Introduction
Dispersal is perhaps the most dangerous period in the life of most parasites (Ward et al., Reference Ward, Leather, Pickup and Harrington1998) and a key process influencing the population dynamics and coevolution of host–parasite interactions (Hassell, Reference Hassell2000; McCoy et al., Reference McCoy, Boulinier, Tirard and Michalakis2003). Yet, little information is available on dispersal and on the factors that affect it. The lack of basic information has to do with the difficulties associated with the observation and experimental approaches during the highly mobile phase of parasites, which hampers further advance.
Opportunities for successful parasite dispersal are closely linked to host biology (e.g. foraging habits of the host – Harper et al., Reference Harper, Marchant and Boddington1992 –, host density or colony size – Brown and Brown, Reference Brown and Brown1996, Reference Brown and Brown2004; Kleindorfer and Dudaniec, Reference Kleindorfer and Dudaniec2009 –) but they also depend on inherent characteristics of the parasite. The dispersal ability of arthropods is determined largely by morphology. Thus, for the insects that have evolved wings, the distance of dispersal is related to their flight ability. For instance, within the Diptera, the minute and delicate wings of midges and gnats make long-distance dispersal less likely than for strong fliers like horse flies or blowflies (Botzler and Brown, Reference Botzler and Brown2014). The ability of the parasite to survive longer periods off the host also affects its dispersal opportunities. Hence, some arthropods, such as astigmatid mites, survive a short time off their hosts while others (e.g. ticks, fleas) are able to survive for long periods between meals (Botzler and Brown, Reference Botzler and Brown2014). Size is also important for both survival and dispersal ability. Trade-offs between insect body size and life history parameters such as lifespan have been demonstrated (Roff, Reference Roff1993).
To know the lifespan of parasites during their dispersal stages as well as factors affecting longevity (e.g. food provisioning, temperature) during this phase is critical for evaluation of their dispersal ability. For instance, it is known that for many adult insects, including several mosquito species, carbohydrate-rich food is an important source of energy for longevity, fecundity and mobility (Clements, Reference Clements1955; Briegel and Horler, Reference Briegel and Horler1993; Winkler et al., Reference Winkler, Wäckers, Bukovinszkine-Kissa and van Lenteren2006). Moreover, whether insects feed or not during the adult phase is related to other critical traits such as size. In insects that do not feed as adults, a large size often contributes positively both to fecundity and longevity (Nilssen, Reference Nilssen1997). However, in species that feed as adults, there may be a selection towards small male body size due to energy constraints or as a response to host defences (Blanckenhorn et al., Reference Blanckenhorn, Preziosi and Fairbairn1995; Clayton et al., Reference Clayton, Lee, Tompkins and Brodie1999, and references therein).
Here we investigate the lifespan of the free-living stage of the nidicolous, non-contact-transmitted ectoparasitic fly, Carnus hemapterus. Carnus is a widespread bird parasite (Grimaldi, Reference Grimaldi1997; Brake, Reference Brake2011) whose entire cycle, except for dispersal, takes place in its host's nest, where adult flies feed mainly on nestling birds. Pupae overwinter in the nests and after diapause nymphs emerge at the time after nest sites are reoccupied by birds, thus allowing the perpetuation of the parasite in the nest (unless the nest is not occupied by any host). Adult flies are initially winged but lose their wings as soon as they locate a suitable host (Roulin Reference Roulin1998), therefore limiting their dispersal ability from then onwards. Thus Carnus hemapterus flies are not transmitted by the host (Grimaldi, Reference Grimaldi1997) but rather colonize host nests actively during the winged phase (Grimaldi, Reference Grimaldi1997; Roulin, Reference Roulin1998; Reference Roulin1999). To our knowledge, there is no information about the dispersal stage of this parasite, which is a critical episode to understand its infectious potential, nor about the factors that can affect its lifespan during this period.
