INTRODUCTION
Interactions between different species of animals, or functional guilds of animals with shared or very similar niches, are the cornerstone of community ecology (Pedersen and Fenton, Reference Pedersen and Fenton2006; Suttle et al. Reference Suttle, Thomsen and Power2007; Graham, Reference Graham2008), and indeed it has been argued that such interactions define communities, contrasting them with assemblages of animals where the presence or absence of species is considered to be a purely random event (Janovy, Reference Janovy2002).
Most species of free-living hosts are subject to infection by a range of parasitic organisms and in nature, it is rare to encounter completely uninfected hosts. Indeed most wild animals, and also humans, particularly those living in impoverished communities in developing regions of the world (Keusch and Migasena, Reference Keusch and Migasena1982), harbour several species of parasites concurrently, ‘polyparasitism’ in the medical literature (Buck et al. Reference Buck, Anderson and MacRae1978a, Reference Buck, Anderson and MacRaeb). Whether multiple species infections actually constitute communities of helminths within hosts, so called infracommunities, has been a subject of debate for several decades. In some hosts, notably birds that harbour species-rich helminth communities, the evidence is indisputable, but in others such as fish and wild rodents characterized by relatively depauperate helminth communities, evidence in support of a role for interactions in structuring communities has been elusive (reviewed Behnke et al. Reference Behnke, Bajer, Sinski and Wakelin2001). This contrasts markedly with laboratory studies where interactions between helminths have been clearly demonstrated and mostly assigned to interactions mediated through the immune response of the host (Behnke et al. Reference Behnke, Bajer, Sinski and Wakelin2001; Behnke, Reference Behnke2008). The search for evidence of interactions among wild-caught hosts, has been hampered by a number of difficulties; for example, the statistical analyses required to dissect out the evidence for interactions is complex (Haukisalmi and Henttonen, Reference Haukisalmi and Henttonen1998; Howard et al. Reference Howard, Donnelly and Chan2001; Bottomley et al. Reference Bottomley, Isham and Basáñez2005), and there is a lack of support for any specific interaction between particular parasites being a consistent enough feature wherever both species of parasites co-occur with the host. In contrast, a good example of a positive relationship that has been recorded many times in the medical literature, is that between the human intestinal nematodes Trichuris trichiura and Ascaris lumbricoides which not only co-occur but also show a positive quantitative relationship wherever the two species are endemic in human communities (Holland et al. Reference Holland, Asaolu, Crompton, Stoddart, MacDonald and Torimiro1989; Booth and Bundy, Reference Booth and Bundy1992; Howard et al. Reference Howard, Donnelly, Kabatereine, Ratard and Brooker2002; Tchuem Tchuenté et al. Reference Tchuem Tchuenté, Behnke, Gilbert, Southgate and Vercruysse2003; Behnke, Reference Behnke2008).
In our earlier paper (Behnke et al. Reference Behnke, Gilbert, Abu-Madi and Lewis2005) we demonstrated 2 marked interactions among helminths of wild wood mice in the UK in 3 ecologically contrasting habitats; the positive relationship (evident as both co-occurrence and as a positive quantitative relationship) between Heligmosomoides polygyrus and the tapeworm Catenotaenia pusilla; and the positive relationship between H. polygyrus and species richness of other helminths. Both relationships are consistent with the idea that the long-lived H. polygyrus (Gregory et al. Reference Gregory, Keymer and Clarke1990), like its close relative H. bakeri (which, being a parasite of house mice, Mus musculus, has been explored extensively in laboratory work; Behnke, Reference Behnke1987; Behnke et al. Reference Behnke, Barnard and Wakelin1992; Monroy and Enriquez, Reference Monroy and Enriquez1992), is aided in its survival strategy in wood mice by suppressing its host's capacity to mount protective immune responses in the intestine, and consequently provides an opportunity for other species to benefit from the altered host environment and to establish in and survive for longer than they otherwise might (reviewed by Behnke et al. Reference Behnke, Bajer, Sinski and Wakelin2001).
Whilst evidence of interactions between helminths in wild systems is not rare in the parasitological/ecological literature (Kisielewska, Reference Kisielewska1970; Montgomery and Montgomery, Reference Montgomery and Montgomery1990; Haukisalmi and Henttonen, Reference Haukisalmi and Henttonen1993), to date no relationship has stood the test of re-examination and been substantiated in other ecosystems where both the same host and parasite exist. In this paper, building on our initial report that the presence of H. polygyrus increases the likelihood that wood mice will harbour other species of helminths, we explore 2 entirely new datasets together with an extended dataset already partially analysed, with the specific aim of testing the robustness of our earlier findings. The first concerns helminths in wood mice from Portugal collected over a single calendar year, but from 6 ecologically contrasting habitats across all 4 seasons; the other 2 are from uniform habitats collected at the same time of the year (late summer) for 9 (Egham) and 16 (Malham) years. The extrinsic factors (e.g. year, season, site) known to influence helminth burdens differed between these 3 datasets, as did helminth species richness. Hence, if the relationship between H. polygyrus and the species richness of other helminth species can be demonstrated in these 3 independently collected datasets, then for the first time we will have demonstrated a robust element of the infracommunity structure of the helminths of A. sylvaticus in Europe.
MATERIALS AND METHODS
Datasets
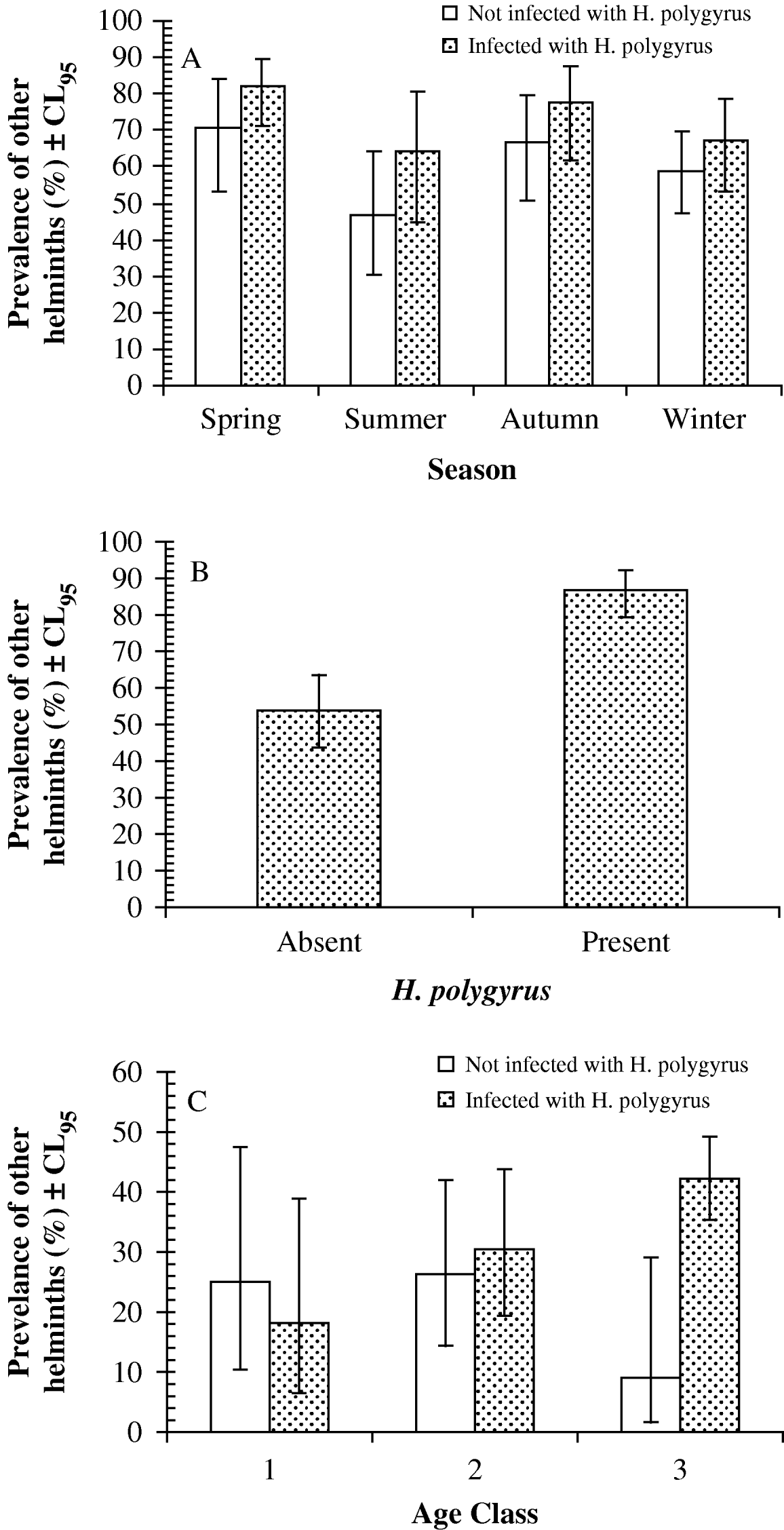
The first dataset is derived from wood mice trapped in a coastal sand dune region (Dunas de Mira) in Portugal and localities in close proximity. In this ‘Portuguese’ dataset, wood mice were caught in 6 ecologically distinct sites represented by sand dunes, dune meadows, coastal pinewoods, pinewoods burned in 1993, lake margins and agricultural fields. The mice analysed were caught between autumn 2004 and the summer of 2005, structured into 4 seasons corresponding to autumn, winter, spring and summer in the region. For full details of the ecology of each of the sites and the methods used for trapping and necropsy of animals, see Eira et al. (Reference Eira, Torres, Vingada and Miquel2006), where the methods used in the field and in the laboratory are comprehensively detailed. The range of helminth species identified in these mice and the number of factors recorded, together with the levels of each factor, are summarized in Table 1.
