heterogeneous and shows sclerotic nests containing thick-walled cells that are generally smaller than the surrounding parenchyma cells. Both specimens show the emission of small vascular traces that are ephemeral and interpreted as leaf traces.
Specimen MD106 is larger than specimen 600/2/3; its stele is also slightly larger and its growth rings are thicker (compare Figures 2A and 2C, 2B and 2D). These two specimens may therefore represent two successive orders of branches, specimen 600/2/3 itself producing a branch. This observation indicates that the new species of Callixylon would have had at least three orders of branches, all bearing leaves.
Despite the larger size of its stele, the primary xylem strands and the sclerotic nests of specimen MD106 tend to be smaller than those of specimen 600/2/3 (Table 2; Fig. 2B, D). In contrast, the diameter of the secondary xylem tracheids can be slightly larger in specimen MD106 and its rays have a larger range of heights, 1–59 cells instead of the 1–21 cells in specimen 600/2/3 (Table 2). The significance of these differences is currently uncertain, but in no case are they taken as evidence for any specific distinction between the two specimens. The size of these elements is not a good indicator of the size or the rank of the branches.
4.2. Comparison with previously described Callixylon species
The 19 species of Callixylon described to date (Table 1) are primarily distinguished by wood characteristics, such as the seriation and comparative height of the rays, the shape and arrangement of the ray tracheids when present, and the orientation of the pit apertures. A majority, represented by 13 species out of 19, possess rays that are predominantly uniseriate like the new specimens from Morocco. The steles of only eight of them are known with enough information for comparison. The main characters of the 13 Callixylon species with narrow rays are listed in Table 3 and discussed below.
Table 3 Compared features of the species of Callixylon showing narrow rays, including C. wendtii.

Callixylon petryi is a species created for roots. Its primary vascular system is protostelic and the radial pits have distinctive horizontal apertures. Four other species of Callixylon with narrow rays, C. henkei, C. clevelandensis, C. huronensis, and C. brownii, differ from the Moroccan specimens in a lack of ray tracheids. Moreover, the pith of the two latter ones is entirely parenchymatous and the apertures of the radial pits of C. brownii are vertical rather than oblique, according to Chitaley & Cai (Reference Chitaley and Cai2001).
The presence of ray tracheids in C. whiteanum and C. trifilievii is either controversial or uncertain (Arnold Reference Arnold1934; Zalessky Reference Zalessky1909, Reference Zalessky1911; Lemoigne et al. Reference Lemoigne, Iurina and Snigirevskaya1983; Beck & Wight Reference Beck, Wight and Beck1988; Trivett Reference Trivett1993; Orlova & Jurina Reference Orlova and Jurina2011). If such elements occur in these species, they are rare, a character that distinguishes them from the new Moroccan specimens. The stele of C. whiteanum, which consists of primary xylem strands detached from the wood at the periphery of an entirely parenchymatous pith, is another feature that differs from C. wendtii. The nature of the thick-walled cells reported in the pith of C. trifilievii is unknown. These elements are scattered and do not occur in large clusters like the sclerotic nests of the Moroccan specimens.
Six species of Callixylon possess wood with narrow rays comprised of ray tracheids. The species C. bristolense, C. velinense and C. schmidtii are based on poorly preserved wood fragments with few distinctive characters. Their stelar structure is unknown. A specific feature of C. bristolense that has not been observed in the Moroccan specimens is the vertical aperture of its radial pits. C. velinense and C. schmidtii have rays that do not exceed 13 cells in height and do not reach the same height as those of C. wendtii. C. erianum, by contrast, is a well-known species. Its high rays, showing evenly arranged ray tracheids, are unique and differ from the shorter rays with randomly distributed tracheids of the Moroccan specimens. The pith of C. erianum does not contain any other elements than parenchyma cells. The two species that share most anatomical characters with C. wendtii are C. beckii and C. zalesskyi, which have been synonymised by Orlova & Jurina (Reference Orlova and Jurina2011). Both have rays of moderate height, showing abundant tracheids arranged in long uninterrupted rows, unevenly alternating with rows of parenchyma cells. Their stele is comprised of primary xylem strands in contact with the secondary xylem. These species, however, differ in the size of their ray tracheids and the cellular composition of their pith. In tangential section, the ray tracheids of C. zalesskyi are similar in size and shape to the parenchymatous ray cells (Arnold Reference Arnold1930), whereas those of C. beckii, like those of the Moroccan specimens, are shorter (Chitaley & Cai Reference Chitaley and Cai2001). The pith in C. beckii is entirely parenchymatous; that of C. zalesskyi is heterogeneous, like the pith of C. wendtii. The pith of C. zalesskyi, however, contains tracheids that are longer and narrower than the other pith cells and differ from the latter by their spiral to scalariform thickenings; these tracheids are either scattered among the parenchyma cells or arranged in clusters of 3–4, more rarely five, elements (Arnold Reference Arnold1930). The occurrence of compact clusters of thick-walled cells forming sclerotic nests like those of the Mader specimens has never been reported in any Callixylon species so far. C. beckii, C. zalesskyi and the new specimens are assignable to the Erianum group of Orlova & Jurina (Reference Orlova and Jurina2011), a morphotaxon with a wide paleogeographical distribution and represented throughout the Late Devonian (Table 1).