In this work, our first aim is to analyse whether adult Carnus hemapterus is sexually dimorphic. If there are size differences between sexes, we would expect a positive relationship between size and longevity (Hasson et al., Reference Hasson, Fanara, Rodriguez, Vilardi, Reig and Fontdevila1993; Sivinski, Reference Sivinski1993; Chen et al., Reference Chen, Onagbola and Fadamiro2005). Furthermore, we experimentally investigate whether Carnus hemapterus lifespan depends on some abiotic factors such as humidity and/or on the availability of feeding resources. It has been demonstrated that higher environmental humidity increases survival in some insects (e.g. Mellanby, Reference Mellanby1932; Tochen et al., Reference Tochen, Woltz, Dalton, Lee, Wiman and Walton2015), or the other way round, drier environments may reduce longevity due to desiccation. Moreover, plants represent a source of food for many insects, mainly those looking for the high energy sugary substances. Floral volatiles have been argued to inform insects about the potential energy gain to be obtained from a flower (Wright and Schiestl, Reference Wright and Schiestl2009). In our experiments, we would thus expect adult Carnus hemapterus flies (i) living longer in a humid than in a drier environment, and (ii) live longer when also food is available. The results of our study provide insights into some of the factors influencing longevity in this parasite during the dispersal stage and contribute to the determination of its colonization abilities.
Material and methods
Study species
Carnus hemapterus (hereafter Carnus) is a 2-mm long, highly mobile ectoparasitic fly that colonizes nestling birds (Grimaldi, Reference Grimaldi1997; Brake, Reference Brake2011). Its life cycle comprises an adult (parasitic) stage, three larval phases and a pupal phase (Guiguen et al., Reference Guiguen, Launay and Beaucournu1983). Diapausing pupae are found in the nests of the host species. After a diapause usually lasting several months (Guiguen et al., Reference Guiguen, Launay and Beaucournu1983) imagoes, initially winged, emerge when host nestlings are available. Emergence continues throughout the whole nestling period (Valera et al., Reference Valera, Casas-Crivillé and Hoi2003). Emerged flies can stay in the nest if occupied by a suitable host. Otherwise, flies are forced to disperse. Once a suitable host is located, adult flies lose their wings and feed on blood, epidermal cells and skin secretions of their host (Kirkpatrick and Colvin, Reference Kirkpatrick and Colvin1989; Papp, Reference Papp, Papp and Darvas1998). This parasite prefers bird species nesting in sheltered areas or cavities and has never been found parasitizing ground or swamp nesters (Grimaldi, Reference Grimaldi1997; Papp, Reference Papp, Papp and Darvas1998).
Flies sampling and experimental design
Nest material (including diapausing pupae) from eight European roller (Coracias garrulus) nest-boxes located at the Desert of Tabernas (Almería, SE Spain, 37°05′N, 2°21′W) was collected between 4th February 2016 and 14th February 2016. The samples were kept in transparent plastic bags and moved to the Estación Experimental de Zonas Áridas (EEZA, Almería, 36°50′N 02°28′W) after collection. They were stored at room temperature until emergence started (mid-April). Then, plastic bags were opened and the material spread in a tray so that emerged carnid flies could fly away. After half an hour, we turned over the material to assure that no adults were left, put the nest material back into the plastic bag, closed it and waited for 1 h. After that, the emerged flies in the bag were collected and then assigned to the different experimental trials (see below). In this way, we were sure that the adult individuals we used in trials had emerged, at most, 1 h prior the experiment. This procedure was repeated several times over the experimental period (between 18th April and 2nd May 2016) for the collection of experimental flies.
To determine lifespan of adult Carnus flies during their free-living stage and the likely effect of both humidity and food provisioning on it, we performed an indoor experiment with the following three treatments:
(i) An untreated control. Adult flies were introduced in glass jars (one per jar; volume = 1000 cc) into which a dry sponge with a plastic flower (8 cm tall) simulating a wild plant was added. During the experiment, the jars were closed to avoid flies to fly off. This situation represents a dry environment with no food available (hereafter control treatment).
(ii) Experimental environment 1. The same experimental set up was used but, in this case, the sponge was maintained wet for the whole experiment. This represents a wet environment with no food available (hereafter moist treatment).
(iii) Experimental environment 2. The same device than in the experimental environment 1 was used, but an 8 cm flowered branch of Cytisus scoparius (Fabaceae) was used instead of the plastic flower. All branches used had two flowers. Flower blossoms were collected during the naturally flowering period of the species in our study area. Branches were inserted in the wet sponge and kept turgid during the whole experiment. Adult Carnus flies have been found on flowers of some plant species (Papp, Reference Papp, Papp and Darvas1998) and adult individuals of the closely related Hemeromyia anthracina have been swept from flowering Retama raetam (Fabaceae) in Israel (Freidberg, pers. comm.). Thus, we assume that in this treatment food is available for adult flies, being labelled hereafter moist-food treatment.