Table 1. Prevalence of helminths from the Portuguese, Egham and Malham sites
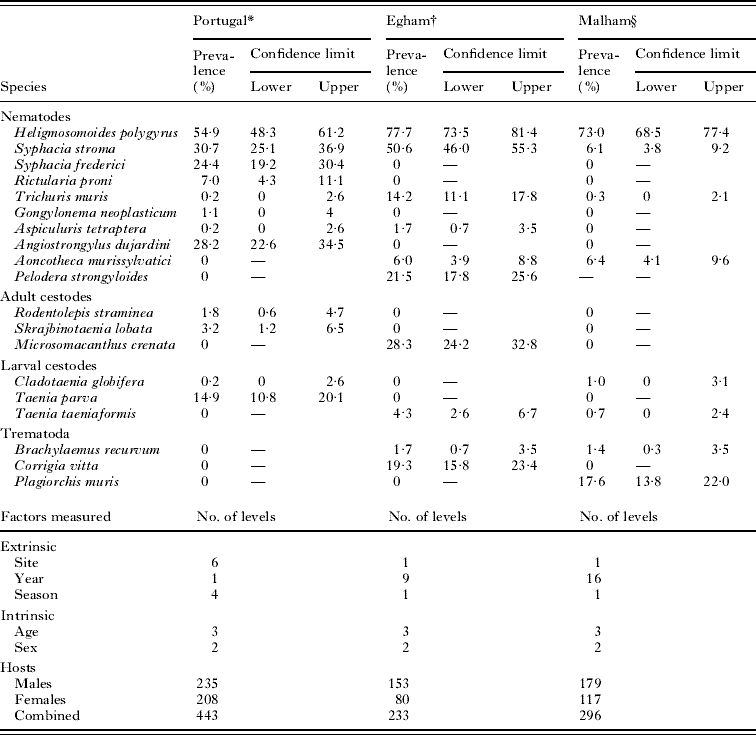
* Eira et al. (Reference Eira, Torres, Vingada and Miquel2006).
† For the first 4 years (1994–1997) see Behnke et al. (Reference Behnke, Lewis, Mohd Zain and Gilbert1999). Data from 1998 to 2002 are in preparation for submission.
§ The full analysis of this dataset has not been published.
The second dataset is derived from wood mice trapped in the grounds of the Royal Holloway, University of London, at Egham in Surrey. This ‘Egham’ dataset is based on mice trapped only during late summer, so it is not affected by seasonal variation. We (Behnke et al. Reference Behnke, Lewis, Mohd Zain and Gilbert1999) analysed the epidemiology of helminths in mice caught in the period 1994 to 1997 and data from these 4 years were also utilized in our earlier analysis of interactions between helminths (Behnke et al. Reference Behnke, Gilbert, Abu-Madi and Lewis2005), although that paper was largely based on another study originally conducted by Abu-Madi et al. (Reference Abu-Madi, Behnke, Lewis and Gilbert1998, Reference Abu-Madi, Behnke, Lewis and Gilbert2000) and the data from Egham played only a minor role. The current analysis is based on the addition of a further 5 years of data to the Egham dataset, now spanning the period from 1994 to 2002. The range of helminth species identified in these mice and the number of factors recorded, together with the levels of each factor, are summarized in Table 1.
The third dataset concerns wood mice trapped in the woodlands north of Malham Tarn in Yorkshire, UK. This ‘Malham’ dataset is based also on mice trapped only during late summer, but across a 16-year period from 1993 to 2008. The range of helminth species identified in these mice and the number of factors recorded, together with the levels of each factor, are summarized in Table 1. Details of the methods used to catch, necropsy mice and allocate them into age classes can be found in Rogan et al. (Reference Rogan, Craig, Hide, Heath, Pickles and Storey2007).
Hypotheses
In contrast to our earlier paper where we looked for evidence of interactions between species employing a range of different analytical procedures, in this paper we set out specifically to test the a priori hypothesis that mice infected with H. polygyrus would be predisposed to infection with other helminths, and should therefore show a higher prevalence and a higher species richness of other helminths. We used 2 response variables. Firstly, each animal was recorded as either infected or not infected with any of the other species of helminths (i.e. excluding H. polygyrus). This binary factor is referred to hereafter as ‘presence/absence of other helminth species’. Secondly we recorded the number of other helminth species in each animal; this factor is referred to as ‘species richness of other helminths’.
Statistical analysis
Binary prevalence data were analysed by maximum likelihood techniques based on log-linear analysis of contingency tables, using the software package SPSS (Version 12.0.1.). Full factorial models incorporated the intrinsic factors age (3 age classes, all data-sets) and sex (2 levels, males and females), and the extrinsic factors of site (6 levels, Portuguese dataset), year of survey (9 levels [1994 to 2002] in the Egham data-set or 16 [1993 to 2008] in the Malham dataset) and season (4 levels, corresponding to 4 seasons in the Portuguese data-set). Infection with H. polygyrus was considered as a binary factor (present/absent), as was also the presence/absence of other helminth species. One of the species of helminths recorded in Portugal, Angiostrongylus dujardini does not inhabit the intestine or peritoneal cavity of the host, and in a final phase of this analysis we also examined the effect of the presence/absence of H. polygyrus on other helminth species but this time excluding A. dujardini. At Egham Pelodera strongyloides was recorded, another species that does not inhabit the intestine, and the same approach was used here.
In each case, beginning with the most complex model, involving all possible main effects and interactions, elements that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion, beginning with the highest-level interaction. A minimum-sufficient model was then obtained, for which the likelihood ratio (L.R.) of χ2 was not significant, indicating that the model was sufficient in explaining the data. The importance of each term in the final model was assessed by the probability that its exclusion would alter the model significantly: these values are given in the text, but only for those terms that included both the presence/absence of H. polygyrus and the presence/absence of other helminth species. The number of other terms is also stated, but for brevity we do not give the details of each (these can be obtained from J.M.B. by request). Prevalence data (percentage of animals infected with various combinations) are shown with 95% confidence limits (CL95), calculated with bespoke software following Rohlf and Sokal (Reference Rohlf and Sokal1995).
Analysis of quantitative data was conducted in 2 phases. Firstly we fitted generalized linear models (GLM) with negative binomial errors, normal errors on Box-Cox transformed data, Poisson errors or quasibinomial errors, as relevant, using R version 2.2.1 (R Core Development Team). Models were fitted using the appropriate GLM routine in the MASS library of R (Venables and Ripley, Reference Venables and Ripley1997). We first fitted full factorial models and if these converged, we employed the STEP procedure in R to simplify the models. We then tested the significance of the highest-order interaction by comparing models with or without that interaction. Next, the significance of each 3-way interaction was tested by comparing models with and without that interaction, but incorporating all the other 3-way interactions, 2-way interactions and main effects. This was repeated for 2-way interactions and then main effects. Minimum-sufficient models were then fitted (all significant interactions and main effects+any non-significant main effects that featured in interactions) and the process was repeated to obtain values for changes in deviance, test statistics and probabilities. The residuals of these models were saved.
