INTRODUCTION
Deforestation and habitat fragmentation are considered to be the main causes of biodiversity loss in terrestrial ecosystems (Foley et al. Reference FOLEY, DEFRIES, ASNER, BARFORD, BONAN, CARPENTER, CHAPIN, COE, DAILY, GIBBS, HELKOWSKI, HOLLOWAY, HOWARD, KUCHARIK, MONFREDA, PATZ, PRENTICE, RAMANKUTTY and SNYDER2005, Sala et al. Reference SALA, CHAPIN, ARMESTO, BERLOW, BLOOMFIELD, DIRZO, HUBER-SANWALD, HUENNEKE, JACKSON, KINZIG, LEEMANS, LODGE, MOONEY, OESTERHELD, LEROY-POFF, SYKES, WALKER, WALKER and WALL2000). Although it has a global dimension, fragmentation has increased in the tropics where vascular epiphytes are an important component of tropical forests because of their richness and diversity (Gentry & Dodson Reference GENTRY and DODSON1987, Krömer et al. Reference KRÖMER, KESSLER, GRADSTEIN and ACEBEY2005, Nieder et al. Reference NIEDER, ENGWALD and BARTHLOTT1999). In communities of vascular epiphytes, forest fragmentation modifies the alpha and beta diversity because some populations disappear, and consequently, different assemblages are produced (Benzing Reference BENZING1990, Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Werner et al. Reference WERNER, HOMEIER and GRADSTEIN2005). Changes in the diversity of vascular epiphytes vary depending on the type of vegetation, the intensity of the habitat transformation, the size of their populations and their habitat preferences (Andrén Reference ANDRÉN1994, Köster et al. Reference KÖSTER, FRIEDRICH, NIEDER and BARTHLOTT2009).
After fragmentation, the remaining trees retain their primary epiphytes; however, many of the epiphytes subsequently die due to the new environmental conditions. Epiphytic orchids that are located in more humid areas with less radiation usually decline in disturbed habitats (Acebey et al. Reference ACEBEY, GRADSTEIN and KRÖMER2003, Hietz Reference HIETZ2005, Holz & Gradstein Reference HOLZ and GRADSTEIN2005). Some species of colonizing epiphytic orchid that establish on the edges of fragments and branches most exposed to drought and insolation are favoured by the disturbance (Hágsater et al. Reference HÁGSATER, SOTO-ARENAS, SALAZAR-CHÁVEZ, JIMÉNEZ-MACHORRO, LÓPEZ-ROSAS and DRESSLER2005, Hietz & Hietz-Seifert Reference HIETZ and HIETZ-SEIFERT1995, Kelly Reference KELLY1985, ter Steege & Cornelissen Reference TER STEEGE and CORNELISSEN1989). These patterns suggest that morphophysiological adaptations are crucial to the retention or establishment of vascular epiphytes in fragmented habitats (Wolf Reference WOLF2005).
Orchids are one of the best-studied groups of plants. However, there is little information regarding their responses to habitat alterations. Some studies have suggested that there are species of epiphytic orchids that can survive in transformed habitats (Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Solis-Montero et al. Reference SOLIS-MONTERO, FLORES-PALACIOS and CRUZ-ANGÓN2005, Williams-Linera et al. Reference WILLIAMS-LINERA, SOSA and PLATAS1995), while other studies have reported the local extinction of epiphytic orchids in certain habitats (Jacquemyn et al. Reference JACQUEMYN, BRYS, HERMY and WILLEMS2005, Olmsted & Gómez-Juárez Reference OLMSTED and GÓMEZ-JUÁREZ1996, Sosa & Platas Reference SOSA and PLATAS1998). However, there is no consensus on the response of epiphytic orchid species to habitat fragmentation. Understanding how the alteration and fragmentation of primary forests affect organisms is critical for conservation (Laurance Reference LAURANCE2007).
The studies addressing the human impact on the diversity of vascular epiphytes, including orchids, have compared their diversity in primary forests, secondary forests, isolated trees in pastures and coffee plantations (Benavides et al. Reference BENAVIDES, WOLF and DUIVENVOORDEN2006, Cascante-Marín et al. Reference CASCANTE-MARÍN, WOLF, OOSTERMEIJER, DEN NIJS, SANAHUJA and DURÁN-APUY2006, Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Hietz et al. Reference HIETZ, BUCHBERGER and WINKLER2006, Hundera et al. Reference HUNDERA, AERTS, BEENHOUWER, OVERTVELD, HELSEN, MUYS and HONNAY2013, Larrea & Werner Reference LARREA and WERNER2010, Nöske et al. Reference NÖSKE, HILT, WERNER, BREHM, FIEDLER, SIPMAN and GRADSTEIN2008, Werner Reference WERNER2011, Woods & DeWalt Reference WOODS and DEWALT2013). Köster et al. (Reference KÖSTER, FRIEDRICH, NIEDER and BARTHLOTT2009) analysed the influence of the area of vegetation fragments and their distance to a continuous forest. However, the response of epiphytic orchids as a function of fragment shape, the edge distance and the matrix that surrounds them has not been studied.
The present study aims to analyse the alpha and beta diversity of epiphytic orchids on a local scale in relation to the area and shape of the fragments, their isolation, edge density and the influence of the matrix. This study hypothesized that smaller, more isolated fragments and those with irregular shape and a larger edge density contain less diversity in epiphytic orchid species and that these species are more affected than the species that inhabit the interior of the fragments.