4.3. Sclerotic nests in lignophytes
The possession of a heterogeneous pith comprised of scattered thick-walled cells is an ancient character of the eustelic lignophytes, reported as early as the Givetian in Callixylon (Cornet et al. Reference Cornet, Gerrienne, Meyer-Berthaud and Prestianni2012). The arrangement of sclerotic cells in large clusters or nests, however, was not recorded in the pith of any Devonian taxa prior to the present paper.
Sclerotic nests are not characteristic of any large clade within the lignophytes, but their systematic significance is widely recognised at the generic or specific level. They are generally associated with taxa showing a tree-sized habit. Sclerotic nests have been described in a small number of pteridosperm species of Mississipian age: Cauloxylon ambiguum from Missouri (Cribbs Reference Cribbs1939); Eristophyton beinertianum and E. waltonii from various localities of Europe (Decombeix et al. Reference Decombeix, Meyer-Berthaud and Galtier2007); and, more recently, Ahnetia conradii from Algeria (Decombeix & Galtier Reference Decombeix and Galtier2017). Sclerotic nests are characteristic of walchian Voltziales such as the Pennsylvanian species Emporia cryptica (Hernandez-Castillo et al. Reference Hernandez-Castillo, Stockey, Rothwell and Mapes2009) and Barthelia furcata, (Rothwell & Mapes Reference Rothwell and Mapes2001) and Hanskerpia hamiltonenis (Rothwell et al. Reference Rothwell, Mapes and Hernandez-Castillo2005) from Kansas. They are widespread in coniferophyte taxa of Permian age reported from various localities of Europe, China and almost all areas of the former palaeocontinent Gondwana, except Antarctica. These Permian coniferophytes correspond to the taxa Scleromedulloxylon (Doubinger & Marguerier Reference Doubinger and Marguerier1975), Kaokoxylon (Kräusel et al. Reference Kräusel, Maithy and Maheshwari1962; Maheshwari Reference Maheshwari1972; Kurzawe et al. Reference Kurzawe, Iannuzzi, Merlotti and Rohn2013), Austroscleromedulloxylon and Atlanticoxylon (Merlotti & Kurzawe Reference Merlotti and Kurzawe2011), Mussaeoxylon Brazil (Merlotti Reference Merlotti1998), Septomedulloxylon sclerotica (Merlotti Reference Merlotti2002) and Macdonaldodendron (Falcon-Lang et al. Reference Falcon-Lang, Kurzawe and Lucas2014).
5. Conclusion
A new species of Callixylon is described from two decorticated specimens collected in the early Famennian locality of Mader el Mrakib, in Anti-Atlas, Morocco. C. wendtii sp. nov. is characterised by a heterogeneous pith containing sclerotic nests. Its secondary xylem, which shows faint growth rings, possesses narrow rays with unevenly arranged ray tracheids. Based on their sizes, the two specimens may correspond to two successive orders of axes, each one producing closely arranged organs interpreted as relatively short-lived leaves. The smallest specimen, which itself produces a branch, shows tangential pits on some tracheids. Its wood also presents rare septate tracheids and axial parenchyma. Other differences between the two specimens include the size of the primary vascular strands, sclerotic nests, rays and tracheids. These differences are interpreted as intra-specific variability.
With the 5 m-long trunk of C. erianum described by Meyer-Berthaud et al. (Reference Meyer-Berthaud, Wendt and Galtier1997, Reference Meyer-Berthaud, Scheckler and Bousquet2000) and a C. zalesskyi-type trunk that retained a thick fragment of secondary phloem (Decombeix & Meyer-Berthaud Reference Decombeix and Meyer-Berthaud2013), C. wendtii sp. nov. is the third species of Callixylon based on aerial axes showing narrow rays and ray tracheids at Mader el Mrakib. This discovery increases the diversity of the archaeopterids that may have inhabited the land close to these marine deposits (Meyer-Berthaud et al. Reference Meyer-Berthaud, Wendt and Galtier1997). It suggests favourable conditions for the establishment of a diverse community of Archaeopteris/Callixylon trees in this part of Gondwana during the early Famennian.
Numerous reports of Callixylon have been made from the palaeocontinent Laurussia, fewer from Siberia and from Xinjiang (Table 1). Callixylon species with narrow rays and ray tracheids have a long stratigraphical record, the oldest known one being C. velinense from the late Givetian of Belgium (Marcelle Reference Marcelle1951). None however, has shown the occurrence of sclerotic nests in the pith, a character easily observable when present. It is too early to speculate concerning the palaeogeographical distribution of the new species, but given the considerations above, it is possible that this species was strictly Gondwanan.
6. Acknowledgements
We thank the Ministère de l'Energie, des Mines, de l'Eau et de l'Environnement du Maroc (Rabat, Morocco) for issuing working permits and the permission to export fossil material. We are greatly indebted to Jobst Wendt, Berndt Kaufman, Martin Rücklin and Christian Klug for their invaluable help in the field. The suggestions and comments from the two reviewers, Olga Orlova and Christopher Berry, contributed to greatly improve the manuscript. AMAP (botAny and Modelling of Plant Architecture and vegetation) is a joint research unit associated with CNRS (UMR 5120), CIRAD (UMR 51), INRA (UMR 931), IRD (R123) and Montpellier University (UM 27); http://amap.cirad.fr/.