Secondly, following Lotz and Font (Reference Lotz and Font1994) and Behnke et al. (Reference Behnke, Gilbert, Abu-Madi and Lewis2005), we confined further analysis to animals that harboured H. polygyrus and at least 1 worm of another species. This approach avoids false conclusions generated by excessive zero values in correlational tests. We assessed the correlation (Pearson's coefficient of correlation) between the residuals of the minimum-sufficient GLM of H. polygyrus and the residuals of GLM of species richness of other helminths. For these we used one-tailed tests, since we were testing a priori hypotheses with clearly predicted positive relationships. In the case of the final analysis of the Malham Tarn dataset, we confined the analysis to mice infected with H. polygyrus and calculated the prevalence of other species, for reasons given in the Results section.
RESULTS
Prevalence of species
The prevalence of species of helminths in all 3 studies is summarized in Table 1. Analysis of the factors influencing the prevalence and abundance of helminths in the Portuguese dataset has been published (Eira et al. Reference Eira, Torres, Vingada and Miquel2006). Behnke et al. (Reference Behnke, Lewis, Mohd Zain and Gilbert1999) provided the analysis of prevalence and abundance of helminths in the period 1994–1997 from Egham, but the analysis of the full dataset to 2002 is still in preparation. Only the data on Plagiorchis muris in the period 1993–2005 have been published for the Malham dataset (Rogan et al. Reference Rogan, Craig, Hide, Heath, Pickles and Storey2007).
The Portuguese dataset was the most species-rich with 12 species of helminths recovered, 5 of which showed prevalence exceeding 10%. The prevalence of H. polygyrus was 54·9%, and the second most common species was Syphacia stroma. One of the listed species, A. dujardini was not associated with the intestine, but rather with the blood vessels connecting the lungs with the heart.
The dataset from Egham was based on fewer animals, and 10 species of helminths were identified. H. polygyrus was again the most common showing a higher prevalence than in the Portuguese dataset (77·7%). S. stroma was again the second most-common species. Six species showed prevalence exceeding 10%. One species, P. strongyloides, was not associated with the intestine, but rather with the skin, nasal passages and the eye sockets (Hominick and Aston, Reference Hominick and Aston1981).
Wood mice from Malham were infected with 8 species of helminths and, as elsewhere, H. polygyrus was the most common (73·0%). Here, S. stroma was rarer than in the first 2 datasets (6·1%) and instead the second most-common species encountered was the digenean P. muris (17·6%). H. polygyrus and P. muris were the only 2 species to occur in more than 10% of the animals. P. strongyloides was present in the population, but infections with this species were only quantified in some years, and therefore the species was excluded from the analysis.
Only 1 species of nematode was common (H. polygyrus) but 3 others (S. stroma, Trichuris muris and Aspiculuris tetraptera), were present in all 3 studies. The last of these was recorded in 1 mouse from Malham but the identity was not confirmed and it was removed from the analysis. S. stroma was common in the Portuguese and Egham datasets but far less so in the Malham wood mice. T. muris was more common in the Egham dataset than in the Portuguese or Malham datasets. None of the adult cestodes overlapped among these studies, but larval T. taeniaeformis was encountered in both Egham and Malham datasets.
Multiple species infections
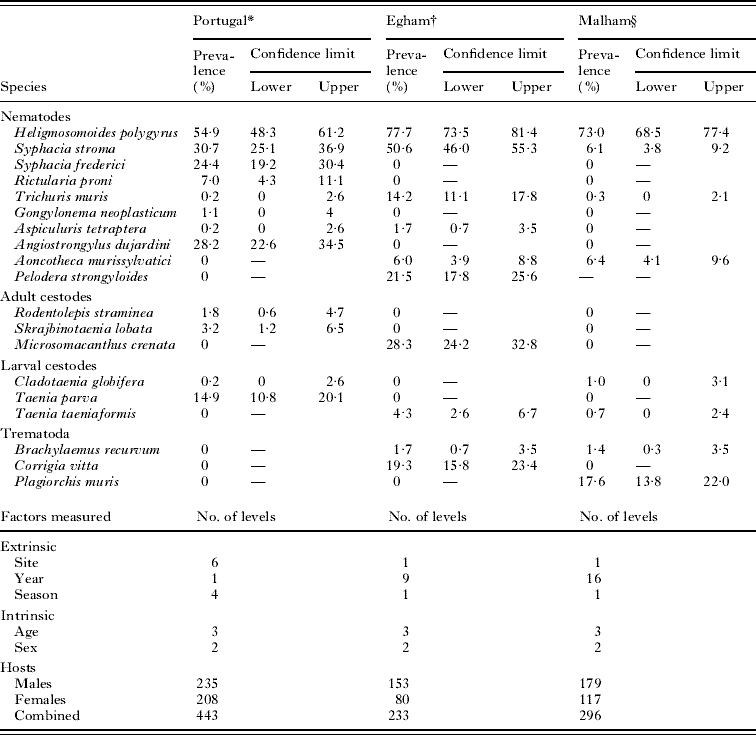
The species density distributions are illustrated in Fig. 1 (Janovy et al. Reference Janovy, Clopton, Clopton, Snyder, Efting and Krebs1995). In the first 2 datasets (Portuguese and Egham) the maximum number of helminth species harboured was 6, whereas at Malham no mice harboured more than 3 species concurrently. In the Portuguese dataset (Fig. 1A), 31·4% (25·5–37·8) were infected with just a single species of helminth, but 50·8% (44·3–57·3) harboured more than a single species. At Malham (Fig. 1C) most wood mice harboured just 1 species, (53·4%, 48·1–58·6) and the percentage harbouring more than 1 species concurrently was correspondingly lower (25·3%, 21·0–30·2). In the Egham dataset (Fig. 1B) 17·2% (13·8–21·1) of mice were infected with 1 species and 72·5% (68·2–76·5) harboured more than just 1 species.
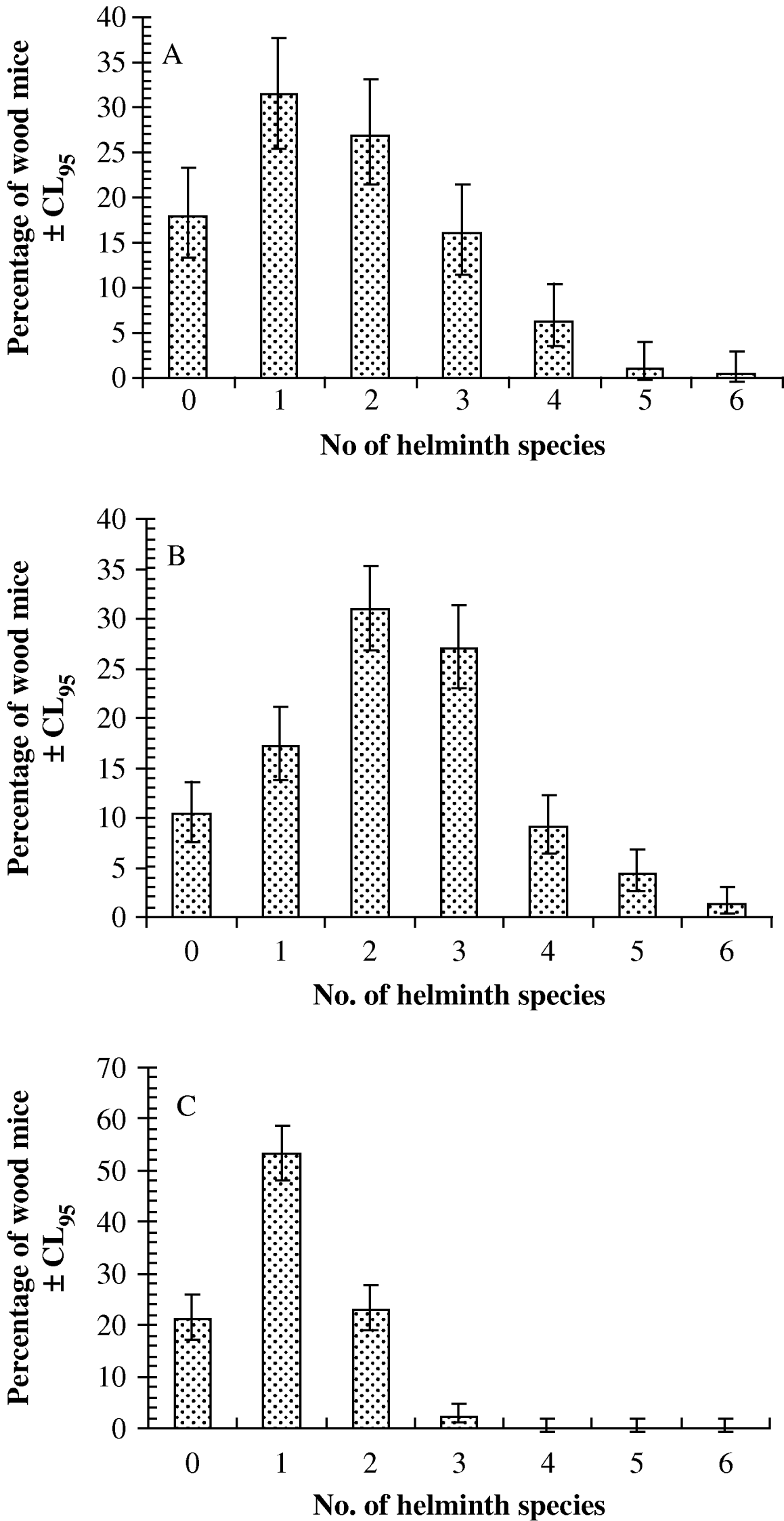
Fig. 1. Species density distributions for the Portuguese (A), Egham (B) and Malham (C) datasets.