METHODS
Study area
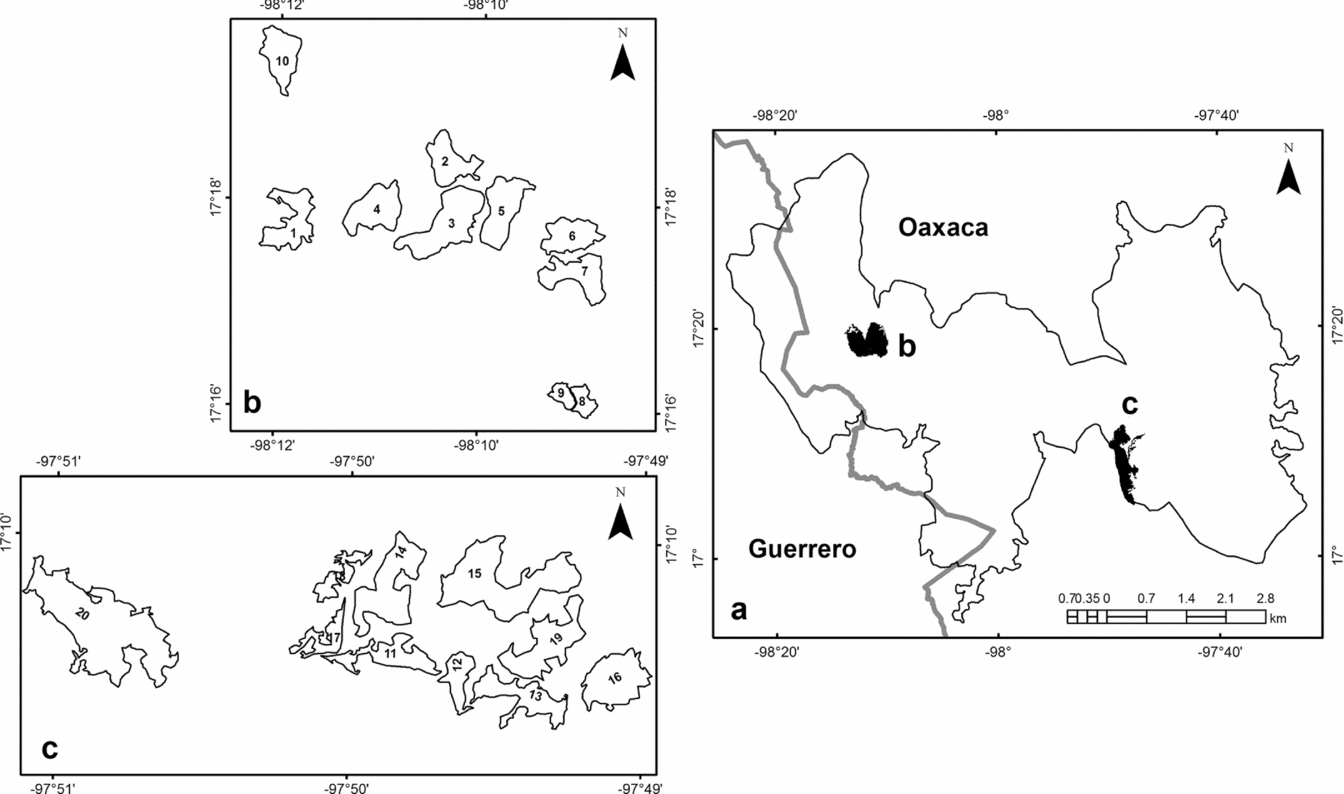
The study was conducted in two localities in the state of Oaxaca, Mexico, located in Priority Terrestrial Region 126, named by the Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, as the Sierras Triqui-Mixteca. The first site was located in San Andrés Chicahuaxtla (17°09′40″N, 97°49′52″W; 2290–2730 m asl), and the second site was in San Martín Peras (17°17′42″N, 98°10′16″W; 2480–2830 m asl) (Figure 1). According to the Köppen climate classification, modified by García (Reference GARCÍA2004), the predominant climate in the study area was sub-humid temperate C(w), with a mean annual temperature of 18°C and an annual rainfall of 1800 mm (Trejo Reference TREJO, García-Mendoza, Ordóñez and Briones-Salas2004). The vegetation consisted of pine and oak forests and fragments of montane cloud forest. The montane cloud forest was distributed in a fragmented formation and ranged between 2300 and 2700 m asl.

Figure 1. Location of the Sierras Triqui-Mixteca study area and sites of sample montane cloud forest fragments studied. Sierras Triqui-Mixteca (a), San Martín Peras (b), San Andrés Chicahuaxtla (c), Oaxaca, Mexico.
Delimitation of the montane cloud-forest fragments
Based on 1:75000 scale aerial photographs and field trips in each of the study areas, the montane cloud-forest fragments were identified. To evaluate the physical characteristics of each fragment, 20 fragments were randomly selected, including 10 in each site. The final selection was based on the permission of the owners and residents of the localities.
Fragmentation index
In each of the fragments, the area, edge density, core area, shape, Euclidean nearest-neighbour distance and the contrast between fragments was quantitated with the FRAGSTATS program version 3.3 (available at http://www.umas.edu/landeco/reasearch/fragstats.html). The patch area (PA) corresponds to its total area. The patch shape (PS) represents its complexity in relation to its perimeter, which is equal to one when the shapes are regular; this value increases when the perimeter of the fragment is irregular. The core area (CA) is defined as the area of the fragment located at a certain distance from the outer limits, that is, the area not affected by edge effects. In this study, the core area of the montane cloud-forest fragments was defined as the area located more than 60 m from the edges. This value was chosen because at this interior distance of 60 m, the influence of the edge was considerably attenuated. The Euclidean nearest-neighbour distance (ENN) is the distance between fragments with the same type of vegetation. The edge density (ED) is the sum of the lengths of all segments of the edge divided by the area of the fragment. The contrast index (CON) describes the degree of difference in ecological attributes between neighbouring fragments. A value between 0 and 1 was assigned for the CON, which describes the degree of dissimilarity of land use between the fragments along its perimeter. A value close to 1 indicates higher contrast and vice versa. According to Ochoa-Gaona et al. (Reference OCHOA-GAONA, GONZÁLEZ-ESPINOSA, MEAVE and SORANI-DAL BON2004), the contrast values used in this study were 0.75 when the area of the fragment was in contact with human settlements and agricultural and livestock areas; 0.50 if the fragment edge was bordered by secondary forest; 0.25 for fragments whose edges adjoined other types of vegetation; and zero when a portion of the edge of the patch was in contact with a piece of forest that was better preserved than the fragment being analysed.
Sampling of epiphytic orchids
Two transects of 2 × 50 m, oriented inward from the edge of the fragment to the core area, were drawn in each fragment. The transects were placed on opposite sides of the fragments. The phorophyte samples with a diameter at breast height (dbh) ≥ 20 cm were collected and were taxonomically identified in each transect. In the phorophyte samples, the number of individuals of each species of epiphytic orchid was quantified and recorded using a single-rope technique to ascend to them (Barker Reference BARKER1997, Barker & Sutton Reference BARKER and SUTTON1997), combined with binocular observation from the ground (Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2001). According to Johansson (Reference JOHANSSON1974) and Sanford (Reference SANFORD1968), the orchids with sympodial growth and vegetative propagation that formed colonies were considered as one individual. The orchid species that were not recognized in the field were collected for identification. All the specimens were collated with the collections of FEZA and MEXU and were subsequently reviewed by a specialist.