Once one individual was introduced into the jar, it was monitored until it died. Previous observations (Calero-Torralbo, Reference Calero-Torralbo2011) and the results of a pilot study with the method described above showed that the life expectancy of carnid flies was at least 24 h. Therefore, the first control to check survival was 24 h after the start of each experiment. Then, each jar was revisited every 4 h (day and night) and we recorded whether the flies were dead or alive.
The experiments were done at room temperature (range: 17–22 °C).
Overall, 55 flies were tested, 16 under the control treatment (seven males and nine females), 20 were assigned to the moist treatment (11 males and nine females) and 19 flies (nine males and 10 females) to the moist-food treatment. Flies entering the experiment were sexed according to their genitalia (Grimaldi, Reference Grimaldi1997; Papp, Reference Papp, Papp and Darvas1998) and measured after death to avoid that manipulation prior to the experiment could harm the individuals. We also sexed and measured nine flies not used for the experiment. As estimators of body size, maximum length and maximum width of the thorax of each fly was measured (Valera and Zídková, Reference Valera and Zídková2012) with the aid of a stereo-microscope (Leica, MZ125) fitted with an ocular micrometer. Two measurements of each variable were taken in ten individuals for assessing the repeatability of our measures (Lessells and Boag, Reference Lessells and Boag1987). Blind measurements of thorax length and width were highly repeatable (F = 245.2, P < 0.001, R = 0.99 and F = 21.1, P < 0.001, R = 0.91 respectively).
Estimating the lifespan
Lifespan was estimated as the number of hours flies were alive. Individuals entering the experiment were at most 1 h old (see above). We considered that death occurred at the midpoint between successive visits. Since the lapse between such visits was 4 h, the error of our estimate of lifespan is, at most, 3 h, thus yielding an accurate measure of longevity.
Statistical analyses
General linear models (GLMs), were used to analyse: (i) sexual size dimorphism: thorax length and width were the dependent variables, gender was a fixed factor and the nest of origin of the flies a random one; (ii) Carnus lifespan in relation to the experimental treatments. Lifespan (hours) was the dependent variable whereas treatment (control, moist, moist-food) and gender were fixed factors. To account for the effect of insect body size (covariate) maximum thorax width (or length) was included as a continuous predictor. Results obtained with thorax length and width are similar so that we report here the ones obtained with the latter variable. The nest of origin of the experimental flies was again included as a random factor. Residuals of the models were normally distributed.
Unless otherwise stated, values reported are means ± s.e.. Analyses were done with STATISTICA version 13 (Dell Inc., 2016).
Results
Sexual size dimorphism
Carnus hemapterus is a sexually dimorphic species in body size, males being smaller than females. The thorax of male flies was significantly shorter (GLM: adjusted R 2 = 0.29, F 1,55 = 17.7, P < 0.001; males: 0.52 ± 0.006 mm, n = 32; females: 0.58 ± 0.009 mm, n = 32) and thinner (GLM: adjusted R 2 = 0.25, F 1,55 = 15.0, P < 0.001; males: 0.40 ± 0.004 mm, n = 32; females: 0.44 ± 0.006 mm, n = 32) than that of female flies.
Longevity of the dispersal stage of Carnus hemapterus and its determinants
Overall, flies lived on average 54.9 ± 1.8 h (n = 55 individuals). The earliest deaths were observed 26 h after emergence (two cases) whereas the two oldest flies lived 82 h. Only six out of 55 flies (10.9%) were alive after 3 days (three males and three females).