Effect of Heligmosomoides polygyrus on prevalence of other helminths
Portuguese sites
In this dataset 52·5% (46·0–60·0) of mice without H. polygyrus were infected with other helminths, but the prevalence was almost 8% higher among mice with H. polygyrus (60·5%, 55·7–65·0). There was no significant difference between the sexes in this respect (otherwise sex would have been retained as a factor in the minimum sufficient model; see below) and not surprisingly the difference was evident in summary data in both males (57·0% of mice without H. polygyrus versus 67·4% of mice with H. polygyrus) and in females (48·0% versus 51·9%, respectively), with the discrepancy greater, but not significantly so among male mice (10·4% vs 3·9%).
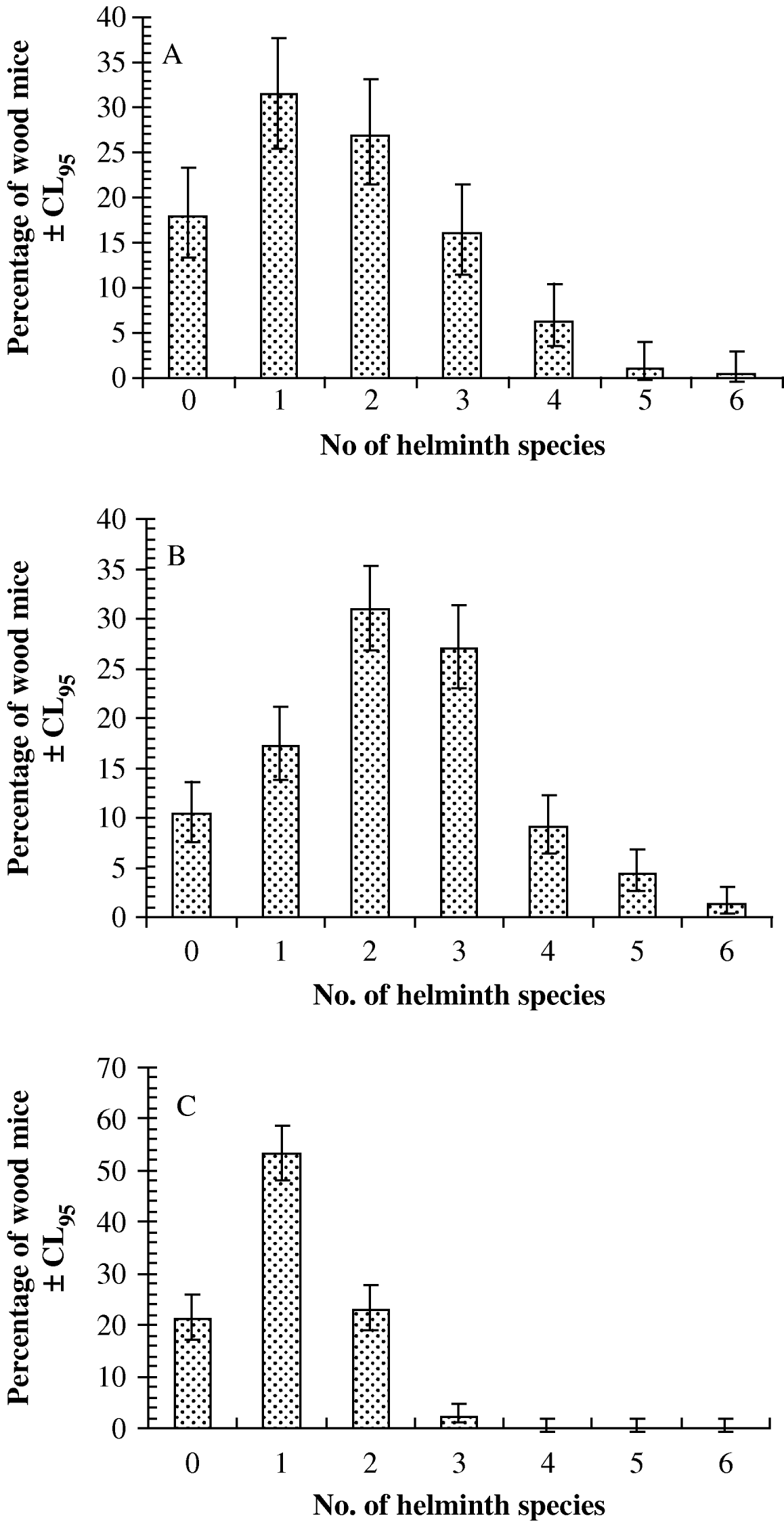
Log-linear analysis revealed just 1 relevant significant interaction term (season∗presence/absence of H. polygyrus∗presence/absence of other helminth species: L.R. χ23=9·43, P=0·024) in the minimum-sufficient model (goodness of fit of model L.R.χ2114=111·55, P=0·55; note that this was a model in which age was not fitted). This interaction is illustrated in Fig. 2A, where it can be seen that in each of the 4 seasons, mice carrying H. polygyrus also showed a higher prevalence of other species of helminths.