Statistical analysis
The first level of alpha diversity represents the number of species of epiphytic orchid on each phorophyte (αt). To establish the differences between levels of diversity, an analysis of covariance (ANCOVA) was performed using the basal area of the trees as a covariate. A correlation analysis was performed to analyse the relationship between the size of the phorophytes and the richness of the epiphytic orchids. The lack of homoscedasticity in the richness (S) of each tree was solved by its transformation with the formula ![]() $\sqrt {S + 1}$ (Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Zar Reference ZAR1996). A correlation analysis was performed to analyse the variation in the punctual alpha diversity of the epiphytic orchids from the edge to the interior of the fragments.
$\sqrt {S + 1}$ (Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Zar Reference ZAR1996). A correlation analysis was performed to analyse the variation in the punctual alpha diversity of the epiphytic orchids from the edge to the interior of the fragments.
Based on the abundance of epiphytic orchids, the species turnover between trees (βt) of each transect was quantified by calculating the dissimilarity between pairs of trees using the reciprocal of the Jaccard similarity index (1 − IJ). According to Flores-Palacios & García-Franco (Reference FLORES-PALACIOS and GARCÍA-FRANCO2008), the reciprocal of this index is used because the punctual beta diversity expresses species turnover. To establish the differences between the species composition of orchids among phorophytes using the dissimilarity values obtained, an analysis of similarities (ANOSIM) with the PAST program (Hammer et al. Reference HAMMER, HARPER and RYAN2001) was performed.
At the next level of diversity, alpha diversity is the sum of the species of epiphytic orchid found in the phorophytes of each fragment, while beta diversity is the species turnover between the fragments. The observed richness was compared with the estimated richness using the Clench species accumulation function to differentiate between the alpha diversity of the fragments (Michaelis–Menten richness estimator). The input order of the sampling effort units (number of phorophytes per fragment) and the number of species observed were randomized 500 times with 95% confidence intervals using the program EstimateS 7.5 developed by Robert K. Colwell (available at http://purl.oclc.org/estimates). The functions a and b were fitted for each fragment, in which a is the growth rate of new species at the start of the inventory, and b relates to the shape of the curve. The fitting of these functions was performed by a non-linear estimation with the Quasi-Newton algorithm of the Statistica 7.0 software. The total number of species estimated was calculated as a/b in the Clench model. The sampling effort was quantified by calculating the slope at the end of the curve with the equation Nq = q/[b(1 − q)], where Nq represents the sampling effort, and q represents the number of observed species.
To determine the differences in the composition of epiphytic orchids between fragments, a dissimilarity matrix (1 − IJ) was created, and an analysis of non-metric multidimensional scaling (NMDS, Gauch Reference GAUCH1982) was conducted for each fragment. The interpretation of the axis was performed by an analysis of correlation between the fragmentation indices and the axis of the NMDS. The relationship between the geographic distance of the fragments and the beta diversity of the epiphytic orchids was determined by a Mantel test.
The effect of fragmentation on the alpha diversity of epiphytic orchids was determined with a multiple linear regression. In this analysis, the alpha diversity was used as the dependent variable, and the fragmentation indices were used as the independent variables. All variables underwent a natural logarithm transformation before analysis. A fragmentation index with a variance inflation factor (VIF) greater than 10 was considered to have high multicollinearity (Graham Reference GRAHAM2003). High multicollinearity indices were excluded from the model. The influence of fragmentation indexes in the species distribution of epiphytic orchids was quantified by a canonical analysis of principal coordinates in the CAP program (Canonical Analysis of Principal Coordinates) (Anderson Reference ANDERSON2004). In this analysis we considered the correlations of individual species with canonical axis to characterize the multivariate effect. We did not include any species that occurred in fewer than 10 observations.
RESULTS
Fragments characteristics
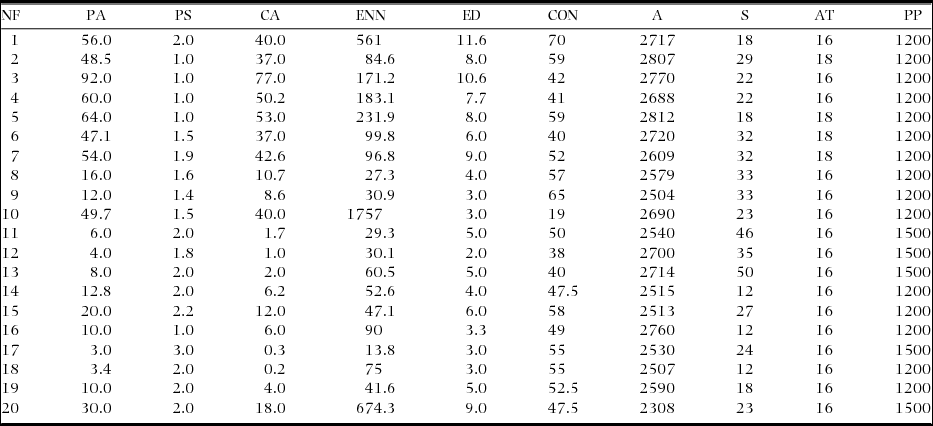
The 20 montane cloud-forest fragments analysed in this study had areas of 3 to 48 ha (mean ± SD; 30.3 ± 26.2 ha). These fragments were in matrices of contrast mediums ranging between 38% and 70% (49.8% ± 11.2%). The Euclidean distances to the nearest neighbour fragment were recorded to be between 13 and 1756 m (402 ± 217 m). The shape of the fragments had an average value of 1.6 ± 0.52. The edge density between the fragments ranged from 3 to 11.6 m ha−1 (5.8 ± 2.8 m ha−1). The fragments had an inner area of 1 to 77 ha−1 (22.3 ± 22.6 ha−1). In the montane cloud-forest fragments analysed, the average altitude was 2629 ± 129.7 m asl, and the average slope was 26% ± 10.4% (Table 1).
Table 1. Characteristics of 20 fragments of montane cloud forest located in the south of Mexico. Fragments 1–10 located in San Martín Peras, Oaxaca, Mexico. Fragments 11–20 located in San Andrés Chicahuaxtla, Oaxaca, Mexico. NF, Number of fragments; PA, Patch area (ha−1); PS, Patch shape; CA, Core area (ha−1); ENN (m), Euclidean nearest-neighbour distance; ED (m), Edge density; CON (%), Contrast index; A, Altitude (m asl); S, Slope (degrees); AT, Mean annual temperature (°C); PP, Mean annual precipitation (mm).