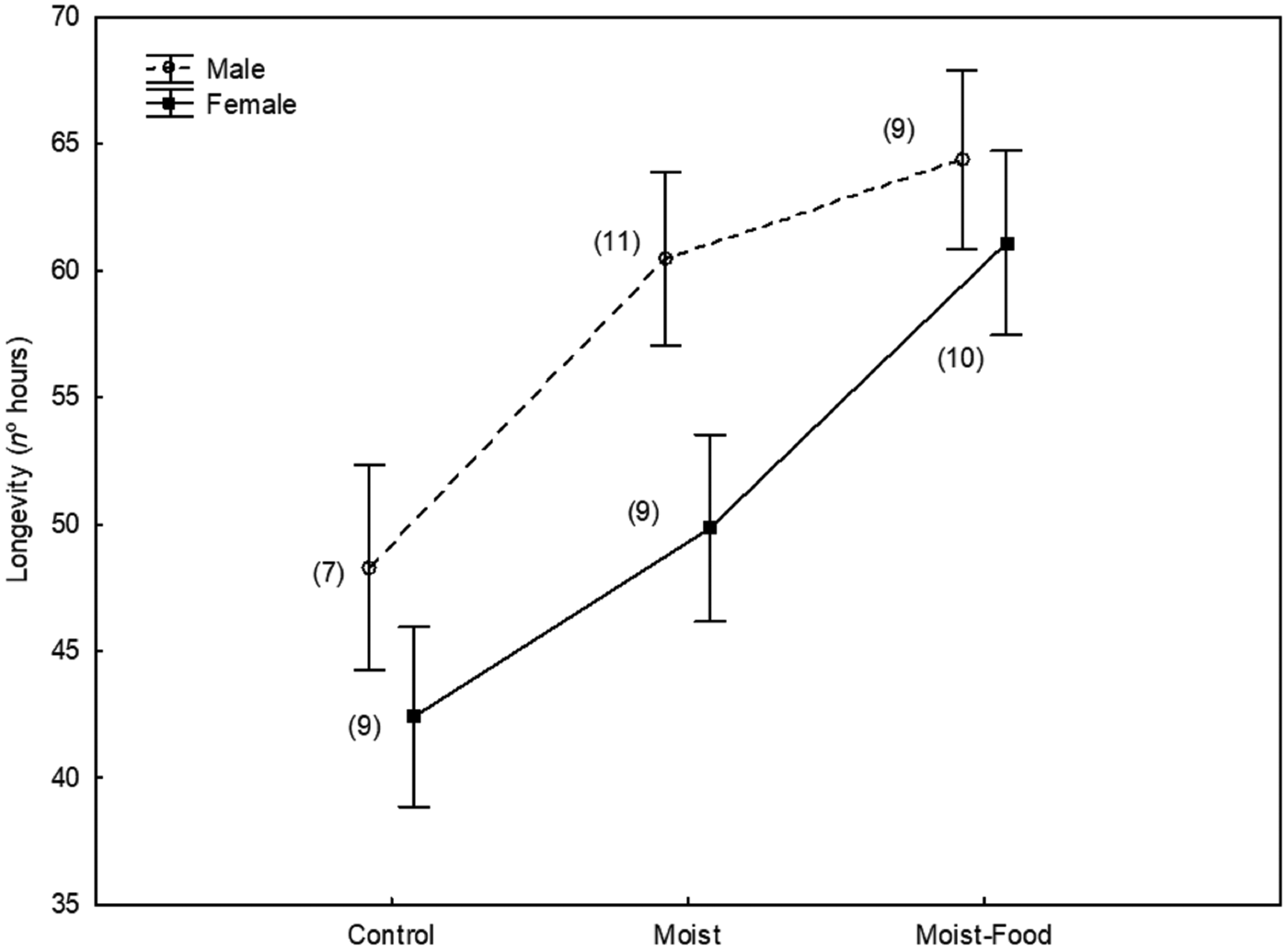
Lifespan was significantly explained by the experimental treatment (GLM: adjusted R 2 = 0.49, F 13,41 = 5.02, P < 0.001; treatment: F 2,41 = 12.1, P < 0.001) so that flies in the control group lived shorter than the ones in the two experimental groups (post hoc Tukey Honestly Significant Difference test, P < 0.01 in both cases, Fig. 1) and flies in the moist treatment lived significantly shorter than those in the moist-food treatment (post hoc Tukey Honestly Significant Difference test, P = 0.024). Lifespan was significantly and positively related to thorax width (F 1,41 = 19.7, P < 0.001).