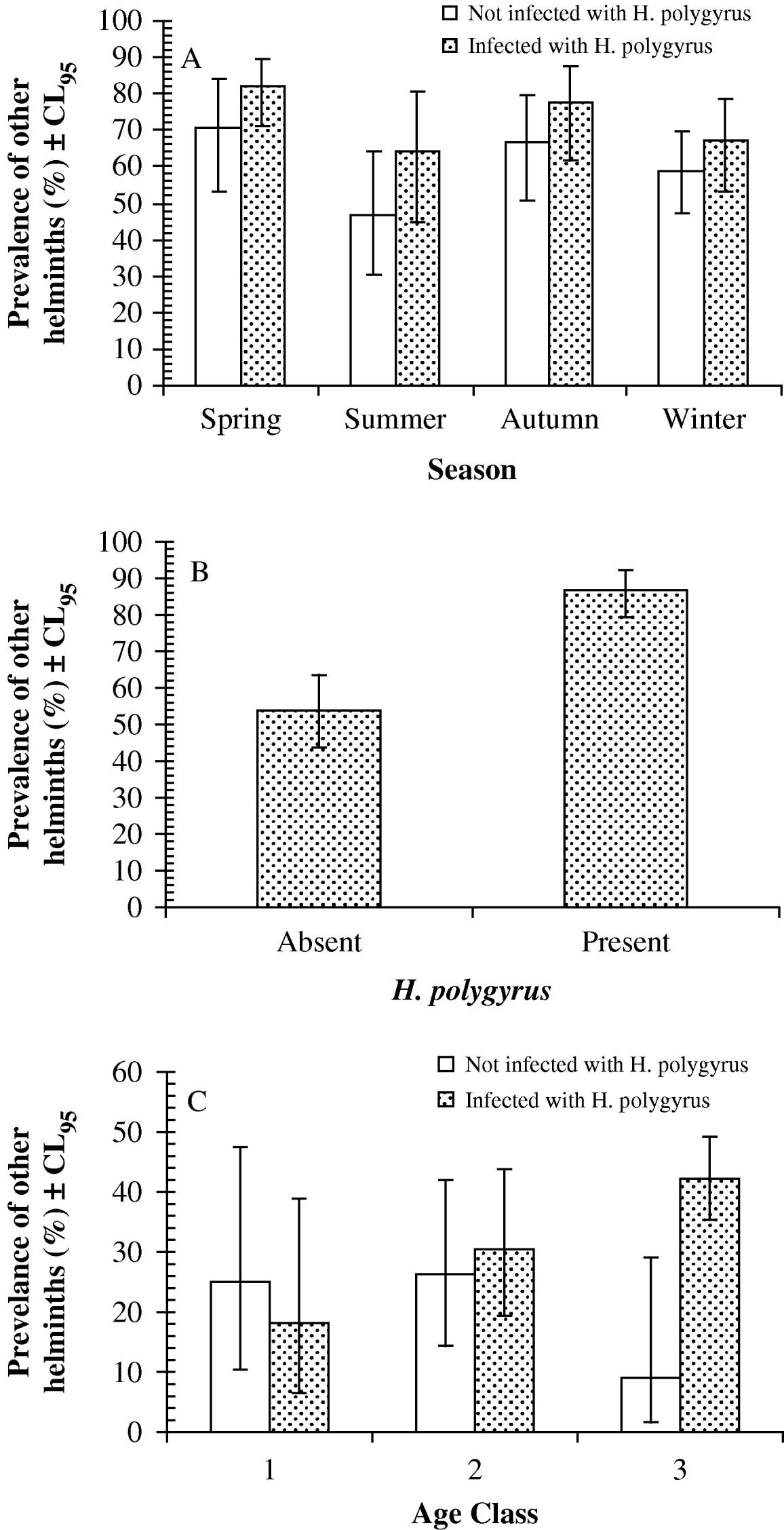
Fig. 2. Prevalence of other species of helminths in wood mice with or without Heligmosomoides polygyrus. (A) Interaction with season in the Portuguese dataset. The sample sizes were 48, 45, 39 and 68 for mice without H. polygyrus, and 78, 28, 40 and 97 for those with H. polygyrus in spring, summer, autumn and winter, respectively. (B) Unaffected by other factors in wood mice at Egham. The sample sizes were 52 for mice without H. polygyrus and 181 for those with H. polygyrus. (C) Interaction with age in the Malham dataset. The sample sizes were 20, 38 and 22 for mice without H. polygyrus, and 22, 92 and 102 for those with H. polygyrus in age classes 1, 2 and 3 respectively. For statistical analysis see text.
Exclusion of A. dujardini (because it is not an intestinal species) from the category ‘other helminth species’ yielded a more complex model with an interaction between site, season and presence/absence of H. polygyrus (L.R. χ215=30·26, P=0·011), but it was not possible to probe this further because with 48 possible combinations (season=4, site=6, presence/absence of H. polygyrus=2, therefore 4×6×2=48) some cells had no data and others were based on very small sample sizes. However, examining the relationship with just season (excluding site) gave much the same outcome as earlier, with mice infected with H. polygyrus generally showing higher prevalence of other helminth species (excluding A. dujardini) in all months except the winter (prevalence of other helminths was 69·2% vs 56·3% in spring, 57·1% vs 42·2% in the summer, 67·5% vs 59·0% in the autumn for mice with and without H. polygyrus respectively: in the winter, prevalence was almost identical at 51·5% in mice with H. polygyrus compared with 52·9% in those without H. polygyrus).
Egham site
The Egham dataset showed a larger divergence in prevalence with other helminth species between wood mice infected (86·7%, 79·3–92·1) and not infected (53·9%, 43·7–63·5) with H. polygyrus (Fig. 2B). Analysis by log-linear procedures generated a minimum-sufficient model with 4 terms (L.R.χ2125=82·36, P=0·99), one of which was presence/absence of H. polygyrus∗presence/absence of other helminth species: this was highly significant and not confounded by other factors (L.R.χ21=23·7, P<0·0001).
The difference in prevalence was also clearly apparent in both sexes. In male wood mice not infected with H. polygyrus the prevalence of other helminth species was 55·3% (39·4–69·9) compared to 88·7% (83·1–92·7) among those with H. polygyrus. In females the values were very similar (50·0% [23·8–76·2] versus 83·3% [73·5–90·2]).
Here again, 1 species was not intestinal. P. strongyloides has a phoretic relationship with mice, exploiting them only for temporary refuge when environmental conditions are detrimental to survival (Hominick and Aston, Reference Hominick and Aston1981). It occurs in the lachrymal ducts, eye sockets and skin during its residence in mice, before emerging and completing its life cycle externally when the mice have died. Therefore, we repeated the analysis excluding P. strongyloides from the category ‘other helminth species’. The outcome was much the same as earlier. The most-parsimonious model (L.R. χ2151=125·8, P=0·933), included 4 terms, of which 1 was presence/absence of H. polygyrus∗presence/absence other helminth species (excluding P. strongyloides), not confounded by other factors, and an essential component of the final model (L.R.χ21=22·95, P<0·0001).
Malham Tarn site
As in the other datasets, prevalence of other helminths was higher among mice carrying H. polygyrus (34·7%, 30·49–39·16) compared with those without H. polygyrus (21·3%, 12·62–33·35), and this difference was evident in both males (34·5% [26·95–42·75] versus 24·3% [12·83–39·76]) and females (35·1% [24·58–47·05] versus 18·6% [8·38–35·09]).
Log-linear analysis generated a minimum-sufficient model (L.R. χ2350=269·2, P=1·0) with just 2 terms 1 of which was relevant (presence/absence of H. polygyrus∗presence/absence of other helminths∗age class; L.R.χ22=7·0, P=0·029), indicating that the prevalence of other helminth species depended on both the prevalence of H. polygyrus and host age (Fig. 2C). Although there was no difference in prevalence of other helminths among wood mice in age classes 1 and 2, among the oldest mice (age class 3) prevalence of other helminths was more than 4-fold higher in mice with, compared to those without, H. polygyrus.
Effect of Heligmosomoides polygyrus on species richness of other helminths
Abundance of infections and species richness
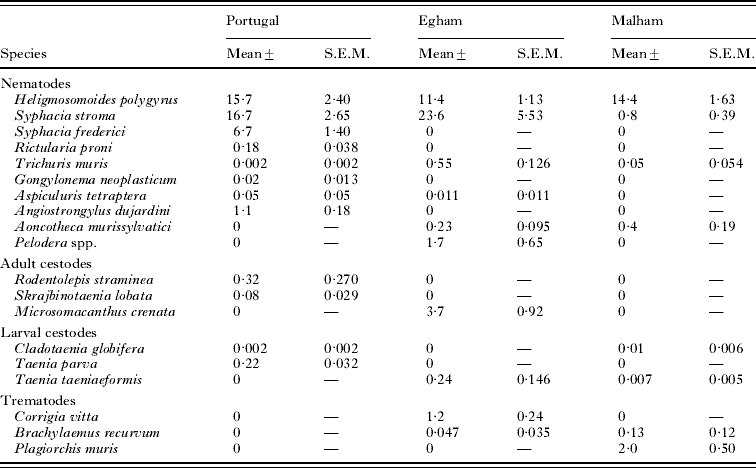
The mean abundance of each of the species recovered from each study site is summarized in Table 2. This shows quite clearly the remarkably similar abundance of H. polygyrus in these 3 study sites, despite their geographical separation and climatic differences. It also shows the degree to which this species dominated the infracommunities of the wood mice. Only S. stroma showed comparable abundances in 2 of the study sites, and the other species were far less abundant.
Table 2. Abundance of helminths from the Portuguese, Egham and Malham sites
(The table shows the pooled abundance across all subsets of data.)
Mean species richness was highest at Egham (2·25±0·087), lower in Portugal (1·67±0·058) and lowest at Malham (1·06±0·043). When H. polygyrus was excluded these values became 1·48±0·074, 1·12±0·050 and 0·33±0·030, respectively. Clearly at Malham very few animals were infected with helminths other than H. polygyrus (31·1%, 26·34–36·20), compared with 67·5% (61·11–73·35) in the Portuguese dataset and 79·4% (75·35–82·91) in Egham.