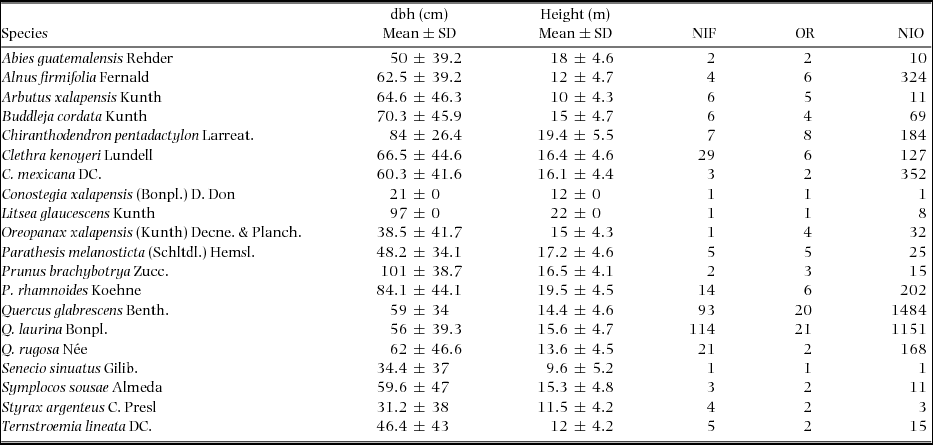
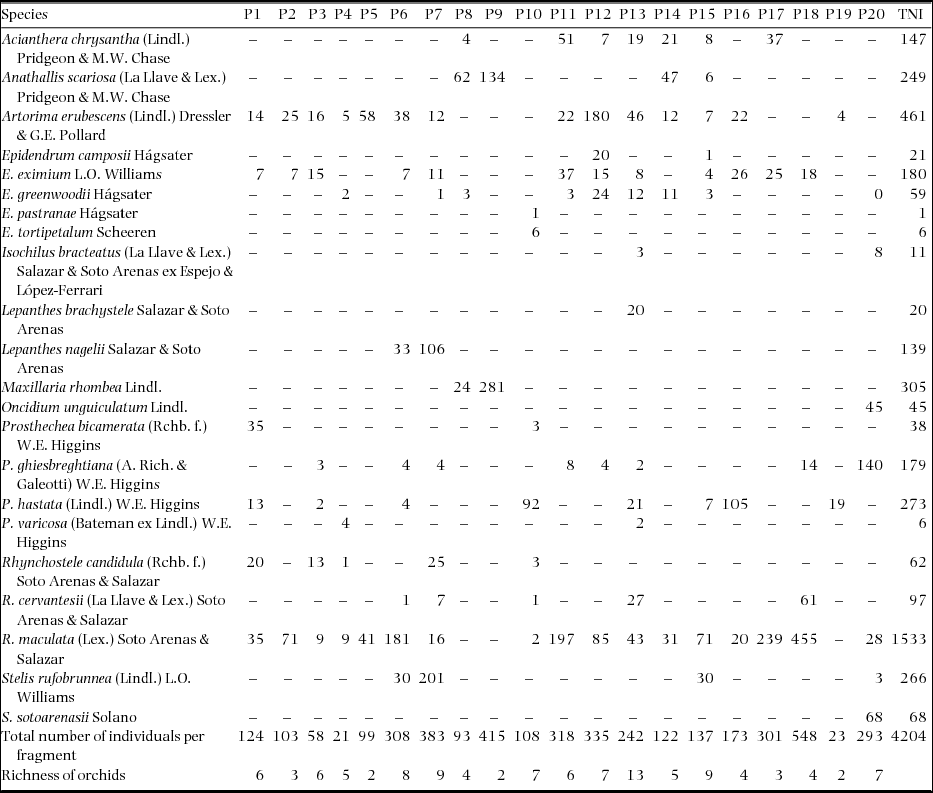
In both locations, 356 trees with an average dbh of 62.4 ± 39.2 cm and an average height of 15.3 ± 4.6 m were inventoried. In fragments 1 and 20, the trees with the largest dbh (116.3 and 108.8 cm, respectively) were recorded, and the trees with the smallest dbh were recorded in fragments 14 and 19 (27 and 38.4 cm, respectively). A total of 4204 individuals corresponding to 11 genera and 23 species of epiphytic orchid (Appendix 1) were recorded on 234 phorophytes belonging to 16 families, 14 genera and 20 species (Appendix 2).
Alpha and beta diversity of epiphytic orchids between phorophytes
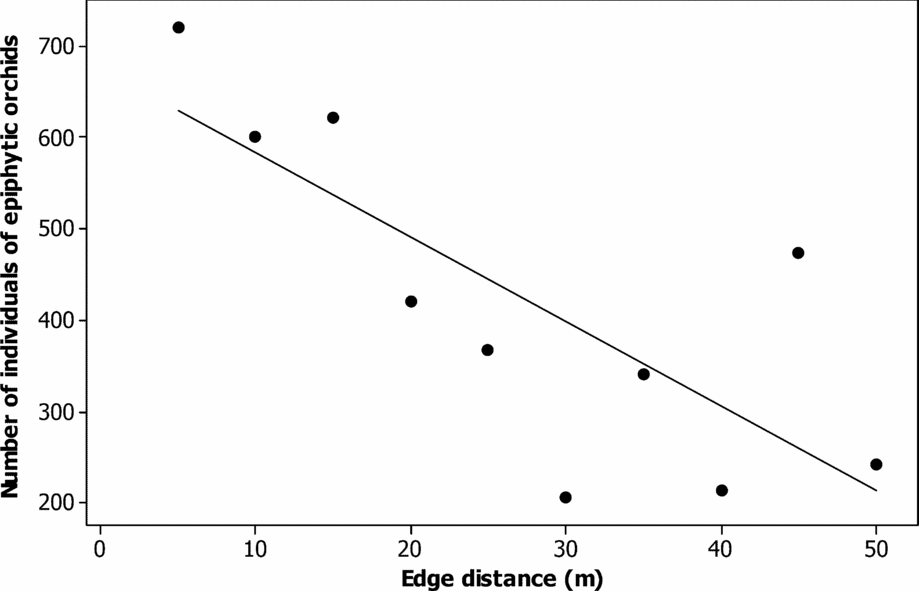
The epiphytic orchid richness per tree was different between the phorophytes (F = 4.93, P < 0.0001) and was positively correlated with the basal area (r = 0.30, P < 0.0001). The average species turnover between phorophytes was 0.70 ± 0.08. Species turnover between trees was different (ANOSIM R-statistic = 0.62, P < 0.0001). The epiphytic orchid richness per tree was independent of the distance from the edge to the core area of the fragments of montane cloud forest (r = 0.3, P = 0.26). The number of individuals of epiphytic orchid was negatively correlated with the distance from the edge to the interior of the fragments analysed (r = −0.77, P = 0.009) (Figure 2).