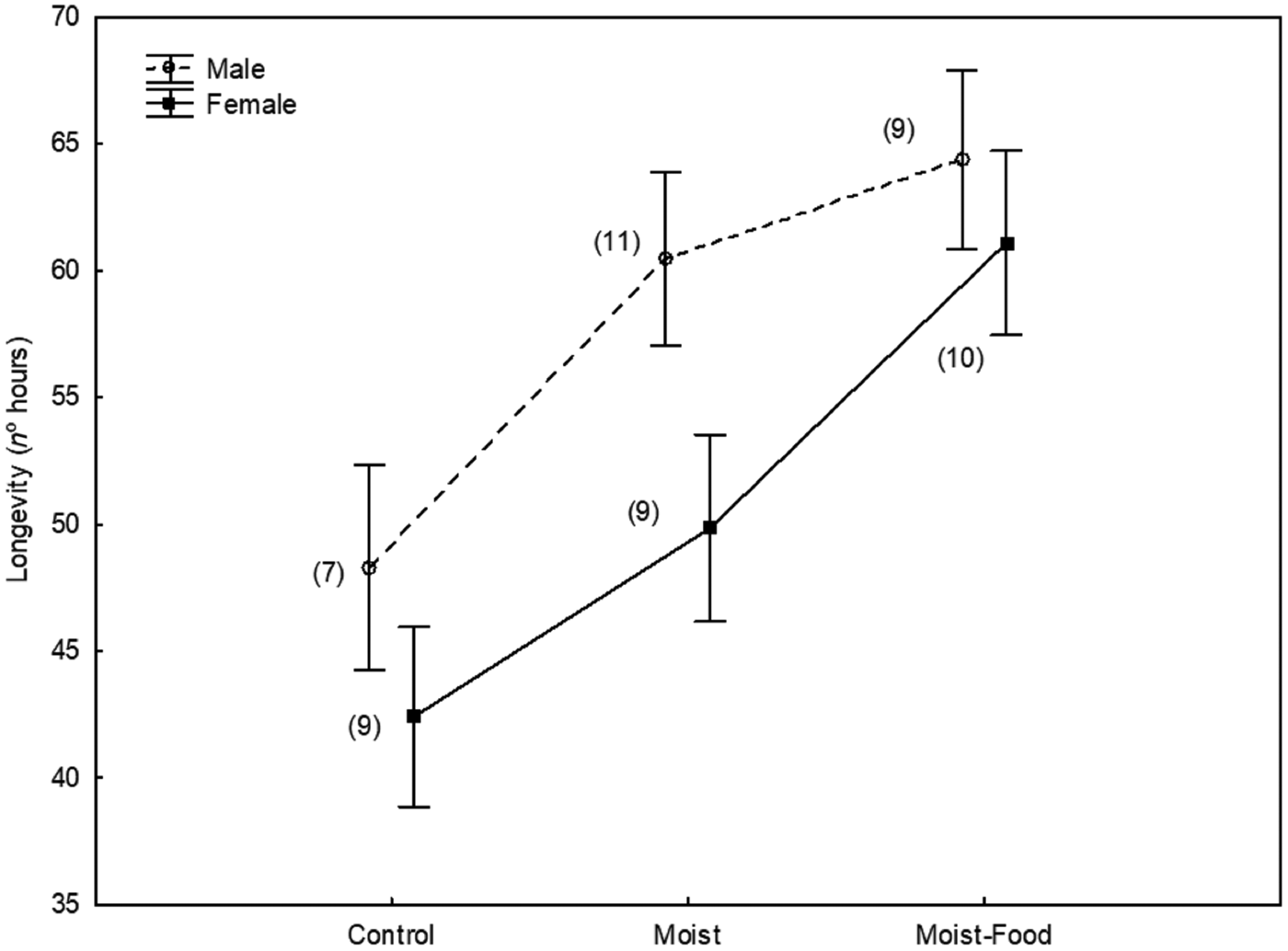
Fig. 1. Lifespan (n° hours) of male and female Carnus hemapterus flies in three experimental setups (control, with moist environment, with moist environment and food) after controlling for body size (maximum thorax width, covariate means: 0.42). s.e. are shown. Figures in brackets are the number of flies used in each case.
Gender had a significant effect on lifespan (F 1,41 = 4.2, P = 0.047), so that, after controlling for body size, males lived longer than females (Fig. 1).
The interaction between gender and treatment was not significant (P > 0.50).