Portuguese sites
The species richness of other helminths was analysed by a GLM model with Poisson errors, initially fitting all 4 factors (site, season, host age and sex) in a full factorial model. However, the data were best accounted for by a minimum-sufficient model that comprised only the main effects of season (χ23=10·3, P=0·02), site (χ25=27·1, P<0·00001) and host age (χ22=56·1, P<0·00001). The precise effect of each of these factors on species richness is described fully by Eira et al. (Reference Eira, Torres, Vingada and Miquel2006). The residuals from this analysis were saved.
Attempts to fit a model with negative binomial errors for the abundance of H. polygyrus failed to converge, so the data were Box-Cox transformed (Box-Cox lambda=−1·018, Cl95=−1·011 and −1·027; Likelihood=−754·635) and analysed by a model with normal errors. The minimum-sufficient model was season (F 3,431=15·9, P<0·00001)+site (F 5,431=28·9, P<0·00001)+sex (P=NS)+age (F 2,431=15·7, P<0·00001)+season∗site (F 13,429=4·47, P<0·00001)+age∗sex (F 2,418=3·59, P=0·028). The residuals from this model were saved.
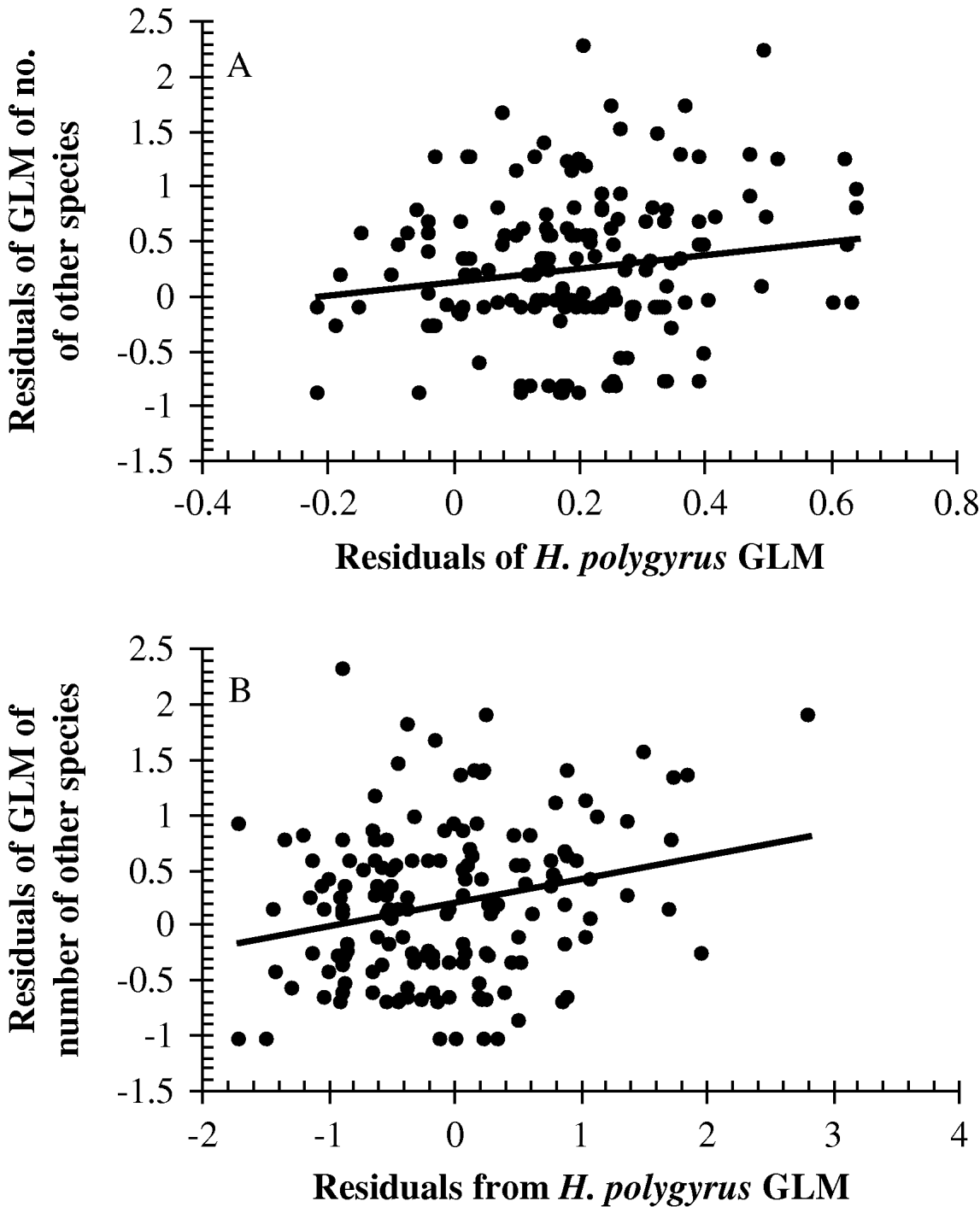
Finally the relationship between the H. polygyrus residuals and the species richness residuals was examined by correlation using data from mice with at least 1 individual of H. polygyrus and 1 worm of another species. These were significantly correlated (r p=0·161, one-tailed test, n=178, P=0·016), and are illustrated as raw values in Fig. 3A and arranged in convenient range classes in Fig. 4A.

Fig. 3. Correlation between the intensity of Heligmosomoides polygyrus infection and number of other species of helminths harboured by wood mice. (A) The relationship in the Portuguese dataset between the residuals of a minimum-sufficient GLM of the species richness of other helminths (Poisson errors, with season, site and age as explanatory factors) and the residuals of a minimum-sufficient GLM of the abundance of H. polygyrus (Box-Cox transformed data and model with normal errors, and season, site, sex, age, season∗site and age∗sex as explanatory factors and interactions). (B) The relationship at Egham between the residuals of a minimum-sufficient GLM of the species richness of other helminths (Poisson errors with year and host age as explanatory factors) and the residuals of a minimum-sufficient GLM of the abundance of H. polygyrus (model with negative binomial errors and year and host age as explanatory factors). Both analyses were confined to mice which harboured at least 1 H. polygyrus and 1 worm of another species and the full statistical analysis is given in the text. Lines of best fit are provided to guide the eye, and these were fitted by a least squares routine in Microsoft Excel.