Figure 2. Linear regression showing the relationship between number of individuals of epiphytic orchid and the distance from the edge to core area in 20 montane cloud-forest fragments in southern Mexico (r = −0.77, P = 0.009).
Alpha and beta diversity of epiphytic orchids between fragments
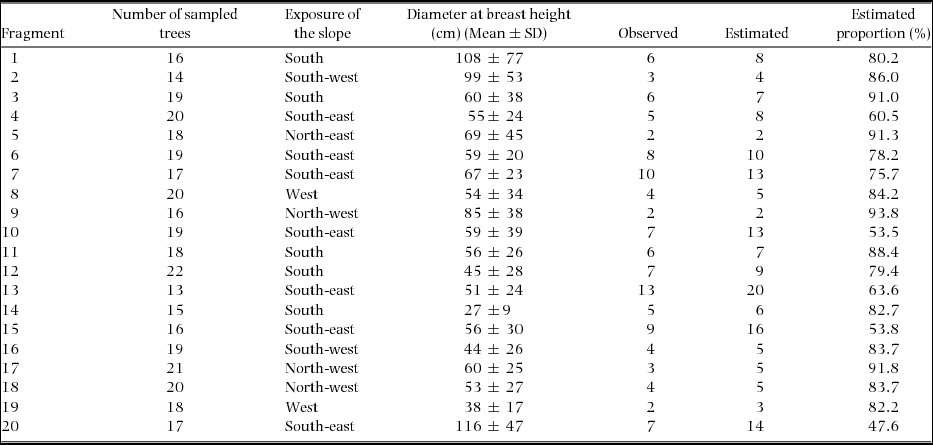
The Clench model recorded between 50% and 90% of the species of epiphytic orchids in the 20 montane cloud-forest fragments analysed (Table 2). The coefficient of determination indicated an adequate fit of the data (R2 = 0.99). The size of the phorophytes did not differ between the fragments (F = 0.9, P = 0.1). The highest alpha diversity was recorded in fragments 13, 7 and 15, and the lowest was recorded in fragments 5, 2, 9, 17 and 19. In the fragments where the largest number of epiphytic orchid species was recorded, the exposure was oriented to the south-east, while in the fragments with the lowest richness, the slope exposure was to the west and north-east.
Table 2. Number of sampled trees (dbh ≥ 20 cm), exposure of the slope of the fragments, mean tree size (diameter at breast height) and alpha diversity of the epiphytic orchids observed and estimated (Clench model asymptote) in the 20 montane cloud-forest fragments in southern Mexico.

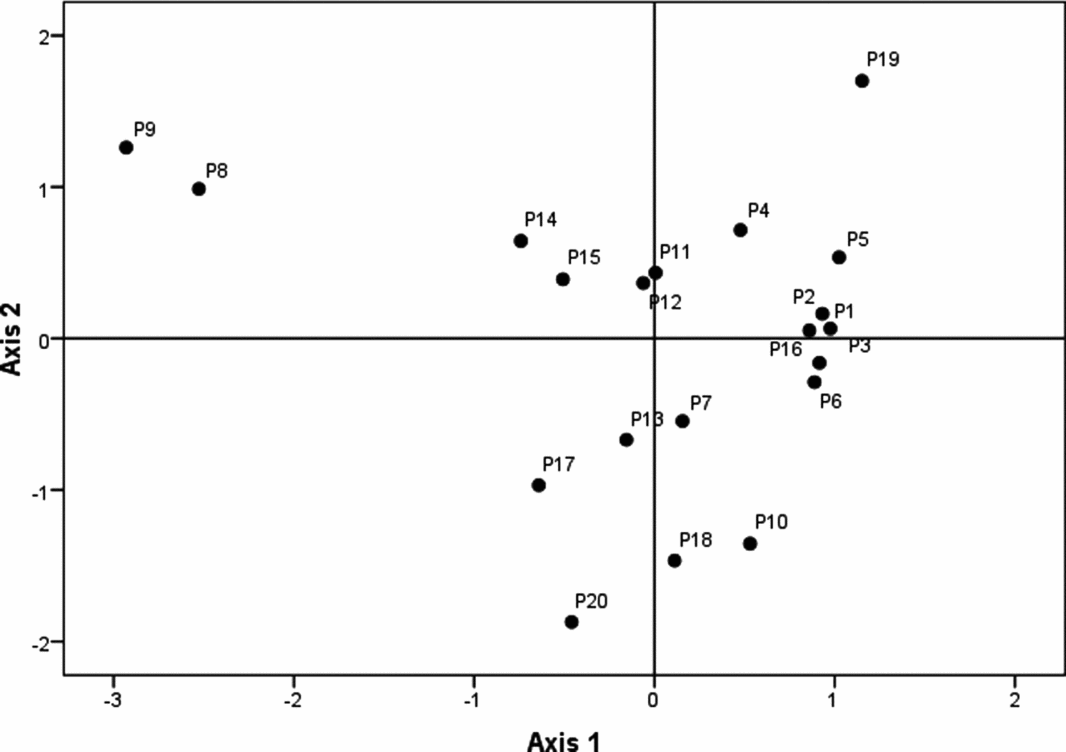
The NMDS showed that dimensions 1 and 2 pooled the fragments whose floristic composition of epiphytic orchids was similar (stress = 0.22, R2 = 0.80). Axis 1 was positively correlated with ENN (r = 0.47, P < 0.05), and axis 2 was negatively correlated with ENN (r = −0.457, P < 0.05); both dimensions were independent of the shape of the fragment, core area, edge density and contrast index (P > 0.05). Figure 3 shows two large groups of fragments, with the first located in the positive values of dimension 1 where there were widespread species (Artorima erubescens and Rhynchostele maculata). The second group was located in the negative values of dimension 2 where the restricted species were located (Epidendrum greenwoodii and Anathallis scariosa). The Mantel test results indicated that beta diversity and the distance between the fragments were significantly correlated (r = 0.26, P = 0.0001).