Discussion
Our study revealed that Carnus hemapterus is sexually dimorphic with females being larger than males, which is similar to many other insects (Fairbairn, Reference Fairbairn1997). We also found that the length of the dispersal stage was short, lasting less than 4 days. We could also demonstrate that longevity was influenced by body size (see also Hasson et al., Reference Hasson, Fanara, Rodriguez, Vilardi, Reig and Fontdevila1993, Sivinski, Reference Sivinski1993, Chen et al., Reference Chen, Onagbola and Fadamiro2005), so that larger flies namely females lived longer. Yet, statistically controlling for body size, males were longer-lived than females. In sexually dimorphic species, the fitness of the male is typically limited by the quantity of mates acquired while that of females depends more often on the quantity of other resources such as food (Darwin, Reference Darwin1871). Thus, while selection may favour larger females due to a generally positive relationship between body size and fecundity in ectotherms (Roff, Reference Roff1993, see Valera and Zídková, Reference Valera and Zídková2012 for Carnus), smaller males may be favoured because their lower energy requirements frees time to be invested in mating (Blanckenhorn et al., Reference Blanckenhorn, Preziosi and Fairbairn1995). In Carnus, the necessity to actively disperse or not is unpredictable (Calero-Torralbo et al., Reference Calero-Torralbo, Václav and Valera2013), as it depends on whether the nest is occupied during the following breeding season. If dispersal is not necessary, smaller males may have some advantages (Blanckenhorn et al., Reference Blanckenhorn, Preziosi and Fairbairn1995; Clayton et al., Reference Clayton, Lee, Tompkins and Brodie1999). In contrast, if the nest is not occupied and dispersal is necessary, larger males with longer lifespan might increase dispersal success. These opposing selection forces suggest that lifespan differences between males and females are not only the by-product of sexual and natural selection on body size and that other factors (e.g. metabolic differences or differences in patterns of resource allocation between males and females, see, for instance, Fox et al., Reference Fox, Dublin and Pollitt2003) probably account for sexual differences in lifespan.
Likewise, since small insects are particularly prone to water loss (Tochen et al., Reference Tochen, Woltz, Dalton, Lee, Wiman and Walton2015; Bujan et al., Reference Bujan, Yanoviak and Kaspari2016), thus an increase in longevity with humidity can be expected. We found that the lifespan of flies in the humid treatment increased 22.8% (for males) and 27.7% (for females) in comparison with the one observed for flies in a dry environment. The impact of humidity on the lifespan of adult Carnus flies is likely to vary along the broad range of the species, being probably higher in drier latitudes and lower in more mesic ones. Similarly, food provisioning has been described as an influential factor on the longevity of insects, including haematophagous ones (e.g. Foster, Reference Foster1995; Yu et al., Reference Yu, Ding, Mo, Liu, Li and Mo2016) and, as predicted, had a significant effect on the longevity of carnid flies: the lifespan of flies in the humid-food treatment increased between 11 and 19% (for males and females, respectively) in comparison with the lifespan observed in a humid environment without food.
One important question is to what extent our estimation of the longevity of Carnus under laboratory conditions resembles the lifespan of dispersing flies in the wild. On one side differences in influential abiotic factors (e.g. temperature, see Taylor, Reference Taylor1981; Lessard and Boivin, Reference Lessard and Boivin2013) between field conditions and our experimental set up (with similar mean temperature but lower thermal oscillation) could have an effect on the calculated longevity. Some authors have reported a shorter lifespan of insects under alternating temperature regimes than under constant temperatures (e.g. Carroll and Quiring, Reference Carroll and Quiring1993; but see Cônsoli and Parra, Reference Cônsoli and Parra1995). Ambient conditions during the experiment were not strenuous in the wild (maximum and minimum temperature during the period: 26.5 and 5.1 °C, respectively) nor they were at the experimental room so that we do not expect major differences. If so, the real lifespan of carnid flies would be even shorter in nature. This could be the case in parts of the range of the species given its broad distribution along the Holarctic. Thus, we would expect geographical differences in the infectious capacity of the parasite. Studies of the colonization ability of Carnus along its range could shed light on the effect of abiotic factors on the epidemiology of this parasite. On the other side, the food plant offered in our experiment could not be the most adequate one. Until now, it was ignored whether carnid flies could feed during their free-living phase. The significant increase in longevity of winged Carnus in presence of flowers suggests that this parasite can refuel during dispersal feeding on plant substrates. The plant species used in our experiment is a Fabaceae and species of this family have been reported to host Hemeromyia anthracina, a closely related species to Carnus (Freidberg, pers. comm.). It is known that the lifespan and dispersal ability of a broad spectrum of dipteran species depends on their diet during adulthood (Clements, Reference Clements1955; Briegel and Horler, Reference Briegel and Horler1993; Foster, Reference Foster1995; Tochen et al., Reference Tochen, Walton and Lee2016). Moreover, previous studies have demonstrated that the plants chosen by mosquitoes for their sugar meals are those that maximize survival and fecundity (Manda et al., Reference Manda, Gouagna, Foster, Jackson, Beier, Githure and Hassanali2007; Yu et al., Reference Yu, Ding, Mo, Liu, Li and Mo2016). Thus, the lifespan of carnid flies during dispersal could be longer than our results suggest given that they very likely find more appropriate food sources in their environment.