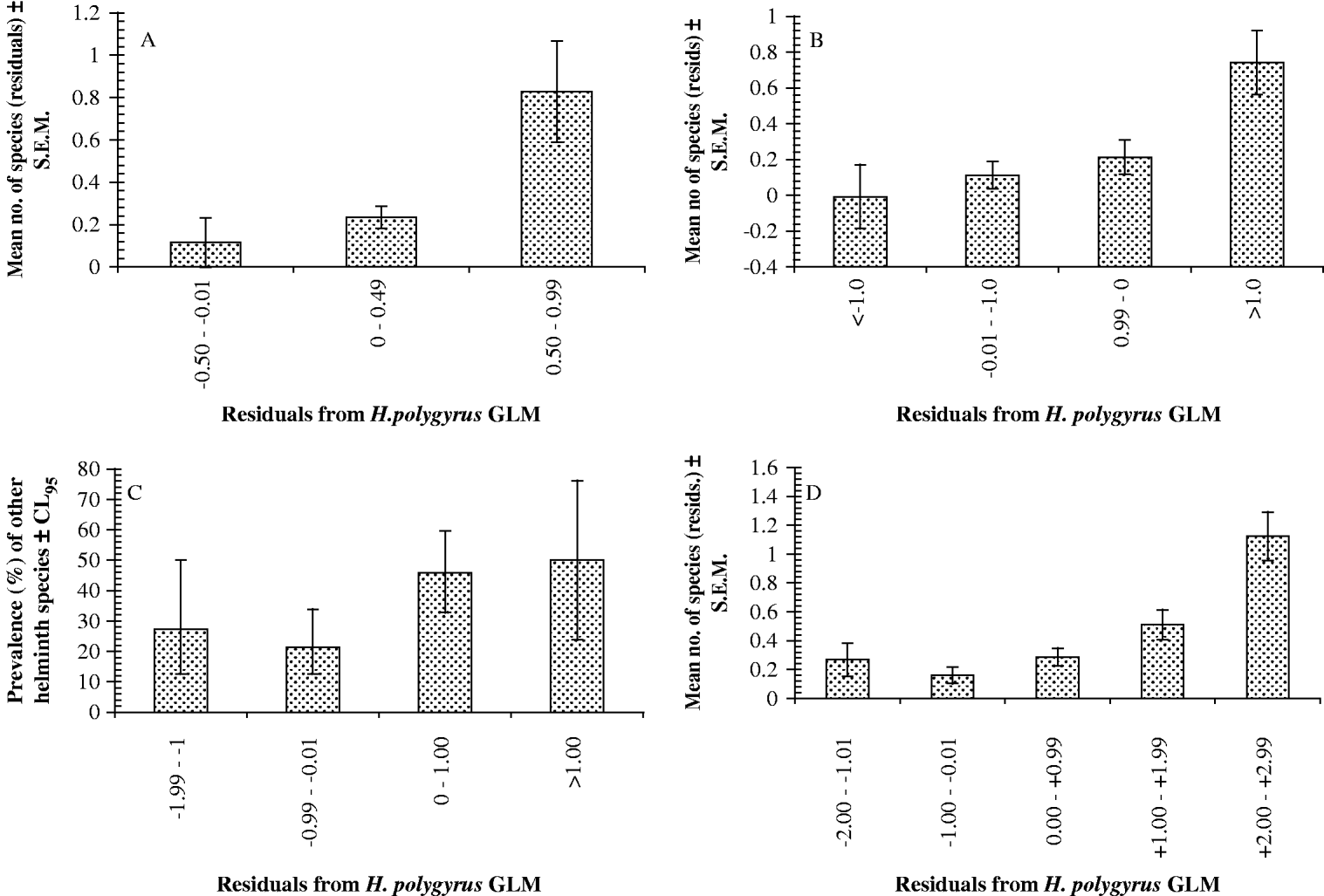
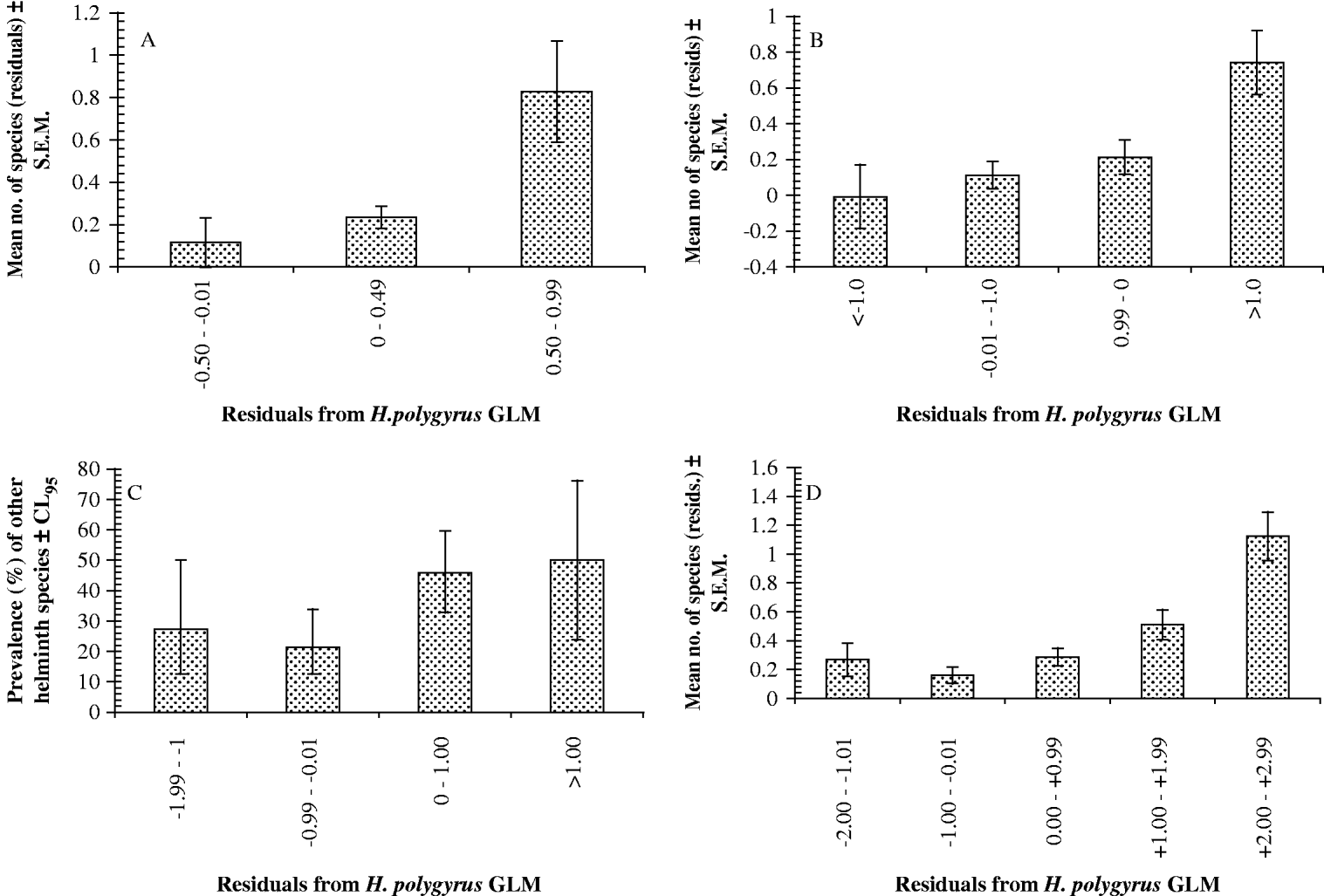
Fig. 4. Relationship between the intensity of Heligmosomoides polygyrus infection and number or prevalence of other species of helminths harboured by wood mice expressed in the form of histograms, with the residuals of the H. polygyrus GLMs in class ranges as shown on the abscissa. (A) Portuguese dataset (no. of mice in columns from left to right=21, 148 and 9). (B) Egham dataset (no. of mice in columns from left to right=14, 73, 56, and 14). (C) Malham dataset (prevalence of other helminth species; no. of mice in columns from left to right=22, 84, 96 and 14). (D) For comparison with the above, data reproduced from Behnke et al. (Reference Behnke, Gilbert, Abu-Madi and Lewis2005) showing the same relationship in the dataset of Abu-Madi et al. (Reference Abu-Madi, Behnke, Lewis and Gilbert1998, Reference Abu-Madi, Behnke, Lewis and Gilbert2000) collected from wood mice in 3 different habitats in the south of the UK (no. of mice in columns from left to right=16, 59, 76, 22 and 6).
Egham site
The species richness of other helminths was analysed by a GLM model with Poisson errors, initially fitting all 3 factors (year, host age and sex). Only age played a significant role in explaining the species richness of other helminth species (χ22=28·4, P=6·9×10−7) with the effect of year just outside significance (χ28=14·5, P=0·069): neither host sex nor any interactions were significant predictors. Accordingly, to err on the side of caution, we saved the residuals of the Poisson model with just host age and year as the explanatory factors, and preferentially conducted further analyses using this model.
Analysis of the abundance of H. polygyrus was carried out by GLM with negative binomial errors. The minimum-sufficient model was year (LRχ28=26·4, P=0·00088) plus age (LRχ22=39·1, P=3·3×10−9). The main effect of host sex and all possible interactions were not significant. The residuals of this model were saved.
The species-richness residuals and the H. polygyrus abundance residuals were then correlated using mice with at least 1 individual of H. polygyrus and 1 worm of another species. They were significantly correlated (r p=0·24, n=157, P=0·0015 one-tailed test) and are illustrated as raw values in Fig. 3B and arranged in convenient range classes in Fig. 4B.
Additionally we also examined the relationship between H. polygyrus abundance residuals and the species-richness residuals of a model with just age as the explanatory factor. This gave a lower value for r p but still significant (r p=0·191, one-tailed test, n=157, P=0·008).
Malham Tarn site
The Malham dataset could not be examined in exactly the same manner because species richness was low, and as explained earlier only 31·1% of mice harboured helminths other than H. polygyrus. Moreover, the maximum number of other helminth species was 2, and only 7 mice fell into this category. Therefore, we fitted a full factorial GLM model with negative binomial errors to the abundance of H. polygyrus. This model could not be simplified, comprising year (LRχ215=27·3, P=0·026)+age (LRχ22=35·2, P=2·299×10−8)+year∗sex (LRχ215=27·8, P=0·023)+year∗age (LRχ229=62·1, P=0·0003)+sex∗age (LRχ22=7·2, P=0·028)+sex∗age∗year (LRχ217=28·6, P=0·038). The main effect of sex was not significant but this was retained because sex featured in significant interactions. The residuals from this model were saved.