Figure 3. Floristic dissimilarity of epiphytic orchids in 20 study montane cloud-forest fragments in southern Mexico. Two-dimensional scatter plot of non-metric scaling based on Jaccard's dissimilarity values (stress = 0.22, R2 = 0.80 for non-metric fit). Points represent montane cloud-forest fragments.
Fragmentation indices and alpha diversity of epiphytic orchids
The multiple linear regression analysis indicated that the fragmentation indices had significant effects on alpha diversity (F = 3.18, P < 0.05, R2 = 0.459). The area and the core area of the fragments were excluded from the model because they showed a high multicollinearity (VIF > 10).
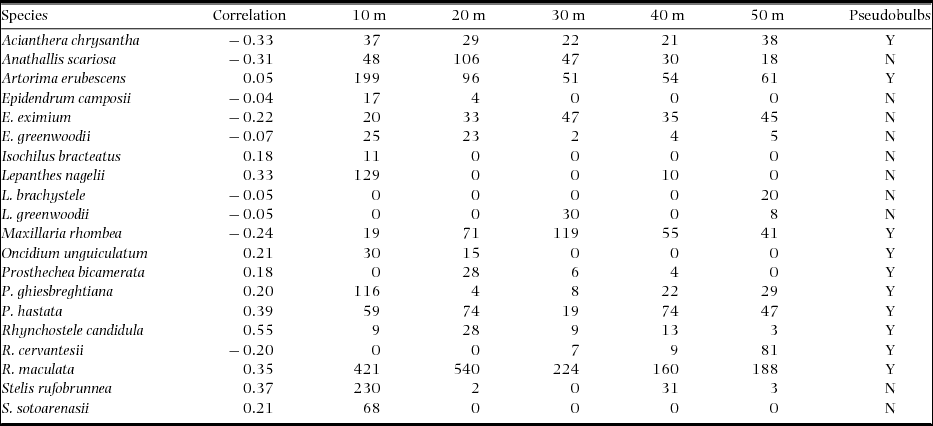
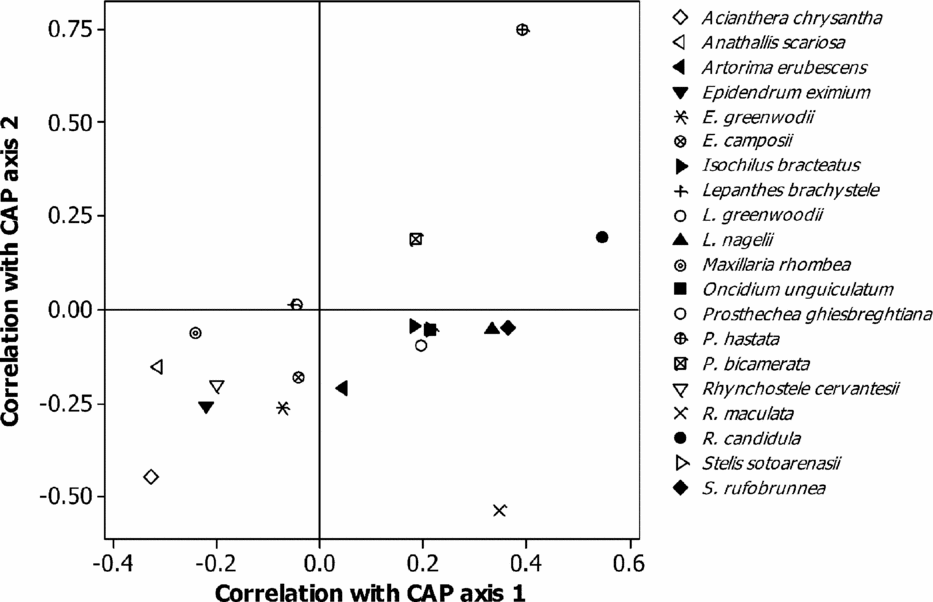
The canonical analysis of the principal coordinates reported a significant difference between the canonical correlations (δ2 = 0.72, P = 0.045). The first axis explained 22.1% of the variability in the original dissimilarity matrix and was positively correlated with edge density. The second axis (17.3%) was positively correlated with ENN (Table 3). Positive correlations of the species with axis 1 indicate that the number of individuals increased with increasing edge density, while negative correlations indicate that the number of individuals decreased with a reduction of this attribute (Table 4). By relating the richness of epiphytic orchids with the first canonical axis, two groups were formed (Figure 4). The species of group I (Artorima erubescens, Isochilus bracteatus, Lepanthes nagelii, Oncidium unguiculatum, Prosthechea bicamerata, P. ghiesbreghtiana, Rhynchostele candidula, Stelis rufobrunnea and S. sotoarenasii) had an increasing number of individuals as the edge density increased. In the species of group II (Acianthera chrysantha, Anathallis scariosa, Epidendrum camposii, E. greenwoodii, E. eximium, Lepanthes brachystele, L. greenwoodii, Maxillaria rhombea, Rhynchostele cervantesii and R. maculata), the number of individuals decreased when the edge density increased; however, Prosthechea hastata maintained the same number of individuals in both the edge and the interior of the fragments (Table 4).
Table 3. Matrix of correlations values of first two axes of canonical analysis of principal coordinates (CAP) with fragmentation indices to characterize effect multivariate in 20 montane cloud-forest fragments in southern Mexico.

Table 4. Species of epiphytic orchids showing correlations with canonical axis 1 of the canonical analysis of principal coordinates, that separated the five different distances from the edge to core area of the fragments (10–50 m) in 20 montane cloud-forest fragments in southern Mexico. Y, with pseudobulbs; N, without pseudobulbs.


Figure 4. CAP ordination diagram to illustrate the correlations of epiphytic orchid species with first two axes of the canonical analysis of principal coordinate in 20 montane cloud-forest fragments in southern Mexico. The symbols represent the epiphytic orchid species.