Nonetheless, even if enlarged by fuelling during dispersal, the time carnid flies have for finding and colonizing a host is seemingly brief. Short free-living stages have been described for other parasites. For instance, trematode cercariae possess only limited energy reserves and have to invade their hosts within a short lifespan of a few hours to 3 days (Haas, Reference Haas2003). Adult fig wasps have never been kept alive for more than 48 h (Kjellberg et al., Reference Kjellberg, Doumesche and Bronstein1988) and most are likely to die the day they emerge unless they manage to find a fig quickly. The spatial dispersal of Carnus can also be hampered by its low flight ability. Given that controlling the direction of flight in moving air is problematical for small insects (Dudley, Reference Dudley2000; Compton, Reference Compton and Bullock2002), carnid flies probably cannot effectively control their own flight direction, particularly in windy weather, therefore decreasing the probabilities of successful dispersal.
Data available for this parasite suggest that it can reach high prevalences and loads. High infestations have been reported for colonial species (Hoi et al., Reference Hoi, Kristofík, Darolová and Hoi2010) but also for bird species breeding at low densities (Roulin, Reference Roulin1998; Václav et al., Reference Václav, Calero-Torralbo and Valera2008). Yet, this information can be misleading since carnid pupae can accumulate in the nest during successive breeding seasons (Roulin et al., Reference Roulin1998; Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006). To our knowledge, just two studies report the colonization of clean nests by Carnus. Liker et al. (Reference Liker, Markus, Vozár, Zemankovics and Rózsa2001) reported 94% of infected nests in a colony of Common starlings (Sturnus vulgaris) and Soltész et al. (Reference Soltész, Seres and Kovács-Hostyánszki2018) found that 76% of Red-footed falcon nests (Falco vespertinus) were infected by this parasite. Both studies suggest that the colonization ability of carnid flies is high. Whereas such a high colonization rate can be due to the high density of nest boxes (see Liker et al., Reference Liker, Markus, Vozár, Zemankovics and Rózsa2001), colonization of new, isolated nest boxes has been observed frequently (Veiga et al., in prep.). Exceptional dispersal distances have also been reported for aphids and fig wasps, which share with Carnus the problems with aerial dispersal faced by small, delicate insects (Johnson, Reference Johnson1969).
It is known that dispersal may be either temporal or spatial and that temporal dispersal via developmental mechanisms (especially diapause) is functionally equivalent to spatial dispersal (Hairston, Reference Hairston2000; Hairston and Kearns, Reference Hairston and Kearns2002) and negatively correlated with migration (Hanski, Reference Hanski1988; Bohonak and Jenkins, Reference Bohonak and Jenkins2003). Given Carnus’ short lifespan of the dispersal stage, the seemingly low flight ability and frequently prolonged diapause (Valera et al., Reference Valera, Casas-Crivillé and Calero-Torralbo2006), one could assume that spatial dispersal is not imperative and that temporal dispersal is more important for this species. Yet, the non-occupation of infected cavities, nest failure (i.e. loss of feeding resources) or asynchrony between the emergence of the parasite and the occurrence of the hosts (Calero-Torralbo et al., Reference Calero-Torralbo, Václav and Valera2013) are important pressures forcing emerging flies to disperse spatially, what suggests that this species should be well-suited for detecting and reaching other hosts (i.e. occupied nests) in the surroundings. More studies on the colonization ability of Carnus on non-colonial or solitary host species could shed light on the dispersal ability and infectious potential of this parasite.
Acknowledgements
M.A. Calero-Torralbo provided interesting suggestions. Junta de Andalucía kindly provided permits to sample birds’ nests.
Financial support
F.V., E.M. and J.B. received financial support from the Spanish Ministry of Economy and Competitiveness (grant no CGL2014-55969-P) and the European Regional Development Fund. J.V. was funded by the Spanish Ministry of Economy, Industry and Competitiveness by means of a predoctoral grant (BES-2015-075951).
Conflict of interest
None.
Ethical standards
Not applicable.