We then sorted the residuals of the H. polygyrus GLM into 3 convenient abundance classes, and for animals that were infected with H. polygyrus we calculated the prevalence of other helminth species in each of the 3 classes (Fig. 4C). These indicate that after controlling for age, sex and year and their interactions, the prevalence of other species increased as H. polygyrus abundance increased. In order to test the significance of this relationship, we fitted the fixed factors year, sex and age, and the residuals of H. polygyrus as a covariate in a GLM with quasibinomial errors, with the dependent variable being the binary presence/absence of other helminths species: the data were confined to mice infected with H. polygyrus. Full factorial models did not converge but fitting just the main effects simplified to a model with just age and H. polygyrus residuals (age, χ22=8·1, P=0·018; H. polygyrus residuals, χ21=9·8, P=0·002).
In Fig. 4D we have reproduced the results of our earlier published analysis (Behnke et al. Reference Behnke, Gilbert, Abu-Madi and Lewis2005) based on 3 study sites in the south of the UK, assessed in a single calendar year, to emphasize the similarity of the findings of all 4 studies.
DISCUSSION
In this study we tested the specific a priori hypothesis that the presence of the dominant intestinal nematode of wood mice, H. polygyrus, predisposes wood mice to infection with other helminths. We predicted that the prevalence of other helminth species would be higher in the presence of H. polygyrus, and that the species richness of other helminths would also increase with increasing worm burdens of H. polygyrus. These two predictions were based on the findings of Behnke et al. (Reference Behnke, Gilbert, Abu-Madi and Lewis2005) from 3 ecologically contrasting sites in the UK. Here we tested the robustness of this finding in 3 additional datasets unrelated to one another. Since the current analysis was focused on just 2 predictions, each analysed by a well-defined statistical approach, it was free of the confounding problems of multiple comparisons (including Type I errors) that invalidate many other studies of interactions between species. It is clearly evident from our results that our predictions were both borne out. Both relationships (the presence/absence of other helminth species and the species richness of other helminths) held up in 2 datasets, and there was strong support for a similar relationship in the third dataset (Malham) where overall species richness was poor in comparison to the first 2 study sites. The data were collected in geographically different extremes of Europe and in ecologically quite distinct habitats with different combinations of helminths. On the basis of these results we are confident in concluding that indeed H. polygyrus has an effect on other co-infecting helminths in wood mice, and therefore that quantitative interactions between helminths infecting wild wood mice exist and contribute to infracommunity structure at the individual host level, and to component community structure at the population level.
Perhaps counter-intuitively, the current analysis does not indicate that the mice that are most heavily infected with H. polygyrus in any population are those most likely to carry also a wide range of other helminths. Rather, our data show that mice more heavily infected with H. polygyrus for their own particular subset (i.e. relative to the average burden for a particular year, site, season, age and sex) are the individuals that are most predisposed to being concurrently infected with other helminth species, and the more heavily they are infected for their subset, the more likely they are to carry a greater range of other species than their uninfected or less heavily infected conspecifics.
Whilst correlational analyses such as the current work can never prove cause and effect, there is already a substantial body of information in the experimental field indicating that the very closely related congener H. bakeri has a marked immunodepressive effect on its host, particularly at the local intestinal level, and that as a consequence other helminths reside longer than they normally would in concurrently infected mice (reviewed by Behnke, Reference Behnke1987). Our data are consistent with the idea that H. polygyrus in wild populations of wood mice behaves similarly, not only facilitating its own survival but equally providing an opportunity for other species of helminths to exploit the host's weakened intestinal defences to benefit their own survival and reproductive effort. We are unaware of any experimental assessments of the immunocompetence of wild wood mice carrying heavy burdens of H. polygyrus, but Jackson et al. (manuscript in preparation) have recently reported that the abundance of H. polygyrus in wild wood mice correlates negatively with innate immune responsiveness, implying that this parasite like H. bakeri does have immunomodulatory properties.
In recent years, the question of polyparasitism has attracted considerable attention (Graham, Reference Graham2002; Lello et al. Reference Lello, Boag, Fenton, Stevenson and Hudson2004; Graham et al. Reference Graham, Cattadori, Lloyd-Smith, Ferrari and Bjornstad2007), although in reality the subject has a long history of experimental investigation under laboratory conditions (Christensen et al. Reference Christensen, Nansen, Fagbeni and Monrad1987; Behnke et al. Reference Behnke, Bajer, Sinski and Wakelin2001). The underlying mechanisms that lead to multiple-species infections have come under both theoretical (Dobson, Reference Dobson1985) and experimental scrutiny (reviewed by Behnke et al. Reference Behnke, Bajer, Sinski and Wakelin2001; Behnke, Reference Behnke2008), driven partly by the ideas that limited intrinsic resources leave hosts with life-history choices relating to their defences against infection, and hence resistance to some species of parasites may be traded off against susceptibility to others (Jolles et al. Reference Jolles, Ezenwa, Etienne, Turner and Olff2008). In part this may be mediated by the compartmentalization of the immune system into apparently mutually antagonistic Th1 and Th2 driven arms (the former of which is optimal in protection against intracellular organisms, the latter more appropriate for metazoan parasites such as helminths) with resultant consequences for the epidemiology of important human infections controlled by these two arms of the immune system e.g. exacerbation of malaria by concurrent helminth infections (Druilhe et al. Reference Druilhe, Tall and Sokhna2005; Graham et al. Reference Graham, Cattadori, Lloyd-Smith, Ferrari and Bjornstad2007). Motivation for the study of polyparasitism is also driven by the urgency to understand the epidemiological consequence of immunodepression inducing HIV infection, and other emerging diseases, in the context of an array of already established pathogens and symbionts which the host may harbour simultaneously and/or be exposed to throughout life (Pedersen and Fenton, Reference Pedersen and Fenton2006). In the context of parasites of wildlife, a particular threat may be posed by introduced host species, which once established generally have impoverished helminth communities in foreign ecosystems because some of their parasites are unsuccessful in surviving in a novel environment (Vignon et al. Reference Vignon, Sasal and Galzin2009), but others may spread to indigenous hosts and farmed livestock with resultant consequences for the balance of parasite communities and their influence on host fitness and survival (Hudson et al. Reference Hudson, Dobson and Newborn1998; Gortázar et al. Reference Gortázar, Ferroglio, Höfle, Frölich and Vicente2007). For these reasons models of co-infection, such as those provided by wild rodent systems, provide an important avenue for testing relevant hypotheses; in this case the consequences of a potentially immunodepression inducing intestinal helminth. Field studies involving intervention, for example by treatment to selectively remove H. polygyrus (Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004), are logically the next step in assessing the overall importance of this nematode in facilitating infection with other helminths in wood mice (Pedersen and Greives, Reference Pedersen and Greives2008).
The history of studies of interactions between helminths in wild rodents is long (Elton et al. Reference Elton, Ford, Baker and Gardiner1931; Kisielewska, Reference Kisielewska1970), but until now no relationship has been discovered that is robust enough to be repeatedly demonstrated in different host populations, and under different ecological circumstances. Our study is therefore the first to do so, and given the prevailing view that quantitative interactions between intestinal helminths play a minor if any role in structuring helminth communities (Haukisalmi and Henttonen, Reference Haukisalmi and Henttonen1993; Poulin, Reference Poulin2001; Behnke et al. Reference Behnke, Gilbert, Abu-Madi and Lewis2005), our results, together with those of Behnke et al. (Reference Behnke, Gilbert, Abu-Madi and Lewis2005), provide firm evidence that at the level of species richness, if not at the level of individual species, a highly predictable element of co-infections in wood mice has now been defined: infection with H. polygyrus has detectable consequences for the susceptibility of wood mice to other helminth species, particularly those associated with the intestine.
We thank the many undergraduate students who contributed to the fieldwork at Egham and Malham over the years, our colleagues Professor P.S. Craig, Professor G. Hide, Dr S. Heath and Professor D. Storey, and all SPVS personnel at the Quiaios Field Station (Portugal). We are grateful to Drs P. Harris and J. Jackson for their comments on earlier drafts of this manuscript.