DISCUSSION
The montane cloud-forest fragments analysed had different areas, were irregularly shaped, were surrounded by transformed areas and had a high degree of isolation. Similar results were obtained in other studies (Williams-Linera Reference WILLIAMS-LINERA1993, Williams-Linera et al. Reference WILLIAMS-LINERA, MANSON and ISUNZA2002), which found that these fragmented ecosystems formed a mosaic with a matrix of crops and pastures with scattered trees. Most of the fragments analysed in this study are likely remnants of primary forests and were created by anthropic activities.
Alpha and beta diversity of epiphytic orchids between phorophytes
The differences in the alpha and beta diversity were influenced by the size and species of phorophytes. This relationship has been documented in vascular epiphytes in different studies (Flores Palacios & García Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2006, Hietz Reference HIETZ2005, Hietz & Hietz-Seifert Reference HIETZ and HIETZ-SEIFERT1995, Hirata et al. Reference HIRATA, KAMIJO and SAITO2009, Moorhead et al. Reference MOORHEAD, PHILPOTT and BICHIER2010, Zotz & Schultz Reference ZOTZ and SCHULTZ2008). According to Flores-Palacios & García-Franco (Reference FLORES-PALACIOS and GARCÍA-FRANCO2006), Gradstein et al. (Reference GRADSTEIN, NADKARNI, KRÖMER, HOLZ and NÖSKE2003), Krömer & Gradstein (Reference KRÖMER and GRADSTEIN2003) and Malizia (Reference MALIZIA2003), larger phorophytes generally contain more species than small trees because the former have a greater variety of micro-environments to be colonized. Other studies have reported that the increment of epiphyte diversity with phorophyte size differs between species because each species has particular characteristics (type of bark and architecture, among others) (Burns & Dawson Reference BURNS and DAWSON2005, Callaway et al. Reference CALLAWAY, REINHART, MOORE, MOORE and PENNINGS2002). For epiphytic orchids, the chemical characteristics of the cortex are crucial for the development of mycorrhizae (Hietz & Hietz-Seifert Reference HIETZ and HIETZ-SEIFERT1995, Otero et al. Reference OTERO, ACKERMAN and BAYMAN2002).
Alpha and beta diversity among fragments
The differences between fragments of the observed and estimated alpha diversity of epiphytic orchids on the Clench model, could be related to the species composition and dominance of phorophytes in each fragment. In the fragments which dominate Quercus laurina, Q. glabrescens and Chiranthodendron pentadactylon, contain the largest number of species of epiphytic orchids, this indicates that some species of phorophytes could limit or favour the establishment of these plants. Similar results have been described in different studies (Callaway et al. Reference CALLAWAY, REINHART, MOORE, MOORE and PENNINGS2002, Hietz & Hietz-Seifert Reference HIETZ and HIETZ-SEIFERT1995, Hirata et al. Reference HIRATA, KAMIJO and SAITO2009, Malizia Reference MALIZIA2003, Mehltreter et al. Reference MEHLTRETER, FLORES-PALACIOS and GARCÍA-FRANCO2005, Migenis & Ackerman Reference MIGENIS and ACKERMAN1993, Tremblay et al. Reference TREMBLAY, ZIMMERMAN, LEBRÓN, BAYMAN, SASTRE, AXELROD and ALERS-GARCÍA1998). We also observed that the phorophytes that developed on slopes with exposure along the south direction received more moisture than those located on north-facing slopes, which could influence the greater number of epiphytic orchid species that were recorded in the fragments with this orientation.
The distance between fragments had a negative effect on the diversity of epiphytic orchids because it produced discontinuity in the species distribution patterns. This effect has been reported in isolated trees in Mexican montane cloud forest (Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Hietz-Seifert et al. Reference HIETZ-SEIFERT, HIETZ and GUEVARA1996). Köster et al. (Reference KÖSTER, FRIEDRICH, NIEDER and BARTHLOTT2009) recorded that the distance between fragments had no effect on diversity, suggesting that the effects of fragmentation are related to limitations in the dispersion and size of populations, which act over time. The presence or absence of vascular epiphytes in forest fragments in matrices of anthropogenic use is related to the capacity and type of dispersal of this group of plants (Cascante-Marín et al. Reference CASCANTE-MARÍN, VON MEIJENFELDT, DE LEEUW, WOLF, OOSTERMEIJER and DEN NIJS2009, Snäll et al. Reference SNÄLL, PENNANEN, KIVISTÖ and HANSKI2005). It was thought that epiphytes have a limited dispersal because they tend to colonize phorophytes randomly and are added around the mother plant, but other factors, such as the microclimate and substrate, also have an influence (Cascante-Marín et al. Reference CASCANTE-MARÍN, WOLF, OOSTERMEIJER, DEN NIJS, SANAHUJA and DURÁN-APUY2006, Krömer & Gradstein Reference KRÖMER and GRADSTEIN2003, Laube & Zotz Reference LAUBE and ZOTZ2006).
The absence of re-colonization events due to dispersal limitation causes a decrease in the number of species among fragments and modifies its floristic composition. Several studies have indicated that species turnover in vascular epiphytes may increase or decrease as a result of fragmentation (Köster et al. Reference KÖSTER, FRIEDRICH, NIEDER and BARTHLOTT2009, Nöske et al. Reference NÖSKE, HILT, WERNER, BREHM, FIEDLER, SIPMAN and GRADSTEIN2008, Werner et al. Reference WERNER, HOMEIER and GRADSTEIN2005, Wolf Reference WOLF2005). According to the NMDS analysis, groups of fragments were distinguished that had a similar composition of epiphytic orchids. The assembly of species in these fragments is most likely dominated by pioneer species.
Effects of fragmentation on the diversity of epiphytic orchids
The results of the present study show that variation in alpha diversity is related to the edge effect. This observation had already been reported in other studies in montane cloud forests (Barthlott et al. Reference BARTHLOTT, SCHMIT-NEURERBURG, NIEDER and ENGWALD2001, Flores-Palacios & García-Franco Reference FLORES-PALACIOS and GARCÍA-FRANCO2008, Krömer & Gradstein Reference KRÖMER and GRADSTEIN2003). In this study, the number of individuals increased in the first 30 m of the edge and decreased towards the core area of the fragments studied. However, Werner & Gradstein (Reference WERNER and GRADSTEIN2008) and Werner et al. (Reference WERNER, HOMEIER and GRADSTEIN2005) mentioned that the diversity of vascular epiphytes was lower on phorophytes exposed to edge effects because xeric taxa preferably settle in those areas. In contrast, Köster et al. (Reference KÖSTER, FRIEDRICH, NIEDER and BARTHLOTT2009) described that this species turnover comes from initial stages of the fragmentation process, and at times, hygrophilous epiphytes are able to persist on the edges.
Some epiphytic orchids of the genera Prosthechea and Rhynchostele were recorded in this study that thrived on the edges of the fragments; the same results were recorded by Barthlott et al. (Reference BARTHLOTT, SCHMIT-NEURERBURG, NIEDER and ENGWALD2001) in a montane forest of Venezuela. Rhynchostele maculata was abundant in the montane cloud forest fragments analysed, and hundreds of individuals were observed in only one phorophyte, which already had been referred by Hágsater et al. (Reference HÁGSATER, SOTO-ARENAS, SALAZAR-CHÁVEZ, JIMÉNEZ-MACHORRO, LÓPEZ-ROSAS and DRESSLER2005). According to Salazar-Chávez & Soto-Arenas (Reference SALAZAR-CHÁVEZ and SOTO-ARENAS1996), some species of the genera Lepanthes and Stelis have a wide ecological tolerance; however, in this study, a low diversity was recorded because fragmentation reduced their populations. Artorima erubescens was also abundant at the edges of the fragments studied and had settled in the highest parts of the canopy where microclimatic alterations show greater fluctuations. Krömer et al. (Reference KRÖMER, KESSLER and GRADSTEIN2007) indicated that epiphytic orchids with pseudobulbs have strong preferences for the canopy of phorophytes and can thrive in disturbed sites. According to Flores-Palacios & García-Franco (Reference FLORES-PALACIOS and GARCÍA-FRANCO2008), Krömer & Gradstein (Reference KRÖMER and GRADSTEIN2003) and Nöske et al. (Reference NÖSKE, HILT, WERNER, BREHM, FIEDLER, SIPMAN and GRADSTEIN2008), microclimatic changes in the canopy may explain why epiphyte species more tolerant to drought are present in fragmented landscapes.
In this study, it was observed that the epiphytic orchids less abundant at the edges of the fragments were those without pseudobulbs, such as Acianthera chrysantha, Epidendrum pastranae, E. tortipetalum, E. camposii, E. greenwoodii, Lepanthes brachystele and L. greenwoodii; these orchids were found at the base of the stems and in the primary branches of phorophytes. These results agree with those obtained by Krömer et al. (Reference KRÖMER, KESSLER and GRADSTEIN2007) and ter Steege & Cornelissen (Reference TER STEEGE and CORNELISSEN1989) who studied the diversity of vascular epiphytes in South American cloud forests. However, Johansson (Reference JOHANSSON1974) and Parker (Reference PARKER, Lowman and Nadkarni1995) described that in these areas, light decreases, moisture increases and the microclimate conditions in the trunk are relatively constant. These factors could enable the establishment and development of these types of plants in fragmented habitats. Mehltreter et al. (Reference MEHLTRETER, FLORES-PALACIOS and GARCÍA-FRANCO2005), in montane cloud forests in the state of Veracruz, Mexico, recorded a high diversity of epiphytes that developed on the shaft of the phorophytes. These distribution patterns are related to the tolerance of epiphytes to light and moisture or to their eco-physiological adaptations (Jácome et al. Reference JÁCOME, GALEANO, AMAYA and MORA2004, Krömer et al. Reference KRÖMER, KESSLER and GRADSTEIN2007, ter Steege & Cornelissen Reference TER STEEGE and CORNELISSEN1989).
Based on the results obtained in this study, the drought-tolerant taxa were not dramatically affected by fragmentation and could successfully establish at the edges. According to Tremblay & Salguero-Faría (Reference TREMBLAY and SALGUERO-FARÍA2001), the edge provides an environment that increases the production of fruits and seeds in some species of orchids, including Lepanthes woodburyana. However, in other taxa, the edges negatively affect reproductive success and decrease the efficiency of pollination, such as in the epiphytic orchids Oncidium ascendens and Catasetum viridiflavum (Murren Reference MURREN2003, Parra-Tabla et al. Reference PARRA-TABLA, VARGAS, MAGAÑA-RUEDA and NAVARRO2000).
The hypothesis set forth in this study was confirmed: changes in the diversity of epiphytic orchids at the local scale are related to the combined effects of edge density, contrast index, shape and distance between fragments. The edge density and contrast between fragments are the main attributes of fragmentation affecting the alpha diversity of epiphytic orchids. The isolation between fragments is negatively related to beta diversity mainly because epiphytic orchids are limited in their dispersal. Individuals increase with proximity to the edge of the fragments in some species of epiphytic orchids but decrease in others, indicating a high degree of habitat specialization. In the current landscape, the montane cloud forests remnants are habitats where epiphytic orchids survive. The ecological amplitude of the epiphytic orchids registered in the montane cloud forests studied is crucial to its establishment, development and retention in fragmented habitats, although the degree of adaptation to microclimate conditions that are modified by fragmentation depends on the species of epiphytic orchid. Analysing and explaining the role of the ecological factors that are modified during fragmentation is a complex task because ecological information of the primary forest is generally lacking. This information is essential for a proper analysis that allows for the establishment of differences in the diversity of epiphytic orchids before and after fragmentation.
ACKNOWLEDGEMENTS
T. P. Feria Arroyo and José García-Franco reviewed the manuscript and contributed important comments to improve it. During research development, Irma Trejo Vázquez contributed methodology recommendations. Gerardo Salazar Chávez identified the species of orchids. Ramiro Ríos Gómez helped with the fieldwork. This project was funded by the Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México – PAPIIT (IN225210-3 agreement). The first author thanks the Consejo Nacional de Ciencia y Tecnología for the scholarship during his graduate studies (fellow number: 165051) and the Posgrado en Ciencias Biológicas, UNAM, for the training received during his studies.
Appendix 1. Phorophyte species (dbh ≥ 20 cm), mean diameter at breast height (dbh), mean height and richness of epiphytic orchids observed in 20 montane cloud-forest fragments in southern Mexico. NIF, Number of individuals (phorophytes); OR, Orchid richness; NIO, Number of individuals (epiphytic orchids).

Appendix 2. Data matrix representing the frequencies of observations of epiphytic orchid species in 20 montane cloud forest fragments in southern Mexico. P, patch; TNI, Total number of individuals.